Abstract
Altered expression of the ER-resident aminopeptidases ERAP1 and ERAP2 might play an important role in shaping the MHC class I-presented peptide repertoire, but their function in tumors has not been determined in detail. Thus, the expression of ERAP1, ERAP2 and HLA class I heavy chain (HC) was analysed in various renal tumor types and corresponding kidney parenchyma by immunohistochemistry. Additionally, comparative expression profilings of untreated versus interferon (IFN)-γ-treated RCC cell lines were performed applying qRT-PCR, Western blot and/or flow cytometry. Normal kidney tissues showed strong ERAP1 staining in the proximal tubules of 57.4 % of cases, in the distal tubules of 94.3 % of cases and in the medulla of 88.6 % of cases, whereas high ERAP2 levels were observed in the medulla of 77.1 % of cases and in both, proximal and distal tubules of about 88 % of cases. Imbalanced, downregulated and RCC subtype-specific ERAP1 or ERAP2 expression was detected in 12.7 % or 43.8 % of samples analyzed, respectively. A coordinated downregulation of ERAPs was found in 4.8 %, an upregulation of ERAP1 or ERAP2 in 22.8 % or 2.0 % of RCC lesions. No association exists between ERAP and HLA class I HC expression for any tissue type. A heterogeneous constitutive ERAP expression pattern was also detected in RCC cell lines with lower ERAP2 than ERAP1 expression levels, which was in 11/17 RCC cell lines inducible by IFN-γ. Conclusively, ERAP1 and ERAP2 might be involved in the development of immune escape mechanisms of RCC.
Keywords: Renal cell carcinoma, ERAP, immune escape, HLA class I
Introduction
Antigenic peptides are generated by different proteases in distinct subcellular compartments and are important for the quality control of major histocompatibility complex (MHC) class I antigen presentation [1-3]. Besides the multicatalytic proteasome cleaving intracellularly synthesized, ubiquitinated proteins into intermediate peptide fragments, other cytosolic and endoplasmic reticulum (ER)-resident proteases are required for further trimming. The ER-associated aminopeptidases ERAP1 and ERAP2 either trim residual N-terminal extended peptides to a length fitting into the HLA class I peptide-binding groove [4-6] or even destroy peptides [7-10] suggesting an important role for ERAPs in the generation of antigenic peptide repertoire presented by HLA class I surface molecules [11]. Since the efficacy of ERAP1/2 trimming is important for the rate limiting for MHC class I peptide loading and immunodominance of CD8+ T cell epitope the absence of ERAP might affect the cellular immunogenicity [1,4,12,13]. While the presentation of some selected antigens is unaffected by aberrant ERAP deficiencies, others might be substantially upregulated. The extent by which ERAP expression alter the total peptide repertoire and its immunological consequences, still remains to be determined. However, it has recently been shown that silencing of ERAP caused peptide loading defects resulting in efficient rejection of tumor cells by natural killer (NK) cells [14].
So far, there exists only limited information concerning the expression of ERAP1 and ERAP2 both in murine and human normal or malignant tissues [15]. Immunohistochemical stainings of tissue samples with ERAP-specific antibodies demonstrated ERAP expression in normal kidney [16], extratrophoblasts [17], endometrium [18] as well as in tumors of distinct histotypes including endometrial adenocarcinoma, cervical cancer and melanoma [15,18-21]. While an association between the expression pattern of ERAP1, ERAP2 and/or HLA class I antigens was found in some normal tissues [15], the consequences of deregulated ERAP1 and ERAP2 expression on HLA class I surface expression levels have been controversially discussed. Loss or imbalance of ERAP expression might have clinical relevance and could be associated in some tumor entities with disease progression and poor patients’ outcome [22]. The heterogeneous and discordant expression pattern of both aminopeptidases as well as alterations in their enzyme activity could be caused by structural alterations, transcriptional as well as post-transcriptional regulation [21,23]. In neuroblastoma, the downregulated ERAP expression has been associated with the low availability of the transcription factor NF-κB [24]. Furthermore, constitutive ERAP expression could be upregulated by interferon (IFN) [6], the leukaemia inhibitory factor [17], which could be produced by cells of the tumor microenvironment. Concerning renal cell carcinoma (RCC), only seven RCC lesions of unknown subtype have been analysed suggesting a loss of ERAP1 and ERAP2 expression in kidney cancer [15]. The present study analyzed the constitutive ERAP1 and ERAP2 mRNA and protein expression pattern in normal kidney tissues and RCC lesions of distinct subtypes in order to determine whether (i) there exists an imbalanced ERAP1 and ERAP2 expression pattern in both normal kidney epithelium and RCC lesions as well as between different RCC subtypes, (ii) ERAPs are co-ordinately regulated with the HLA class I heavy chain (HC), (iii) impaired ERAP expression could be associated with tumor grade and stage and (iv) altered ERAP1 and ERAP2 expression could be enhanced by cytokines.
