Abstract
Background
Benign colon polyps may require bowel resection if endoscopic polypectomy cannot be performed to assess adequately for cancer. However, endoscopic removal still may be possible using combined endoscopic and laparoscopic surgery (CELS). The CELS procedure allows for intra- and extraluminal manipulation of the bowel wall to facilitate polyp removal, thereby avoiding bowel resection. This study evaluated the authors’ institutional experience with CELS in this patient population.
Methods
Between August 2008 and October 2012, all patients referred to undergo surgery for a benign colon polyp were retrospectively reviewed for operative characteristics, pathology, and postoperative outcomes. Of 14 patients, five were considered candidates for CELS and were compared with nine patients who underwent resection.
Results
The average patient age was similar between the two groups (CELS, 64.9 years vs. resection, 68.3 years). The mean polyp size was 2.3 cm in the CELS group and 2.9 cm in the resection group. In the CELS group, polyps were successfully removed in all cases. The mean operating room time was 159 min in the CELS group and 205 min in the resection group. The median hospital stay was 1 day in the CELS group and 5 days in the resection group. No complications occurred in the CELS group. Two patients in the resection group (22 %) experienced a wound infection. One patient had a postoperative ileus (11 %). Four patients in the CELS group had a benign adenoma. One patient had a benign frozen section evaluation, but the final pathology showed adenocarcinoma requiring a subsequent colectomy. In the resection group, six patients had a benign adenoma, and three patients had a T1N0 cancer. In the CELS group, repeat endoscopy was performed an average of 9.9 months after CELS. Two patients had a residual polyp, and two patients had new polyps in a different location. All were successfully removed.
Conclusion
For benign-appearing polyps not amenable to endoscopic techniques alone, CELS may be an alternative to formal bowel resection for carefully selected patients. The CELS procedure can be performed safely with minimal morbidity and with outcomes that compare favorably with those of formal colectomy.
Keywords: CELS, Colon polyp, Combined endoscopic, Laparoscopic surgery
Benign-appearing colonic polyps that cannot be removed by conventional endoscopic techniques typically are referred for surgical resection, mainly due to the risk of cancer, estimated at roughly 15 % in this subset of patients [1]. Although generally safe, a segmental colectomy is associated with risks that include wound infection, anastomotic leak, and stoma formation. Furthermore, a large percentage of patients undergoing colectomy for this indication actually will not have cancer.
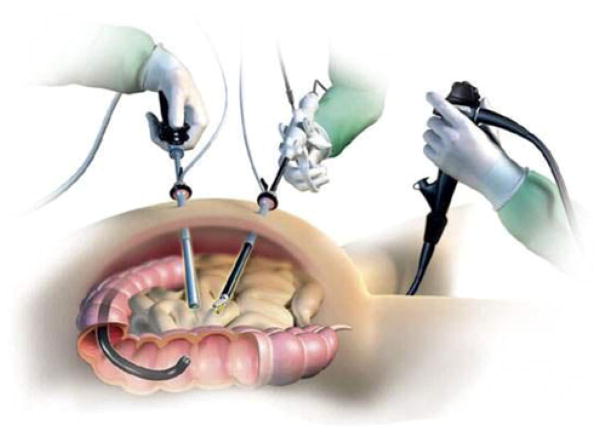
Combined endoscopic–laparoscopic surgery (CELS) has recently been described as an alternative method for addressing these polyps without the need for a formal bowel resection [2]. The technique involves a hybrid, multiteam approach in which endoscopic polypectomy is facilitated with laparoscopic techniques in the operating room (Fig. 1).
Fig. 1.
