Abstract
This paper describes a modeling framework for estimating the acute effects of personal exposure to ambient air pollution in a time series design. First, a spatial hierarchical model is used to relate Census tract-level daily ambient concentrations and simulated exposures for a subset of the study period. The complete exposure time series is then imputed for risk estimation. Modeling exposure via a statistical model reduces the computational burden associated with simulating personal exposures considerably. This allows us to consider personal exposures at a finer spatial resolution to improve exposure assessment and for a longer study period. The proposed approach is applied to an analysis of fine particulate matter of <2.5 μm in aerodynamic diameter (PM2.5) and daily mortality in the New York City metropolitan area during the period 2001–2005. Personal PM2.5 exposures were simulated from the Stochastic Human Exposure and Dose Simulation. Accounting for exposure uncertainty, the authors estimated a 2.32% (95% posterior interval: 0.68, 3.94) increase in mortality per a 10 μg/m3 increase in personal exposure to PM2.5 from outdoor sources on the previous day. The corresponding estimates per a 10 μg/m3 increase in PM2.5 ambient concentration was 1.13% (95% confidence interval: 0.27, 2.00). The risks of mortality associated with PM2.5 were also higher during the summer months.
Keywords: exposure modeling, particulate matter, time series analysis
INTRODUCTION
There exists substantial epidemiological and toxicological evidence on the adverse health effects of ambient air pollution.1–3 Population-based studies have had an important role in establishing regulatory standards, such as the National Ambient Air Quality Standards (NAAQS), to protect public health.4 For exposure assessment, these studies routinely use concentrations measured at outdoor monitors where ambient levels serve as a surrogate for pollution from outdoor sources. However, individuals spend the majority of their time indoor and air pollution exposures include both ambient and non-ambient sources. Consequently, there has been a continual effort to understand the relationship between ambient concentrations and personal exposures,5,6 as well as the bias in risk estimates when ambient concentrations are used to derive exposure metrics.7 Studies have also suggested that factors affecting ambient contribution to personal exposure may modify air pollution health risks.8,9
Exposures from both ambient and non-ambient sources depend on the locations (microenvironments) and the amount of time spent at the locations. Exposure levels can vary in different microenvironments, such as vehicle, restaurant or home, depending on how much ambient pollutant penetrates indoor and the presence of indoor sources (e.g., environmental tobacco smoke, cooking, cleaning). Empirical findings from panel studies have provided considerable knowledge on how different sources contribute to personal exposures.10–13 These results, along with data on human daily activity patterns,14 have led to the development of several personal exposure simulators. Examples include the probabilistic NAAQS Exposure Model,15 the Air Pollutants Exposure Model16 and the Stochastic Human Exposure and Dose Simulation (SHEDS).17
An exposure simulator predicts daily exposure for a hypothetical individual by first randomly assigning a time sequence of activity pattern that describes the amount of time the individual spends in different microenvironments. The activity pattern is selected to match the characteristic of the individual such as age, sex and occupation. Simplified versions of the exposure simulators based on statistical models have also been proposed.18–20 Exposure simulators were originally developed to assess the impacts of regulatory policies. The ability to obtain exposure distribution for the at-risk population has encouraged their application in health studies. For example, Shaddick et al.21 and Reich et al.22 used simulated exposures to characterize daily group-level exposures to particulate matter (PM) and examined their effects on daily mortality. Also, Berrocal et al.23 utilized SHEDS in an analysis of birth weight and fine PM exposure during pregnancy.
This paper describes a modeling framework for estimating the short-term health effects of personal exposures to ambient air pollution in a time series design. The proposed approach is applied to an analysis of fine PM of <2.5 μm in aerodynamic diameter (PM2.5) and daily mortality in the five-county New York City metropolitan area during the period 2001–2005. We choose to focus on the associations between mortality and personal exposures to PM2.5 of ambient origin, whereas previous studies have used total personal exposures as the exposure metrics. Studies have shown that exposures to ambient and non-ambient fine PM have low correlations. Also, ambient and non-ambient PM often differ in size range and composition.24 Moreover, the NAAQS regulates ambient concentrations and estimating specifically the health effects attributable to outdoor sources enables risk assessment that is policy relevant.
We follow previous approaches to link simulated personal daily exposure to PM2.5 from SHEDS and the health outcome; but we also consider several extensions aimed at reducing computational burden, and improving exposure assessment for the at-risk population. First, in a time series analysis, the outcomes are daily counts of adverse events aggregated over a geographical region that typically involves a large at-risk population and a long study period. Simulating personal exposures for each day is computationally intensive, which limits their use in health studies. We address this challenge by considering a statistical emulator approach. Specifically, personal exposures are only simulated for a subset of the study days. We then build a model between ambient concentrations and simulated average personal exposures in order to impute exposures for the entire study period. The modeling approach is carried out in a Bayesian framework such that uncertainty in exposure estimation can be easily propagated into risk estimation. Using longer study period also allows us to examine seasonal differences in the health effects.