Material and methods
Antibodies
The following monoclonal antibodies (mAb) were used in this study: the murine mAb 4D2 raised against recombinant human ERAP1 [16], mAb 3F5 directed against human recombinant ERAP2 [25], the mAb HC10 recognising the β2-microglobulin (β2-m)-free HLA class I HC [26] and the b-actin-specific mAb (ab 6276, Abcam, Cambridge, UK). The activities of the mAb preparations were all tested on lymphoid cell lysates.
Tissue specimen and immunohistochemistry (IHC)
Tumor specimens from clear cell, chromophobe and papillary RCC lesions as well as from benign oncocytoma and normal tissues were obtained from patients during nephrectomy. Normal kidney tissue was obtained at resection margins as far away from the tumor as possible and examined by two experienced surgical pathologist (AH and SB). Only normal tissue samples without any histologic alterations were included in the study.
All specimens were diagnosed according to the WHO classification of tumors [27], staging was performed according to the tumor-node-metastasis (TNM) system [28]. The tissue collection was approved according to the recommendation of the Ethical committee of the different universities. The patients’ mean age was 63.4 years. The clinical-pathological characteristics of the RCC lesions are summarized in Table 1. After slide review by two experienced surgical pathologists (AH and SB), a tissue micro array (TMA) was constructed from representative 1.5 mm large tissue punches as described [29]. The TMA included 98 RCC cases of clear cell, 102 of chromophobe, 76 of papillary subtypes and 58 oncocytoma and 300 normal kidney tissues.
Table 1.
Characteristics of tumor patients and tumor lesions analysed
| characteristics | Number of cases | |
|---|---|---|
| gender | female | 89 |
| male | 167 | |
| unknown | 78 | |
| subtype | clear cell | 98 |
| papillary | 76 | |
| chromophobe | 102 | |
| oncocytoma (benign) | 58 | |
| stage | pT1a | 89 |
| pT1b | 63 | |
| pT2 | 51 | |
| pT3a | 31 | |
| pT3b | 18 | |
| missing | 24 | |
| N | N0 | 189 |
| N1 | 2 | |
| N2 | 5 | |
| missing | 80 | |
| M | M0 | 172 |
| M1 | 9 | |
| missing | 95 | |
| histopathological grade (N): | 1 | 49 |
| 2 | 177 | |
| 3 | 28 | |
| 4 | 1 | |
| missing | 21 | |
| UICC stage | I | 111 |
| II | 27 | |
| III | 31 | |
| IV | 9 | |
Immunohistochemical staining of the formalin-fixed, paraffin-embedded tissues was performed as recently described [30] and all slides were reviewed blinded by two experienced surgical pathologists (AH and MB). One of the pathologists (MB), is highly specialized on nephropathology. Therefore, a discrimination between the different tubuli sections was possible without marker protein staining on serial sections, which would have compromised the study due to tissue limitations. The percentage and intensity of stained tumor cells and tubular cells of normal kidney tissues were evaluated, while other tissue components were ignored. The results were scored as negative (0), heterogeneous (1) and positive (2), when the percentage of stained cells was < 25, between 25 and 75, and > 75, respectively, whereas the staining intensity was classified as negative (0), weak (1), moderate (2) and strong (3). A combined score was generated by addition of both scores: Combinations of “0” for either frequency or percentage of intensity with other values remained “0” were regarded as negative combined scores of “2” as weak, of “3-4” as moderate, and of “5“ as strong expression [31]. For statistical purposes, negative and weak as well as moderate and strong expression levels were grouped together and designated as “low” and “high” expression range, respectively. The expression of the respective marker in each tumor was compared to that of the corresponding normal tissue. The staining pattern of clear cell and papillary RCC was compared to the staining of proximal tubuli in the normal cortical tissue of the same sample, whenever possible, while chromophobe RCC and oncocytoma stainings were compared to the staining pattern of collecting duct, whenever possible. If the required tubuli sections were not available, the sample was omitted from statistical analysis. Negative controls were performed by omitting primary antibodies.