Illustration of combined endoscopic-laparoscopic surgery (CELS)
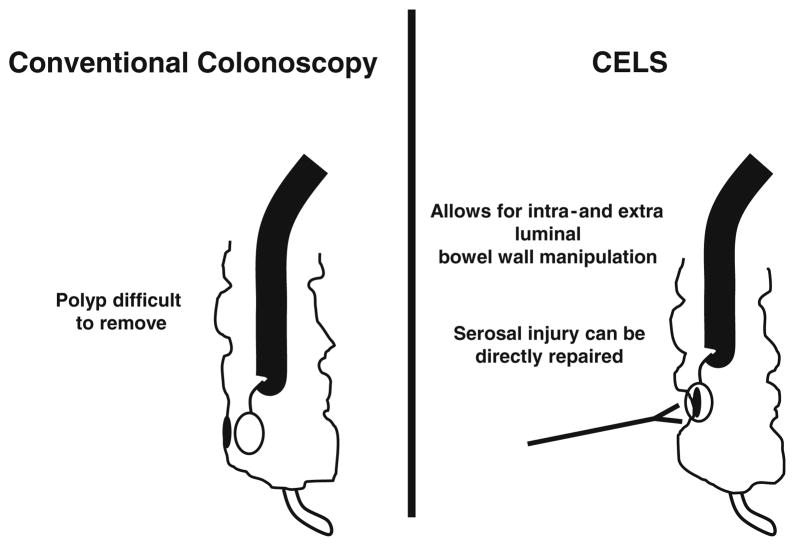
Polyps that cannot be removed endoscopically typically are large or in an anatomically difficult area that is inaccessible, such as behind a mucosal fold or where attempted removal may risk bowel perforation. External manipulation of the bowel wall with laparoscopic instruments may improve the likelihood of endoscopic removal (Fig. 2), and direct laparoscopic visualization of the bowel wall can enable identification and treatment of full-thickness injuries caused by endoscopic polypectomy.
Fig. 2.
CELS allows for extraluminal bowel wall manipulation and repair
Using the CELS approach, we evaluated our institutional experience in the management of colonic polyps that were not removable using standard endoscopic techniques and those that were initially referred to our institution for surgical resection. We demonstrated that for selected patients, CELS is feasible and safe, enhancing our ability to perform endoscopic polypectomy and avoid bowel resection for a large majority of these patients.
Methods
In this retrospective, nonrandomized, single-institution study, all patients referred to the Section of Colorectal Surgery at the Ronald Reagan UCLA Medical Center for surgical resection of a benign colon polyp from August 2008 to October 2012 were reviewed. Medical records were reviewed for age, polyp size and location, operative characteristics, pathology, hospital stay, postoperative outcomes, and follow-up endoscopic results. The study was approved by the University of California at Los Angeles (UCLA) institutional review board through the Office of the Human Research Protection Program.
Patients were considered candidates for CELS based on previously established selection criteria [2], which specified a large polyp not removable by an experienced endoscopist, previous endoscopic biopsies that were benign or demonstrated only high-grade dysplasia (no carcinoma present), an endoscopic appearance consistent with a benign polyp, and surgery performed on an elective basis. The CELS group was compared with patients not considered candidates for CELS who underwent resection (resection group).
Informed consent was obtained from all the patients after a careful discussion about the risks and benefits of the CELS approach, which currently is not considered standard therapy. Included in the discussion was the risk that cancer may be present in the polyp (~15 %), the need for follow-up endoscopic surveillance, the potential need for laparoscopic repair of the bowel wall or a bowel resection if endoscopic polyp removal was not possible, and the need for surgical resection at a later date if the frozen section analysis was not consistent with the final pathology.
The surgical procedure was performed in the following fashion. After establishment of general endotracheal anesthesia, a urinary catheter and orogastric tube were placed. The patient was placed in modified lithotomy position with appropriate padding and then prepped and draped in the usual sterile fashion.
Pneumoperitoneum was established, and the peritoneal cavity was carefully inspected. With the endoscopist standing between the legs of the patient, a colonoscopy then was performed using carbon dioxide (CO2) as the insufflating gas. The polyp was identified and carefully examined. The involved segment of bowel often required laparoscopic mobilization with release of lateral, omental, or retroperitoneal attachments to allow for adequate visualization and external manipulation of the bowel wall. Endoscopic polypectomy then was performed using saline lift techniques and snare polypectomy, with laparoscopic visualization, and manipulation of the serosal surface of the colon wall as needed.