We also consider simulating exposures at a finer spatial resolution compared with previous studies. For example, Holloman et al.18 generated individuals across a county, while Shaddick et al.21 and Reich et al.22 generated individuals in small areas around air quality monitors. Similar to most time series analyses, these studies assumed that the pollutant concentration is relatively smooth across the study area. However, exposure to air pollution can still vary spatially because of: (1) spatial variation in demographic characteristics that can contribute to different activity and commuting patterns; and (2) spatial variation in housing type that can contribute to different ambient contributions to indoor concentration. Moreover, ambient concentration of air pollution can exhibit fine-scale spatial variation, especially in an urban setting. We therefore choose to simulate exposures across all census tracts in the five-county study region. Tract-level exposures are then combined to derive an overall population-weighted exposure for risk estimation.25,26
In this paper, our goal is not to determine which metric (ambient concentration versus personal exposure) is optimal. Instead, we wish to compare the risk estimates obtained from the two exposure metrics. Empirical results from panel studies have demonstrated that ambient concentration differs from personal exposure on the individual level. Our work addresses an important scientific question because true personal exposures cannot be obtained directly in a large population-based study such as a time series analysis.
METHODS
Mortality and Air Pollution Data
Detailed mortality data were obtained through the National Center for Health Statistics. For the period 2001–2005, daily mortality counts for those aged 65 or above were assembled in the New York City metropolitan area. Our study region consisted of the following five counties: Bronx, Kings (Brooklyn), New York (Manhattan), Queens and Richmond (Staten Island). Based on the International Statistical Classification of Diseases 10th revision, we considered deaths because of cardiovascular (I00–I79) and respiratory diseases (J10–J18, J21–J47 and J69–J70). Total population count by sex, and housing type (single-unit detached, single-unit attached, multiple-unit attached and other) were obtained from Census 2000 for 2105 tracts.
Mean daily temperature and dew-point temperature were obtained from the National Oceanic Atmospheric Administration's National Climatic Data Center. Daily ambient PM2.5 data were obtained from the Statistically Fused Air Quality database (http://www.epa.gov/esd/land-sci/lcb/lcbsfads.html). The database contains predicted daily PM2.5 concentration averaged over contiguous 12 km by 12 km grid cells. Predictions are based on a Bayesian space-time hierarchical model27 that combines (1) PM2.5 data from the Air Quality System network and (2) outputs from the Models-3/Community Multiscale Air Quality model.28
Personal exposures to PM2.5 because of outdoor sources were obtained from the SHEDS version 3.7.17 First, we generated 23 hypothetical individuals for each census tract (a total of 48,415 across the study region). The simulation was conducted such that the 23 individuals reflect the demographic proportions of sex, age and residential housing type in each census tract. By simulating individuals within each tract, this approach captured the variation in exposure associated with different at-risk population compositions across census tracts. A smoking status was also randomly assigned using sex-specific smoking prevalence statistics obtained from the New York City Department of Health and Mental Hygiene.29,30 Then for each day in April, July, October and December of the year 2002, the activity pattern of each individual was randomly matched to a diary from EPA's Consolidated Human Activity Database. The diary describes the amount of time an individual spends in various microenvironments for a particular season, day of the week and individual characteristics. In-vehicle exposures are estimated based on a revised approach recommended by Liu and Frey.31
Let Xi (t,s) denote the simulated personal exposure to ambient PM2.5 from SHEDS for individual i on day t in census tract s. Daily exposures were calculated by averaging time-weighted exposure to ambient PM2.5 in nine microenvironments across a 24-h period. Specifically, let Cik(t,s) and Tikh(t,s) denote the concentration of ambient PM2.5 and time spent in microenvironment k during hour h. SHEDS considers nine microenvironments including outdoor, home, office, school, store, restaurant, bar, in-vehicle and all other indoor. Then,
For non-residential microenvironments, Cik(t,s) was determined by a linear function based on empirical analysis of concurrent indoor and outdoor PM2.5 measurements for these microenvironments:
where W(t,s) is the predicted ambient level obtained from the FSD database linked to the tract centroid. The slope parameters were assumed known where boutdoor = 1.0, boffice = 0.18, bschool = 0.60, bstore = 0.75, brestaurant = 1.0, bbar = 1.0, bvehicle = 1.0 and bother = 0.85.17 The coefficient for home was determined by the mass balance equation
where P represents the penetration factor; k represents the deposition rate; and ach represents the air exchange rate. Uncertainty in the contribution of ambient PM2.5 at home was accomplished via a two-stage Monte Carlo approach by assigning probabilistic distribution to the parameters. For each individual, SHEDS randomly selects values based on the following distributions. We assumed P to be triangular (0.70, 0.78, 1.0) and k to be normal with mean 0.40 and SD 0.01. We assumed ach to be log-normal with season-specific geometric mean (spring: 0.40, summer: 0.64, fall: 0.22, winter: 0.45) and geometric SD (spring: 1.82, summer: 2.09, fall: 1.72, winter: 2.03).32–34
Exposure Estimation
Denote X̃(t,s) the sample mean calculated across the 23 simulated personal exposures for each tract. We considered the following random-effect model to relate the tract-level population-average exposures to ambient PM2.5 concentrations. We refer to this model as the emulator for SHEDS. We allowed all parameters in the model to be season specific because in the SHEDS simulation, contribution of ambient PM2.5 at home and the associated uncertainty are season specific. To simplify notation, in the following discussion we drop the index for seasons on the model parameters.