Cell lines, cell culture and cytokine treatment
A panel of 17 human RCC cell lines and 1 cell line representing normal kidney epithelium [32] grown in RPMI 1640 supplemented with 10 % fetal calf serum (FCS, BRL, Life Technologies, Karlsruhe, Germany), 2 % glutamine and 1 % penicillin, streptomycin in a humified atmosphere with 5 % CO2 at 37°C, was either left untreated or treated for 24 and 48 hours with 20 µg/ml recombinant IFN-g (R&D Systems; Minneapolis, USA).
Semi-quantitative RT-PCR analysis
Total cellular RNA of cell lines was extracted employing the RNeasy Mini Kit (Qiagen, Hilden, Germany) followed by digestion with DNase I (Invitrogen GmbH, Darmstadt, Germany). cDNA was synthesized from 500 ng of total RNA employing the RevertAidTM H Minus First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany) according to the manufacturer’s instructions. The primer pairs for ERAP1 and ERAP2 along with the conditions and parameters used for semiquantitative and/or comparative qRT-PCR have been recently described [21].
Western blot analyses
20 µg protein/lane of total extracts isolated from untreated and IFN-g-treated RCC/control cell lines were separated by 10 % SDS PAGE and then transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The membranes were sequentially incubated with mAbs directed against ERAP1 [16], ERAP2 [25] or HC10 [26] at 4°C overnight followed by incubation with suitable HRP-conjugated secondary antibodies as recently described [21]. Immunostainings of the blots with an anti-b-actin mAb (Abcam) served as loading controls.
Statistical analysis
The results of immunohistochemical stainings were correlated with the histopathological characteristics of the RCC lesions and with the clinical characteristics of the patients utilizing two-sided exact χ2-test (Pearson). P values < 0.05 were considered to be significant. Statistical analyses were performed utilizing the Statistical Package for the Social Sciences (SPSS) version 17.0 (SPSS, Chicago, USA).
Results
Detection of ERAP1 and ERAP2 in normal kidney tissues
Normal kidney tissues (n = 300) were investigated for the expression of ERAP1 and ERAP2 as well as HLA class I heavy chain (HC) by staining of TMAs with respective mAbs. As representatively shown in Figure 1A heterogeneous ERAP1 and ERAP2 expression was detected in the distinct regions of the kidney: High levels of ERAP1 were found in the proximal tubules of 57.4 % (147/256) of cases, in the distal tubules of 94.3 % (283/300) of cases and in the medulla of 88.6 % (147/256) of cases, whereas high ERAP2 expression levels were found in the proximal tubules of 88.7 % (39/445) of cases, in the distal tubules of 88.3 % (264/299) of cases and in the medulla of 77.1 % (37/48) of cases. However, neither ERAP1 nor ERAP2 expression were associated with HLA class I HC expression levels, which were high in the proximal tubules of 39.6 % (105/265) of cases, in the distal tubules of 99.6 % (256/257) of cases and in the medulla of 59.4 % (41/69) of cases. These data demonstrate for the first time a heterogeneous, but also discordant expression pattern of both aminopeptidases in the different localizations of the normal kidney epithelium.
Figure 1.
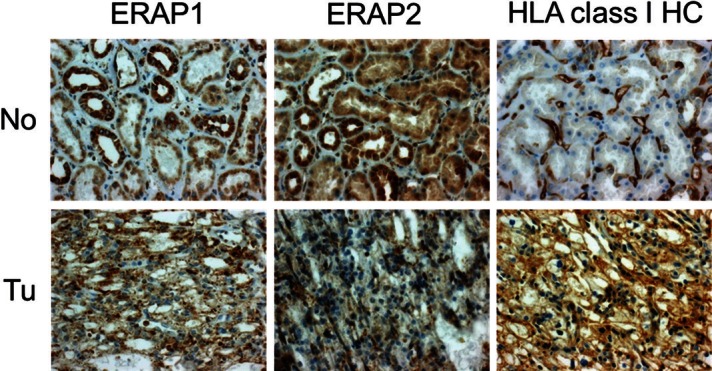
Representative example of ERAP1, ERAP2 and HLA class I HC expression in normal renal tissue (No) and corresponding tumor tissue (Tu). Expression of the three markers in the tumor tissue independently varies relative to the proximal tubules which are the tissue of tumor origin: ERAP1 expression is unchanged, while ERAP2 expression is reduced and HLA class I HC expression is increased (original magnification: 400x). All photographs display the same case of clear cell RCC.