After completion of the polypectomy, a frozen section analysis was performed. If the frozen section analysis was consistent with adenocarcinoma, a formal bowel resection was performed. Otherwise, the bowel wall was carefully inspected and repaired if any bowel wall injury was detected.
For patients who underwent a CELS polypectomy, follow-up surveillance typically was performed within the next 6–12 months to evaluate for complete removal of the polyp. Additional follow-up assessment was dependent on the previous endoscopic findings.
Results
Of the 14 patients referred for surgery, five were considered candidates for CELS based on the endoscopic appearance. The polyp had to appear grossly benign and unresectable by current endoscopic methods. The indications for CELS included polyps that spanned a mucosal fold (n = 3) and large or complex polyps (n = 2). The five patients who underwent CELS had polyps located throughout the colon including the cecum (n = 1), hepatic flexure (n = 1), transverse colon (n = 2), and sigmoid colon (n = 1). The CELS group was compared with the nine patients who underwent resection.
The average patient age was similar in both groups (CELS group, 64.9 years; range, 46–79 years vs. resection group, 68.3 years; range, 30–82 years). The mean polyp size was 2.3 cm (range, 1–4 cm) in the CELS group and 2.9 cm (range, 1.5–5.2 cm) in the resection group (Table 1).
Table 1.
Demographics, intraoperative and post operative data
| CELS group | Resection group | |
|---|---|---|
| n | 5 | 9 |
| Average patient age: years (range) | 64.9 (46–79) | 68.3 (30–82) |
| Mean polyp size: cm (range) | 2.3 (1–4) | 2.9 (1.5–5.2) |
| Mean OR time: min (range) | 159 (60–218) | 205 (121–373) |
| Median LOS: days (range) | 1 (1–4) | 5 (3–8) |
| Complications | None | Wound infection × 2 Ileus × 1 |
| Pathology |
|
|
CELS combined endoscopic and laparoscopic surgery, OR operating room, LOS hospital length of stay, TA tubular adenoma, HGD high-grade dysplasia, LN lymph node, TVA tubulovillous adenoma, AdenoCa adenocarcinoma, VA villous adenoma
In the CELS group, polyps were successfully removed in all cases, and frozen section analysis was routinely performed. The polyps were preoperatively tattooed in four of the five CELS patients. One patient with a polyp adjacent to the ileocecal valve was not tattooed. Laparoscopic mobilization to visualize the bowel wall was required for two of the five patients. One of these patients also required laparoscopic manipulation of the bowel wall to facilitate endoscopic polypectomy, together with subsequent repair of the bowel wall due to the presence of pneumatosis seen during snare polypectomy.
The mean operating room time was 159 min (range, 60–218 min) in the CELS group compared with 205 min (range, 121–373 min) in the resection group. The median hospital stay was 1 day (range, 1–4 days) in the CELS group compared with 5 days (range, 3–8 days) in the resection group. No complications or deaths occurred in the CELS group, whereas in the resection group, two patients (22 %) experienced a wound infection, and one patient (11 %) had a postoperative ileus.
In the CELS group, pathology demonstrated an adenoma in four patients without high-grade dysplasia or cancer. One patient had a benign intraoperative frozen section evaluation, but the final pathology 1 week later showed adenocarcinoma, and a sigmoid colectomy was subsequently performed. The final pathology demonstrated no residual cancer and no involved lymph nodes (0/12).
In the resection group, six patients had a benign adenoma without high-grade dysplasia or cancer. Three patients had a T1N0 adenocarcinoma (0/15, 0/22, and 0/22 lymph nodes).
In the CELS group, repeat endoscopy was performed for four of the five patients an average of 9.9 months (range, 3.3–15.6 months) after CELS. Two patients had residual polypoid tissue at the polypectomy site, and two patients had new polyps in a different location. All were successfully removed.
Discussion
Minimally invasive surgical techniques continue to evolve in an effort to treat patients more effectively with less trauma and minimal morbidity. For patients with benign-appearing colonic polyps not removable by traditional endoscopic techniques, CELS appears to be a reasonable alternative for the management of these polyps without the need for a formal bowel resection, which has been the standard approach. The CELS procedure allows for aggressive endoscopic removal because the bowel wall can be carefully manipulated, inspected, and repaired if necessary, laparoscopically.