We assumed a linear relationship between the log average personal exposure and the log ambient concentration with tract-specific intercept α0(s) and slope α1(s). Daily variation in exposure because of different activity patterns is captured by the residual term ε(t, s), which is Gaussian with mean zero and variance τ2. Exploratory analysis showed that the residual error increases with ambient concentrations and the logarithmic scale was chosen to stabilize heteroskedasticity. Tract-specific intercepts and slopes were modeled with tract-level covariates Z̃(s) for population characteristics that can influence personal exposure to ambient PM2.5. Based on the SHEDS algorithm, we included two variables: percent male in the 23 simulated individuals and percent single-unit detached housing unit from Census 2000. We also considered average age as an additional covariate but found that it did not improve prediction. This may be due to the study population being restricted to those aged 65 or above.
We allowed α0(s) and α1(s) to vary smoothly in space by including spatial random effects θ(s) and an unstructured random effects ν(s) with heterogeneity covariance V. The spatial random effects aim to capture residual effects such as higher-order interactions between the covariates that may exhibit spatial similarity. Specifically, we modeled θ(s) as a multivariate conditionally autoregressive model (MCAR). The model is specified through spatial adjacencies and a conditional covariance Σ. Let s ~ s0 denote that census tract s and s0 are spatial neighbors, and ms be the number of spatial neighbors of tract s. The MCAR model entails that the distribution of θ(s0) given all other locations is Gaussian with conditional mean and conditional variance 1/msΣ.
Parameter estimation for the emulator was carried out under a Bayesian framework using Markov Chain Monte Carlo (MCMC). Parameters β0 and β1 were assigned Gaussian prior with mean zero and variance 1002. The residual variance τ2 followed an inverse-Gamma (0.001, 0.001); Σ and V followed inverse-Wishart distributions with scale matrices of diagonal element 0.12 and 4 degrees of freedom. We used Gibbs sampling to analyze the posterior distributions of all unknown parameters. Analyses were carried out in R 2.8.0 with sub-routines written in C. After 10,000 burn-in samples, we ran an additional 25,000 iterations. To reduce autocorrelation between samples, we saved every fifth sample, resulting in a total of 5000 posterior samples. MCMC convergence was assessed by examining the trace plots of several representative parameters.
We imputed daily tract-level exposures for the complete study period using the emulator as follows. Specifically, let superscript j = 1, ..., 5000 indicate the jth posterior sample. Then the tract-specific logarithmic average exposure X(j)(t, s) can be sampled from a Gaussian distribution with mean and variance τ2,(j). Similarly, at each iteration, the spatially varying bias terms and are sampled from a bivariate Gaussian distribution with covariance V(j), and means and , respectively. The Bayesian estimation approach allows straightforward transformation between the logarithmic and original scale. For imputing tract-specific intercept and slopes, we used the percent male from Census 2000 instead of those calculated from the 23 simulated individuals by SHEDS. We did not use the simulated SHEDS results directly as exposure on days when SHEDS was run. Instead, we used the corresponding predictive samples to incorporate uncertainty in the simulated exposures.
As our daily mortality counts were aggregated across five counties, we computed a weighted-average of the tract-level exposures for the time series analysis. Although we can use tract-level exposures directly to conduct time series analysis within each tract, the number of events on each day is likely to be too small for Poisson regression. In a time series analysis where the health outcome is aggregated over a large geographic unit, the desired exposure should represent the average levels of personal exposure to ambient PM2.5 across the at-risk population. Therefore, for each day t, average exposure across the entire study area was obtained by weighting the tract-specific average exposures by the population at-risk:
where P(s) denote the population size above age 65 in tract s based on Census 2000. We also calculated exposure using ambient concentrations by replace X(j)(t, s) with W(t, s).
To evaluate the predictive performance of our emulator, we conducted an additional analysis by first leaving out five randomly selected days from each census tract. We then calculated the prediction root mean-squared error between the true values and their posterior predicted mean by averaging all imputed X(j)(t) across each missing day. We also examined the empirical 95% coverage probability by calculating the proportions of true values that fell between the 2.5th and 97.5th quantiles of the imputed X(j)(t).