Heterogeneous ERAP1 and ERAP2 expression in RCC lesions
To determine whether ERAP1 and ERAP2 are differentially expressed in tumor lesions when compared to normal tissue, 334 RCC specimen and 300 renal parenchyma tissues were stained in parallel with ERAP1- and ERAP2-specific mAbs. Representative staining patterns for ERAP1 and ERAP2 in renal tumors are shown in Figure 1. All possible ERAP phenotypes could be identified: Single ERAP1 or ERAP2 losses were found in 12.7 % (20/158) and 43.8 % (43/98) of RCC lesions, respectively, whereas 22.8 % (36/158) and 2.0 % (2/98) of RCC lesions displayed higher ERAP1 or ERAP2 expression levels compared to normal kidney epithelium. 4.8 % (4/82) of RCC lesions exhibited a coordinated downregulation of both aminopeptidases.
Association of altered ERAP expression with tumor characteristics and disease
The frequencies for loss or gain of ERAP expression were calculated for each cell type. This was important since clear cell and papillary RCC derive from proximal tubule cells, whereas chromophobe RCC and oncocytoma arise from collecting duct. However, a drawback of this approach is the limited number of accessable normal/tumor tissue pairs and respective RCC subtypes. In order to determine whether the frequency of altered ERAP expression is either linked to distinct kidney tumors or to a more aggressive tumor phenotype, ERAP stainings of RCC subtypes and oncocytoma were performed and correlated to that of the tissue of origin and clinical parameters (Table 2A and 2B). Using this strategy the differential ERAP1 expression pattern between chromophobe RCC and oncocytoma was significant (p = 0.022). ERAP2 appears to be more frequently downregulated in renal tumors than ERAP1, whereas ERAP1 was often found to be upregulated in RCC lesions. However, neither an association between the expression profiles of ERAP1/ERAP2 and the tissue origin nor with clinical parameters exists (Table 2B).
Table 2.
Correlation of ERAP and HLA class I HC expression pattern with tumor subtype (A,C) and disease parameters (B, D)
| A | clear cell RCC | papillary RCC | chromophobe RCC | oncocytoma | total no of lesions |
|
| |||||
| ERAP1 downreg. | 15.1% (8/53) | 11.6% (5/43) | 7% (3/43) | 21.1% (4/19) | 158 |
| ERAP1 upreg. | 13.2% (7/53) | 30.2% (13/43) | 34.9% (15/43) | 5.3% (1/19) | |
| p = 0.057 chromophobe RCC vs. oncocytoma p = 0.022 | |||||
|
| |||||
|
| |||||
| ERAP2 downreg. | 50.0% (21/42) | 32.7% (16/49) | 75% (3/4) | 100% (3/3) | 98 |
| ERAP2 upreg. | 2.4% (1/42) | 2.0% (1/49) | 0% (0/4) | 0% (0/3) | |
| p = 0.190 | |||||
|
| |||||
|
| |||||
| B | UICC low stage (stage 1 + 2) | UICC high stage (stage 3 + 4) | low grade (grade 1 + 2) | high grade (grade 3 + 4) | total no of lesions |
|
| |||||
| ERAP1 downreg. | 9.3% (9/97) | 25% (6/24) | 10.7% (13/122) | 22.2% (4/18) | 121 (UICC stage) |
| ERAP1 upreg. | 22.7% (22/97) | 16.7% (4/24) | 25.4% (31/122) | 22.2% (4/18) | 140 (grade) |
| UICC stage p = 0.122; grade p = 0.450 | |||||
|
| |||||
|
| |||||
| ERAP2 downreg. | 35.8% (24/67) | 52.2% (12/23) | 42.9% (36/84) | 36.4% (4/11) | 90 (UICC stage) |
| ERAP2 upreg. | 3.0% (2/67) | 0% (0/23) | 2.4% (2/84) | 0% (0/11) | 95 (grade) |
| UICC stage p = 0.268; grade p = 0.807 | |||||
|
| |||||
|
| |||||
| C | clear cell RCC | papillary RCC | chromophobe RCC | oncocytoma | total no of lesions |
|
| |||||
| HLA class I HC loss/downreg. | 7.7% (4/52) | 11.1% (5/45) | 18.2% (2/11) | 0% (0/4) | 112 |
| HLA class I HC upreg. | 36.5% (19/52) | 15.6% (7/45) | 27.3% (3/11) | 25% (1/4) | |
| p = 0.322 | |||||
|
| |||||
|
| |||||
| D | UICC low stage (stage 1 + 2) | UICC high stage (stage 3 + 4) | low grade (grade 1 + 2) | high grade (grade 3 + 4) | total no of lesions |
|
| |||||
| HLA class I HC loss/downreg. | 9.2% (7/76) | 13% (3/23) | 11.5% (11/96) | 0% (0/12) | 99 (UICC stage) |
| HLA class I HC upreg. | 31.6% (24/76) | 13% (3/23) | 29.2% (28/96) | 8.3% (1/12) | 108 (grade) |
| UICC stage p = 0.240; grade p = 0.083 | |||||
Summary of the total number of tumor lesions analyzed for ERAP1, ERAP2 and HLA class I HC expression alterations (A, C) and their association with tumor stage and grade (B, D). Statistical test used: two-sided exact χ2-test (Pearson). P-values should be interpreted carefully due to the small amount of cases in the groups chromophobe, oncocytoma, high stage and high grade.