Our study is the first to compare the outcomes for patients who underwent CELS directly with those for patients who underwent a formal bowel resection. Our early experience with CELS demonstrates successful polyp management and good surgical outcomes with minimal morbidity, together with a significantly shorter hospital stay than experienced by patients who underwent colectomy.
This study also is the first to provide information about what percentage of patients referred to undergo surgery for a benign polyp actually may benefit from CELS. Of the 14 patients referred to our institution for segmental resection, five (35 %) were considered candidates for CELS, and all five patients underwent a successful polypectomy. Our data suggest that the adoption of this technique has the potential to have an impact on a relatively high percentage of these patients.
The feasibility of intraoperative endoscopy has been demonstrated in a number of studies [2–5]. However, combining surgical procedures with conventional colonoscopy, which uses room air for insufflation, was technically difficult in the past because bowel distention from the endoscopic procedure obscured laparoscopic visualization, even when the proximal bowel was occluded to minimize intestinal distention. Fortunately, this problem is now avoided with the use of CO2 rather than room air as the insufflating gas for the endoscopic procedure.
Use of CO2 for endoscopic procedures has been well described and shown to be a safe and effective alternative to the use of room air, resulting in less abdominal distention and decreased postprocedural discomfort [6–8]. This lack of persistent bowel distention occurs because CO2 is absorbed considerably faster than room air. It enters the colonic venous circulation and later is removed from the body via the lungs. This rapid absorption of CO2 and the resultant lack of bowel distention obviates the need to clamp the bowel during CELS [4], permitting laparoscopic and endoscopic procedures to be performed simultaneously.
Currently, the major limitation to this approach involves evaluation of the polyp for the presence of malignancy after polypectomy. At our institution, frozen section evaluation adds at least 30 min to the CELS operative procedure time and may not be consistent with the final pathologic evaluation, as seen once in this study. However, this is a known risk when a CELS approach is attempted. Patients must understand that the risk of invasive cancer is roughly 15 % [1] and that a discrepancy may exist between the frozen section and the final pathologic evaluation, which may necessitate a subsequent bowel resection. In fact, the incidence of invasive cancer in this study was 28 % (4/14).
A careful preoperative discussion with the patient outlining the operative approach together with the potential need for additional surgery at a later date is extremely important as part of the informed consent process. In the future, our outcomes with this technique may improve as newer endoscopic imaging methods such as in vivo confocal laser endomicroscopy combined with narrow-band imaging may better differentiate benign from malignant polyps [9].
Our study had several limitations. It was a retrospective, nonrandomized, single-institution study involving a small number of carefully selected patients. Additionally, a more standardized postoperative endoscopic surveillance program would provide better long-term follow-up data.
Despite these limitations, the results of this study are compelling, and a prospective, multi-institutional study examining the use of this approach would be valuable for better characterization of its potential impact on surgical therapy. The CELS procedure is an emerging surgical technique with many potential applications that may improve surgical outcomes for selected patients. Endoluminal approaches continue to evolve, and use of CELS should be considered for appropriate cases.
Footnotes
Disclosures Minna K. Lee, Formosa Chen, Eric Esrailian, Marcia McGory Russell, Jonathan Sack, and Anne Y. Lin have no conflicts of interest or financial ties to disclose.
Contributor Information
Minna K. Lee, Department of Surgery, David Geffen School of Medicine, University of California at Los Angeles, 10833 LeConte Avenue, 72-253 CHS, Los Angeles, CA 90095, USA
Formosa Chen, Department of Surgery, David Geffen School of Medicine, University of California at Los Angeles, 10833 LeConte Avenue, 72-253 CHS, Los Angeles, CA 90095, USA.
Eric Esrailian, Department of Medicine, David Geffen School of Medicine, University of California at Los Angeles, Los Angeles, CA, USA.