Mortality Model and Risk Estimation
Relative change in the rate of mortality associated with variation in daily PM2.5 exposure was estimated via Poisson regression. We examined the effects of same-day (lag 0), 1-day prior (lag 1), 2-day prior (lag 2) and 3-day prior (lag 3) exposure. We also considered an unconstrained distributed lag model, where exposures at lag 1–3 were included in the mortality model simultaneously.35 A cumulative effect of lag 1–3 was then obtained by summing the 3 coefficients. We also considered season-specific relative risks by including interactions between the exposure and indicators of seasons (winter: December–February, spring: March–May, summer: June–August, fall: September–November). Following Samet et al.,36 we controlled for seasonal trends and weather effects via natural cubic splines with degrees of freedom d, including (1) calendar time (d = 8 per year), (2) current-day temperature (d = 6) and average temperature for the previous three days (d = 6); (3) current-day dew-point temperature (d = 3) and average dew-point temperature for the previous three days (d = 3); and (5) indicators for day of the week.
Given the 5000 imputed time series of X(j)(t), we considered two approaches to estimate relative risks. In the “exposure simulation” approach, we fitted the health model repeatedly with each exposure time series and combined the resultant risk estimates and their standard error in a multiple imputation framework. We also considered a two-stage “Bayesian” approach where we carried out a second MCMC by treating the imputed time series as a prior distribution for the exposures. This second approach differs from exposure simulation in that the health data are used to help learn about the exposures and computation details can be found in Peng and Bell.37 Moreover, we did not conduct a full Bayesian analysis where the emulator and the mortality model are fitted simultaneously. Therefore, here we assume that the mortality data do not provide information to estimate the relationship between ambient concentrations and personal exposures. However, the mortality data may provide information about the exposures through the mortality model, especially if the exposure model is specified incorrectly.
RESULTS
Based on Census 2000, the study population includes approximately 0.94 million persons aged 65 or above with an average 79 cardio-respiratory deaths per day. Table 1 summarizes daily population-weighted exposure metrics calculated using ambient PM2.5 concentrations or predicted personal exposure to ambient PM2.5. Both concentrations and exposures were higher during the summer months. Personal exposures also exhibited smaller temporal variation compared with ambient concentrations. The SDs describe the between-day variation in average personal exposures, not the between-individual variation in personal exposure. The decrease in temporal variation is likely a result of personal exposures representing only a fraction of ambient concentrations.
Table 1.
Mean and SD of daily PM2.5 ambient concentrations (μg/m3) and personal exposures (μg/m3) by season.
| PM2.5 concentration |
PM2.5 exposure |
Ratioa | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | |
| Spring | 14.30 | 7.75 | 7.54 | 3.99 | 0.53 |
| Summer | 17.50 | 9.25 | 10.39 | 5.44 | 0.60 |
| Fall | 13.27 | 7.96 | 5.80 | 3.32 | 0.44 |
| Winter | 15.37 | 7.77 | 8.37 | 4.16 | 0.55 |
Ratio of PM2.5 exposure divided by PM2.5 concentrations and averaged across days.
The daily exposure to concentration ratios also varied across seasons and were higher during the summer months. Although intuitively people spent more time indoor during the winter, this period does not correspond to the lowest concentration–exposure ratio. In our SHEDS simulation, we assumed the air exchange rate parameter (ach) in winter to be relatively high. This follows empirical studies, which have demonstrated that greater indoor–outdoor temperature differences were associated with higher ach because convection may be the dominant mechanism for air exchange.
To summarize the random tract-specific intercepts and slopes in the emulator, Table 2 gives the season-specific posterior means and 95% posterior intervals (PI) of the average α0(s) and α1(s) calculated across all tracts. We present the exponentiated intercepts because it roughly describes the ratio between ambient concentrations and simulated exposure on its original scale when α1(s) is close to 1. Coefficient α1(s) describes the multiplicative bias in ambient concentration on the logarithmic scale. Estimates of the random-effect SDs are also given in Table 2 to describe the between-tract variation (heterogeneity) on the relationship between concentration and exposure. On average, we found the intercepts to be higher during the summer and lower during the fall. The PIs of the intercept means also do not overlap, indicating that on average across census tracts, seasonal effects on the relationship between ambient concentration and personal exposure are significantly different.
Table 2.
Mean and SD of the tract-specific intercepts α0(s) and slopes α1(s) relating ambient concentrations and personal exposures.
| Exponentiated intercepts α0(s) |
Slopes α1(s) |
|||
|---|---|---|---|---|
| Mean across tracts | SD across tracts | Mean across tracts | SD across tracts | |
| Spring | 0.560 (0.557, 0.564) | 0.014 (0.011, 0.017) | 0.977 (0.975, 0.980) | 0.010 (0.008, 0.013) |
| Summer | 0.613 (0.610, 0.615) | 0.016 (0.014, 0.019) | 0.989 (0.988, 0.990) | 0.006 (0.004, 0.008) |
| Fall | 0.492 (0.491, 0.493) | 0.031 (0.029, 0.032) | 0.956 (0.955, 0.958) | 0.018 (0.016, 0.020) |
| Winter | 0.569 (0.566, 0.573) | 0.021 (0.019, 0.024) | 0.984 (0.982, 0.987) | 0.008 (0.006, 0.011) |
The summary statistics were calculated across 2105 census tracts. Posterior means and 95% posterior intervals are presented.