Correlation of ERAP with HLA class I heavy chain expression
It has been recently reported that low ERAP1 and/or ERAP2 expression is often associated with a reduced expression of HLA class I surface antigens in tumor cells, while Fruci and coauthors [33] described a more frequent correlation of ERAP1 than ERAP2 with HLA class I antigen expression. In order to determine whether there exists a correlation of ERAP1 or ERAP2 and HLA class I HC expression levels, the TMA was stained in parallel with ERAP- and HLA class I HC-specific mAbs. As listed in Table 2C a loss or downregulation of HLA class I HC staining was detected in 9.8 %, an overexpression in 26.8 % of renal tumors when compared to respective control tissues. A representative example of discordant ERAP1, ERAP2 and HLA class I HC is shown in Figure 1, an example of HLA class I HC overexpression in papillary RCC in Figure 2 and the correlation with the clinical parameters is summarized in Table 2D. Since the number of RCC lesions with high UICC stage and grade as well as of chromophobe RCC and oncocytoma were very low, the reliability of the statistical tests might be influenced. The HLA class I HC expression is not associated with the ERAP expression pattern in distinct RCC subtypes, nor with the UICC stage or tumor grade.
Figure 2.
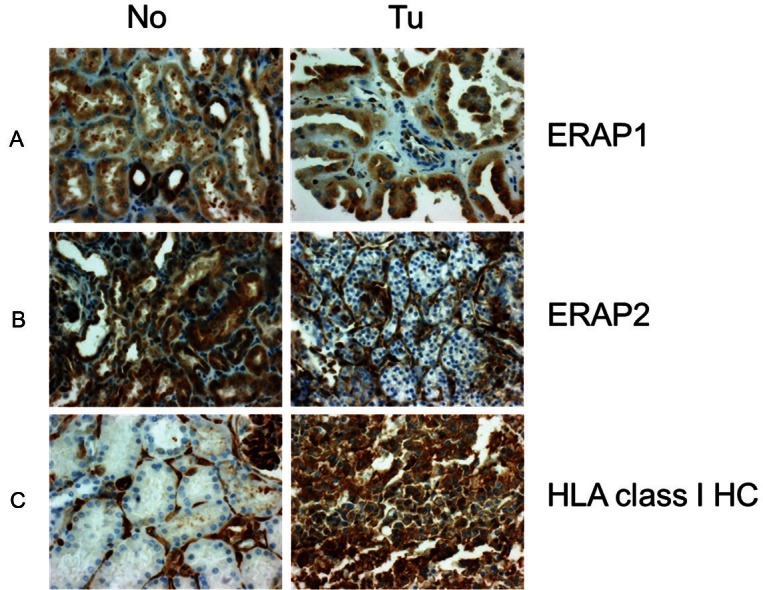
Expression changes of ERAP1, ERAP2 and HLA class I HC in RCC (original magnification: 400x). A. Papillary RCC UICC stage I dispaying higher ERAP1 expression than the proximal tubulues of the corresponding normal renal tissue. B. Clear cell RCC UICC stage III displaying reduced ERAP2 expression relative to the proximal tubules of the corresponding normal renal tissue and C. Papillary RCC displaying higher HLA class I HC expression than the proximal tubules of the corresponding normal renal tissue. No: normal renal tissue, Tu: tumor tissue.