Marcia McGory Russell, Department of Surgery, David Geffen School of Medicine, University of California at Los Angeles, 10833 LeConte Avenue, 72-253 CHS, Los Angeles, CA 90095, USA.
Jonathan Sack, Department of Surgery, David Geffen School of Medicine, University of California at Los Angeles, 10833 LeConte Avenue, 72-253 CHS, Los Angeles, CA 90095, USA.
Anne Y. Lin, Department of Surgery, David Geffen School of Medicine, University of California at Los Angeles, 10833 LeConte Avenue, 72-253 CHS, Los Angeles, CA 90095, USA
James Yoo, Email: jayoo@mednet.ucla.edu, Department of Surgery, David Geffen School of Medicine, University of California at Los Angeles, 10833 LeConte Avenue, 72-253 CHS, Los Angeles, CA 90095, USA.
References
- 1.Jang JH, Balik E, Kirchoff D, Tromp W, Kumar A, Grieco M, Feingold DL, Cekic V, Njoh L, Whelan RL. Oncologic colorectal resection, not advanced endoscopic polypectomy, is the best treatment for large dysplastic adenomas. J Gastrointest Surg. 2012;16:165–171. doi: 10.1007/s11605-011-1746-9. Discussion 171–162. [DOI] [PubMed] [Google Scholar]
- 2.Yan J, Trencheva K, Lee SW, Sonoda T, Shukla P, Milsom JW. Treatment for right colon polyps not removable using standard colonoscopy: combined laparoscopic–colonoscopic approach. Dis Colon Rectum. 2011;54:753–758. doi: 10.1007/DCR.0b013e3182108289. [DOI] [PubMed] [Google Scholar]
- 3.Whelan RL. Laparoscopic-facilitated colonic endoscopic mucosal resection and endoscopic submucosal resection of adenomas: techniques to avoid segmental colectomy. J Gastrointest Surg. 2011;15:1309–1312. doi: 10.1007/s11605-011-1574-y. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima K, Lee SW, Sonoda T, Milsom JW. Intraoperative carbon dioxide colonoscopy: a safe insufflation alternative for locating colonic lesions during laparoscopic surgery. Surg Endosc. 2005;19:321–325. doi: 10.1007/s00464-004-8915-9. [DOI] [PubMed] [Google Scholar]
- 5.Zmora O, Dinnewitzer AJ, Pikarsky AJ, Efron JE, Weiss EG, Nogueras JJ, Wexner SD. Intraoperative endoscopy in laparoscopic colectomy. Surg Endosc. 2002;16:808–811. doi: 10.1007/s00464-001-8226-3. [DOI] [PubMed] [Google Scholar]
- 6.Sumanac K, Zealley I, Fox BM, Rawlinson J, Salena B, Marshall JK, Stevenson GW, Hunt RH. Minimizing postcolonoscopy abdominal pain by using CO2 insufflation: a prospective, randomized, double-blind, controlled trial evaluating a new commercially available CO2 delivery system. Gastrointest Endosc. 2002;56:190–194. doi: 10.1016/s0016-5107(02)70176-4. [DOI] [PubMed] [Google Scholar]
- 7.Church J, Delaney C. Randomized, controlled trial of carbon dioxide insufflation during colonoscopy. Dis Colon Rectum. 2003;46:322–326. doi: 10.1007/s10350-004-6549-6. [DOI] [PubMed] [Google Scholar]
- 8.Bretthauer M, Thiis-Evensen E, Huppertz-Hauss G, Gisselsson L, Grotmol T, Skovlund E, Hoff G. NORCCAP (Norwegian colorectal cancer prevention): a randomised trial to assess the safety and efficacy of carbon dioxide versus air insufflation in colonoscopy. Gut. 2002;50:604–607. doi: 10.1136/gut.50.5.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahid MW, Buchner AM, Heckman MG, Krishna M, Raimondo M, Woodward T, Wallace MB. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow-band imaging for small colorectal polyps: a feasibility study. Am J Gastroenterol. 2012;107:231–239. doi: 10.1038/ajg.2011.376. [DOI] [PubMed] [Google Scholar]