Table 3 gives the predictive performance of the emulator that relates tract-level ambient concentrations and personal exposures. Predictive statistics were calculated using the back-transformed (original) PM2.5 levels. Higher prediction errors are associated for the summer and winter months. Overall the root mean-squared errors were small compared with the daily PM2.5 exposure levels. We also found the emulator to be well calibrated where the 95% prediction intervals are close to the desired coverage probabilities for all seasons.
Table 3.
Predictive performance of the emulator for relating census tract-level ambient concentrations and personal exposures.
| RMSEa (μg/m3) | PI lengthb (μg/m3) | PI Probc (%) | |
|---|---|---|---|
| Spring | 0.37 | 1.38 | 94.6 |
| Summer | 0.74 | 2.43 | 94.8 |
| Fall | 0.35 | 1.30 | 94.6 |
| Winter | 0.45 | 1.61 | 94.5 |
Root mean-squared error.
Average 95% posterior predictive interval length.
Empirical coverage probability of the 95% posterior predictive interval length.
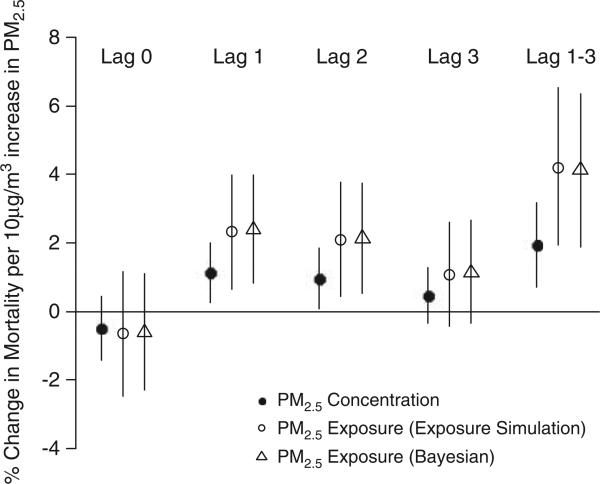
Figure 1 shows the percent increase in mortality associated with ambient PM2.5 exposure. The standard approach where ambient concentrations were used as a surrogate measure is also presented. The two different approaches for utilizing imputed time series of PM2.5 exposure to estimate relative risks were indicated by different symbols. The risks associated with personal exposures were higher than those where ambient concentrations were used directly. The Bayesian approach produced very similar results compared with the exposure simulation approach, the latter of which is more computationally efficient.
Figure 1.
Percent increase in mortality associated with ambient PM2.5 exposure and concentration. Lag 1–3 represents the cumulative effect obtained from an unconstrained distributed lag model. For PM2.5 exposure, two estimation methods (exposure simulation and Bayesian) are considered.
With the exposure simulation approach, we estimated that a 10 μg/m3 increase in PM2.5 exposure was associated with a 2.32% (95% PI: 0.68, 3.94), 2.08% (95% PI: 0.42, 3.73) and 1.09% (95% PI: –0.41, 2.63) increase in daily mortality rate for lag 1, 2 and 3, respectively. The corresponding risk estimates per 10 μg/m3 increase in PM2.5 concentration were 1.13% (95% CI: 0.27, 2.00), 0.95% (95% CI: 0.07, 1.84) and 0.46% (95% CI: –0.35, 1.27). The cumulative effects of lag 1–3 were 4.13% (95% PI: 1.81, 6.45) and 1.94% (95% CI: 0.70, 3.18) per 10 μg/m3 increase in PM2.5 exposure and concentrations, respectively. Table 4 shows the cumulative effects of lag 1–3 stratified by season. We found that the greatest risk occurred during the summer months with a percent increase in mortality of 6.85% (95% PI: 3.10, 10.60) and 3.99% (95% CI: 1.87, 6.12) per 10 μg/m3 increase in PM2.5 exposure and concentrations, respectively.
Table 4.
Percent increase (95% posterior intervals) in mortality associated with ambient PM2.5 exposure and concentration due to lag 1–3 by season.
| PM2.5 exposure | PM2.5 concentration | |
|---|---|---|
| Spring | 1.21 (–3.50, 5.90) | 0.65 (–1.78, 3.09) |
| Summer | 6.84 (3.10, 10.60) | 3.99 (1.87, 6.12) |
| Fall | 3.45 (–1.91, 8.83) | 1.42 (–0.87, 3.72) |
| Winter | 1.90 (–2.52, 6.39) | 1.13 (–1.29, 3.55) |
DISCUSSION
The proposed statistical emulator for population-level average exposure represents a hybrid approach that combines ideas from both a fully statistical and a fully stochastic model. Specifically, stochastic exposure simulators can effectively incorporate state-of-the-art knowledge in various aspects of personal exposure assessment. Statistical emulators then provide a computationally efficient way to estimate exposure for large population-based study and propagate exposure uncertainty as dictated by the stochastic simulation algorithm. To our knowledge, this is the first study that uses personal exposure estimates in a time series design where the analytic approach is comparable to those studies using ambient concentrations. Owing to its computational burden, previous studies that examined the health effects of personal exposures to air pollution could only consider simplified health models or with sample size much smaller than the epidemiologic studies today.