Heterogeneous constitutive ERAP expression in RCC cell ines
In order to get insight into the functional relevance and regulation of ERAP1 and ERAP2 in RCC, constitutive and IFN-γ-regulated expression of both aminopeptidases was monitored in 17 different RCC cell lines and 1 normal kidney epithelium representing cell line. A heterogeneous constitutive and IFN-γ-inducible ERAP1 and ERAP2 expression exists when compared to normal kidney epithelium cells (Table 2). As representatively demonstrated in Figure 3, relatively high ERAP1 and ERAP2 mRNA and/or protein expression levels were detected in 6 RCC cell lines, whereas ERAP1 and ERAP2 expression was downregulated in 11 RCC cell lines. In some cases a coordinated, in others a discordant ERAP1 and ERAP2 expression pattern was detected. IFN-γ treatment induced ERAP1 and ERAP2 mRNA and protein expression in 10/17 RCC cell lines, but to a different extent (Figure 4), which was also accompanied by an increased HLA class I surface antigen expression (data not shown). The lack of IFN-γ-mediated ERAP induction might be at least partially due to an IFN-γ resistance, since the IFN-γ-inducible β2-m was also not upregulated in all IFN-γ-treated RCC cell lines tested (Table 3).
Figure 3.
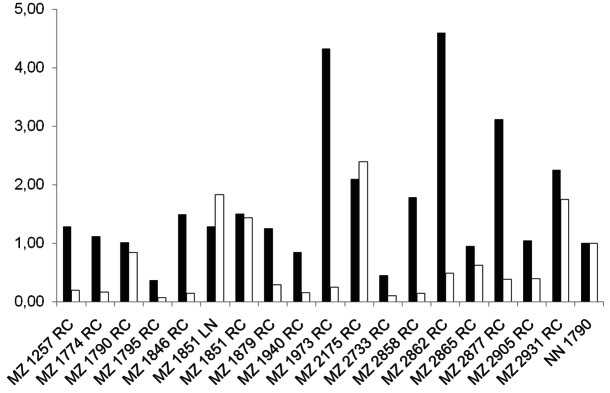
Heterogeneous ERAP1 and ERAP2 expression in RCC cell lines. The constitutive mRNA expression levels of ERAP1 (black bars) and ERAP2 (white bars) were analysed in a panel of renal cell carcinoma cells lines as indicated on the x axes by qRT-PCR using ERAP1 and ERAP2-specific primers. The relative mRNA expression levels were normalized to GAPDH, which served as a control.
Figure 4.
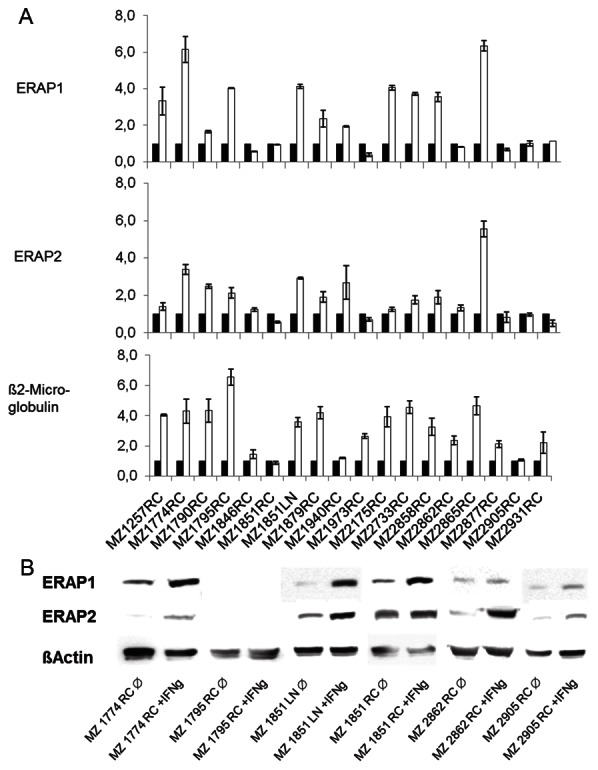
Interferon-γ inducibility of ERAP expression in RCC cell lines. RCC cell lines were either left untreated or treated for 24 or 48 h with IFN-γ before and RT-PCR, Western blot analysis and flow cytometry was performed. A. qRT-PCR of ERAP1, ERAP2 and β2-m in untreated (white bars) and IFN-γ-treated (black bars) RCCs. B. Western blot analysis of representative untreated as well as IFN-γ-treated RCC cell lines.