In this study, the computation time is approximately 5–6 h to simulate exposures for 50,000 individuals in a 30-day time period on a quad-core Window-based PC. The emulator can be fitted within 3 h and imputation is completed within minutes. Our modeling strategy is based on a random-effect specification with second-level covariates and can be fitted in standard software such as SAS or WinBUGS. This will significantly decrease the computation time further and facilitate the use of exposure simulators in future studies of air pollution and health.
We chose to conduct exposure and risk estimation in two stages. This reduces computation and is particularly important because in practice, the health model is often run many times with the same exposures to examine: (1) different health outcomes (e.g., causes of death or hospital admission); (2) different risk-windows in terms lag-effects; (3) and different degrees of confounder control as sensitivity analysis. Stratified or subset analysis are also frequently conducted to examine effect modifications.
Similar to previous studies, we found the risk estimates associated with exposures to be greater than that associated with concentrations, indicating a negative bias in the concentration-response function when ambient levels are used as a proxy for exposure. Another potential explanation to this observation may be related to exposure misclassification, which attenuates the true effects. The magnitude of the bias also approximates the ratio between daily concentration and exposure level. It is possible that additive bias is effectively controlled by the temporal smoothers in the time series model. We believe our approach and results underscore the importance of exposure metric definition and may help shed light on how this choice can impact the risk estimates.
When stratified by season, we found that ambient PM2.5 concentration and exposure were positively associated with mortality in all seasons, but only statistically significant in the summer months. This result agrees with a previous seasonal analysis of PM10 and mortality in northeastern United States.38 As the composition of PM varies both geographically and seasonally, this finding should be explored in other regions and may have important policy implication such as the need for seasonal PM2.5 control strategies. Our results do not exclude the possibility of an adverse PM2.5 effect during non-summer months. For example, during winter months in Northeastern United States, the PM2.5 mass includes a higher proportion of elemental carbon, which has been linked to several health outcomes.39,40 However, elemental carbon often exhibits high spatial heterogeneity in their concentrations. As our time series analysis is conducted over five counties and we used total PM mass as exposure, the winter effect estimate may be attenuated because of larger exposure measurement error. Another possible explanation is statistical power. The summer months had the greatest temporal variation in both concentration and exposure levels. Moreover, during the winter months, it may be difficult to identify the effect of particulate matter from the strong effect of infectious diseases, which is aggressively controlled for using the temporal smoother.
The emulator approach presented here has several limitations that offer potential model extensions. First, we only considered modeling the mean population exposure and not its variation. Time series analysis is ecological in nature where specification bias exists due the aggregation.41 However, the acute health effects of ambient air pollution is typically very small and specification bias is likely to be minor compared with that arises from the using ambient concentrations as personal exposure. In this study, we chose to simulate small number of individual exposures at a finer spatial resolution and did not have sufficient sample to incorporate estimated exposure variances. Approaches to model population exposure variance efficiently, especially when spatial dependence is considered, warrant further investigation. Another limitation is the use of Census 2000 data both in fitting the exposure model and for predicting daily exposures. We assumed that the tract-specific relationships between ambient concentrations and exposures are season specific and do not vary between years. With the availability of Census 2010 data, one potential extension is to incorporate temporal changes in the demographic variables that influences α0(s) and α1(s). Another source of demographic data is the American Community Survey where yearly, 3-year and 5-year statistics are available at different spatial resolutions.
There are additional challenges in estimating personal exposure to ambient air pollution that our approach does not consider and warrant further investigation. First, SHEDS, like other stochastic and statistical simulators, requires ambient concentration as an input. We chose US EPA's fusion product because it provides daily concentrations with complete spatial coverage. However, the 12 km by 12 km grid cell cannot capture heterogeneity at finer spatial scale and better characterization of ambient concentration, especially in urban community, may improve exposure estimation. We also did not consider uncertainty in the concentration measurements. The EPA fused database provides SD for the predicted concentration and motivates additional methodological work to incorporate this uncertainty in the risk estimates. Our results also rely on the validity and applicability of the dairies in CHADS, as well as the assumptions in the concentration–exposure relationships dictated by SHEDS. For example, we simulated exposures using SHEDS only for 4 months to describe the season-specific concentration–exposure relationships. Therefore, imputation carried out in months where simulations were not run relied on the distributional assumptions of the season-specific SHEDS parameters. To our knowledge, previous studies have not considered how these parameters may influence population-based exposure and risk estimation in a time series design. We believe the computational advantage of our emulator approach can be applied to examine the robustness of the risk estimates through sensitivity analysis.
ACKNOWLEDGEMENTS
The research is supported by Grant DMS-0635449, DMS-0706731, DMS-0706731, DMS-0353029 from the National Science Foundation, US EPA Grant RD-83329201-4, US EPA STAR Research Assistance Agreement No. R833863, and Grant No. 1 R01 ES014843-01A2 from the National Institutes of Health. The authors thank Lucas M Neas and Judy Schmid of the National Health and Environmental Effects Research Laboratory of the US Environmental Protection Agency for providing the mortality data. Janet M Burke and Haluk Ozkaynak of the National Exposure Research Laboratory of the US Environmental Protection Agency provided access to SHEDS-PM and guidance regarding its use.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Pope CA, III, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 2.Gotschi T, Heinrich J, Sunyer J, Kunzli N. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008;19:690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- 3.Katsouyanni K, Samet JM, Anderson HR, Atkinson R, Le Tertre A, Medina S, et al. Air pollution and health: a European and North American approach (APHENA). Res Rep Health Eff Inst. 2009;142:5–90. [PubMed] [Google Scholar]
- 4.Dockery DW. Health effects of particulate air pollution. Ann Epidemiol. 2009;11:257–263. doi: 10.1016/j.annepidem.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery CL, Mills KT, Williams R, McGraw KA, Poole C, Smith RL, et al. Estimating error in using ambient PM2.5 concentrations as proxies for personal exposures: a review. Epidemiology. 2010;21:215–223. doi: 10.1097/EDE.0b013e3181cb41f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarnat JA, Wilson WE, Strand M, Brook J, Wyzga R, Lumley T. Panel discussion review: session 1–exposure assessment and related errors in air pollution epidemiologic studies. J Expo Sci Environ Epidemiol. 2007;17:S75–S82. doi: 10.1038/sj.jes.7500621. [DOI] [PubMed] [Google Scholar]
- 7.Dominici F, Zeger SL, Samet JM. A measurement error model for time-series studies of air pollution and mortality. Biostatistics. 2000;1:157–175. doi: 10.1093/biostatistics/1.2.157. [DOI] [PubMed] [Google Scholar]
- 8.Janssen NA, Schwartz J, Zanobetti A, Suh HH. Air conditioning and source-specific particles as modifiers of the effects of PM10 on hospital admission for heart and lung disease. Environ Health Perspect. 2002;110:43–49. doi: 10.1289/ehp.0211043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell ML, Ebisu K, Peng RD, Dominici F. Adverse health effects of particulate air pollution: modification by air conditioning. Epidemiology. 2009;20:682–686. doi: 10.1097/EDE.0b013e3181aba749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ozkaynak H, Xue J, Spengler J, Wallace L, Pellizzari E, Jenkins P. Personal exposure to airborne particles and metals: results from the Particle TEAM study in Riverside, California. J Expo Anal Environ Epidemiol. 1996;6:57–78. [PubMed] [Google Scholar]
- 11.Williams R, Suggs J, Creason J, Rodes C, Lawless P, Kwok R, et al. The 1998 Baltimore Particulate Matter Epidemiology-Exposure Study: part 2. Personal exposure assessment associated with an elderly study population. J Expo Anal Environ Epidemiol. 2000a;10:533–543. doi: 10.1038/sj.jea.7500108. [DOI] [PubMed] [Google Scholar]
- 12.Williams R, Suggs J, Zweidinger R, Evans G, Creason J, Kwok R, et al. The 1998 Baltimore Particulate Matter Epidemiology-Exposure Study: part 1. Comparison of ambient, residential outdoor, indoor and apartment particulate matter monitoring. J Expo Anal Environ Epidemiol. 2000b;10:518–532. doi: 10.1038/sj.jea.7500107. [DOI] [PubMed] [Google Scholar]
- 13.McBride SJ, Williams RW, Creason J. Bayesian hierarchical modeling of personal exposure to particulate matter. Atmos Environ. 2007;41:6143–6155. [Google Scholar]
- 14.McCurdy T, Glen G, Smith L, Lakkadi Y. The national exposure research laboratory's consolidated human activity database. J Expo Anal Environ Epidemiol. 2000;10:566–578. doi: 10.1038/sj.jea.7500114. [DOI] [PubMed] [Google Scholar]
- 15.Zidek J, Shaddick G, White R, Meloche J, Chat eld C. Using a probabilistic model (pCNEM) to estimate personal exposure to air pollution. Environmetrics. 2005;16:481–493. [Google Scholar]
- 16.US EPA Total Risk Integrated Methodology TRIM.Expo Inhalation User's Document Volume I: Air Pollutants Exposure Model (APEX, version 3) User's Guide. 2003.