Table 3.
Heterogeneous ERAP1 and ERAP2 expression in RCC cell lines
| Cell lines analysed | ERAP1 | ERAP2 | ||
|---|---|---|---|---|
|
| ||||
| constitutive | IFN inducible | constitutive | IFN inducible | |
| MZ1257RC | + | yes | - | - no |
| MZ1774RC | + | yes | - | yes |
| MZ1790RC | + | yes | + | yes |
| MZ1795RC | - | yes | - | yes |
| MZ1846RC | + | no | - | no |
| MZ1851RC | + | no | + | no |
| MZ1851LN | + | yes | ++ | yes |
| MZ1879RC | + | yes | - | yes |
| MZ1940RC | + | yes | - | yes |
| MZ1973RC | +++ | no | - | no |
| MZ2175RC | ++ | yes | ++ | no |
| MZ2733RC | - | yes | - | yes |
| MZ2858RC | ++ | yes | - | yes |
| MZ2862RC | +++ | no | - | yes |
| MZ2865RC | ++ | yes | - | yes |
| MZ2877RC | ++ | no | - | no |
| MZ2905 | + | no | - | yes |
| MZ2931RC | ++ | no | + | no |
| MZ1790NN* | + | no | + | no |
MZ1790NN is a normal kidney epithelium representing cell line.
The ERAP expression pattern was normalized to GAPDH. ERAP expression levels of MZ1790NN was set 1.
Discussion
Since data on ERAP expression in renal tumor lesions are very limited, the present study determined the protein expression of ERAP1 and ERAP2 in the four main renal tumor types, clear cell RCC, papillary RCC, chromophobe RCC and oncocytoma by refering the results of tumor stainings to the different cell types of origin. Comparative immunohistochemical stainings of a TMA composed of 334 RCC lesions of distinct origin and if available corresponding adjacent normal renal parenchyma was performed. The heterogeneous ERAP1 and ERAP2 expression pattern in both, different localizations of the kidney tissue as well as in RCC lesions suggested an involvement of these aminopeptidases in shaping the peptide repertoire under physiological and pathophysiological conditions. In addition, differential expression of ERAPs within the distinct regions of adjacent kidney tubuli was detected, which is in accordance with some reports [34]. Based on this important information, ERAP expression of RCC subtypes should be compared to the appropriate control, i.e. the tubular segment regarded as the cellular origin of the tumor. However, the studies available analysing MHC class I and/or APM components in RCC have not yet been taken this aspect into account [30,34]. Losses and gains of ERAP1 and ERAP2 protein expression were identified in RCC lesions with an altered frequency in different renal tumor types. While increased ERAP1 expression was more common in clear cell RCC, ERAP2 was more frequently downregulated in this subtype and rarely elevated when compared to the respective control tissue. For chromophobe and papillary RCC and oncocytoma the number of lesions analysed was too low for reliable conclusions. The same problem arose for correlations of tumor grade and UICC stage with ERAP expression levels. Further studies are needed to evaluate significance and biological relevance of ERAP1 and ERAP2 up-/downregulation frequencies among those tumors. However, the IHC results are consistent with the ERAP mRNA and protein expression in RCC cell lines ranging from lack to high expression levels. The partially discordant ERAP mRNA and protein levels suggested a transcriptional as well as a posttranscriptional control of both aminopeptidases as recently demonstrated in melanoma [21], but the underlying mechanisms of altered ERAP expression have not yet been determined in RCC.
ERAP1 and ERAP2 mRNA and protein levels were upregulated by IFN-γ treatment in the majority of RCC lines suggesting a deregulation rather than structural alterations of ERAP. The lack of an IFN-γ-mediated increase of ERAP expression might be partially due to an IFN-γ resistance since the expression of other IFN-γ-controlled APM components was also not upregulated by this cytokine. This might be caused by defects in components of the IFN-γ signal transduction pathway [35]. The heterogeneity of ERAP and MHC class I HC expression in the different RCC subtypes independent of tumor grade and stage suggest a subtile regulation of these components, which might be mediated by the altered tumor microenvironment due to changes in the frequency of the composition of immune cells infiltrating the tumor lesion as well as an altered metabolism of the tumor itself. This might not only influence the ERAP and MHC expression, but also the repertoire of peptides presented and the immune response mounted. Thus, the immune cell infiltration of the RCC lesions should be determined, since high levels of infiltrating T lymphocytes may modulate the tumor microenvironment leading to an increase of ERAP as well as HLA class I HC expression due to IFN-γ secretion of these cells.