- 17.Burke JM, Zufall MJ, Ozkaynak H. A population exposure model for particulate matter: case study results for PM2.5 in Philadelphia, PA. J Expo Anal Environ Epidemiol. 2001;11:470–489. doi: 10.1038/sj.jea.7500188. [DOI] [PubMed] [Google Scholar]
- 18.Holloman CH, Bortnick SM, Morara M, Strauss WJ, Calder CA. A Bayesian hierarchical approach for relating PM2.5 exposure to cardiovascular mortality in North Carolina. Environ Health Perspect. 2004;112:1282–1288. doi: 10.1289/ehp.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calder CA, Holloman CH, Bortnick S, Strauss W, Morara M. Relating ambient particulate matter concentration levels to mortality using an exposure simulator. J Am Stat Assoc. 2008;103:137–148. [Google Scholar]
- 20.Blangiardo M, Hansell A, Richardson S. A Bayesian model of time activity data to investigate health effect of air pollution in time series studies. Atmos Environ. 2011;45:379–386. [Google Scholar]
- 21.Shaddick G, Lee D, Zidek JV, Salway R. Estimating exposure response functions using ambient pollution concentrations. Ann App Sta. 2008;2:1249–1270. [Google Scholar]
- 22.Reich BJ, Fuentes M, Burke J. Analysis of the effects of ultrafine particulate matter while accounting for human exposure. Environmetrics. 2008;20:131–136. doi: 10.1002/env.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berrocal VJ, Gelfand AE, Holland DM, Burke J, Miranda ML. On the use of a PM2.5 exposure simulator to explain birthweight. Environmetrics. 2011;22:553–571. doi: 10.1002/env.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long CM, Suh HH, Koutrakis P. Characterization of indoor particle sources using continuous mass and size monitors. J Air Waste Manag Assoc. 2000;50:1236–1250. doi: 10.1080/10473289.2000.10464154. [DOI] [PubMed] [Google Scholar]
- 25.Ivy D, Mulholland JA, Russell AG. Development of ambient air quality population-weighted metrics for use in time-series health studies. J Air Waste Manag Assoc. 2008;58:711–720. doi: 10.3155/1047-3289.58.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strickland MJ, Darrow LA, Mulholland JA, Klein M, Flanders WD, Winquist A, et al. Implications of different approaches for characterizing ambient air pollutant concentrations within the urban airshed for time-series studies and health benefits analyses. Environ Health. 2011;10:36. doi: 10.1186/1476-069X-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillan NJ, Holland DM, Morara M, Feng J. Combining numerical model output and particulate data using Bayesian space-time modeling. Environmentrics. 2009;21:48–65. [Google Scholar]
- 28.Byun DJ, Schere KL. Review of the governing equations, computational algorithms, and other components of the Models-3 Community Multiscale Air Quality (CMAQ) modeling system. Appl Mech Rev. 2006;59:51–77. [Google Scholar]
- 29.Cao Y, Frey HC. Assessment of inter-individual and geographic variability in human exposure to fine particulate matter in environmental tobacco smoke. Risk Anal. 2011a;31:578–591. doi: 10.1111/j.1539-6924.2010.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao Y, Frey HC. Geographic differences in inter-individual variability of human exposure to fine particulate matter. Atmos Environ. 2011b;45:5684–5691. doi: 10.1016/j.atmosenv.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Frey HC. Modeling of in-vehicle human exposure to ambient fine particulate matter. Atmos Environ. 2011;45:4745–4752. doi: 10.1016/j.atmosenv.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koontz MB, Rector HE. Estimation of distribution of residential air exchange rates (Report #600R95180) U.S. Environmental Protection Agency; 1995. [Google Scholar]
- 33.Murray DM, Burmaster DE. Residential air exchange-rates in the United States empirical and estimated parametric distributions by season and climatic region. Risk Anal. 1995;15:459–465. [Google Scholar]
- 34.Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, et al. Relationships of Indoor, Outdoor, and Personal Air (RIOPA). Part I. Collection methods and descriptive analyses. Res Rep Health Eff Inst. 2005;130:1–107. discussion 109–127. [PubMed] [Google Scholar]
- 35.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11:320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 37.Peng RD, Bell ML. Spatial misalignment in time series studies of air pollution and health data. Biostatistics. 2010;11:720–740. doi: 10.1093/biostatistics/kxq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng RD, Dominici F, Pastor-Barriuso R, Zeger SL, Samet JM. Seasonal analyses of air pollution and mortality in 100 US cities. Am J Epidemiol. 2005;161:585–594. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 39.Mar TF, Norris GA, Koenig JQ, Larson TV. Associations between air pollution and mortality in Phoenix, 1995–1997. Environ Health Perspect. 2000;108:347–353. doi: 10.1289/ehp.00108347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng RD, Belle ML, Geyh AS, McDermott A, Zeger SL, Samet JM, Dominici F. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ Health Perspect. 2004;117:957–963. doi: 10.1289/ehp.0800185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sheppard L. Acute air pollution effects: consequences of exposure distribution and measurements. J Toxicol Environ Health A. 2005;68:1127–1135. doi: 10.1080/15287390590935987. [DOI] [PubMed] [Google Scholar]