There exists no association of ERAP expression with HLA class I HC expression in RCC lesions. However, it is noteworthy that staining of tissues with the HC10 mAb does not give information about the MHC class I surface antigen expression since the HC 10 mAb only recognises the HLA class I HC, but not the trimeric complex consisting of the HC, β2-m and peptide. Therefore, the impact of ERAP on MHC class I cell surface expression has to be tested on fresh frozen tissue samples. This is further strengthened by the fact that the results obtained in a number of RCC cell lines suggest an association of HLA class I surface antigen levels with ERAP1 and/or ERAP2 expression. The coordinated ERAP and HLA expression is in accordance with a recent study in melanoma, in which ERAP-specific siRNAs were employed [21]. However, the impact of ERAP alterations on the HLA class I-presented peptide repertoire and mounting of peptide-specific T cell response in human tumors requires further studies.
Acknowledgements
This work was sponsored by the DFG grant SE-581-9.1 and 9.2. The authors would like to thank Sylvi Magdeburg for excellent secretarial help and Rudolf Jung and Christiane Kellert for excellent technical assistance and Peter van Endert (Inserm, Paris) for providing us with the ERAP-specific antibodies.
Declaration of conflict of interest
The authors have no conflicting financial interest.
References
- 1.van Endert P. Post-proteasomal and proteasome-independent generation of MHC class I ligands. Cell Mol Life Sci. 2011;68:1553–1567. doi: 10.1007/s00018-011-0662-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 3.Wearsch PA, Cresswell P. The quality control of MHC class I peptide loading. Curr Opin Cell Biol. 2008;20:624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan J, Parekh VV, Mendez-Fernandez Y, Olivares-Villagomez D, Dragovic S, Hill T, Roopenian DC, Joyce S, Van Kaer L. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J Exp Med. 2006;203:647–659. doi: 10.1084/jem.20052271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock KL, Farfan-Arribas DJ, Shen L. Proteases in MHC class I presentation and cross-presentation. J Immunol. 2010;184:9–15. doi: 10.4049/jimmunol.0903399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saric T, Chang SC, Hattori A, York IA, Markant S, Rock KL, Tsujimoto M, Goldberg AL. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat Immunol. 2002;3:1169–1176. doi: 10.1038/ni859. [DOI] [PubMed] [Google Scholar]
- 7.York IA, Chang SC, Saric T, Keys JA, Favreau JM, Goldberg AL, Rock KL. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8-9 residues. Nat Immunol. 2002;3:1177–1184. doi: 10.1038/ni860. [DOI] [PubMed] [Google Scholar]
- 8.York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci U S A. 2006;103:9202–9207. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hearn A, York IA, Rock KL. The specificity of trimming of MHC class I-presented peptides in the endoplasmic reticulum. J Immunol. 2009;183:5526–5536. doi: 10.4049/jimmunol.0803663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang SC, Momburg F, Bhutani N, Goldberg AL. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc Natl Acad Sci U S A. 2005;102:17107–17112. doi: 10.1073/pnas.0500721102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammer GE, Gonzalez F, Champsaur M, Cado D, Shastri N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat Immunol. 2006;7:103–112. doi: 10.1038/ni1286. [DOI] [PubMed] [Google Scholar]
- 12.Hammer GE, Gonzalez F, James E, Nolla H, Shastri N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat Immunol. 2007;8:101–108. doi: 10.1038/ni1409. [DOI] [PubMed] [Google Scholar]
- 13.Tenzer S, Wee E, Burgevin A, Stewart-Jones G, Friis L, Lamberth K, Chang CH, Harndahl M, Weimershaus M, Gerstoft J, Akkad N, Klenerman P, Fugger L, Jones EY, McMichael AJ, Buus S, Schild H, van Endert P, Iversen AK. Antigen processing influences HIV-specific cytotoxic T lymphocyte immunodominance. Nat Immunol. 2009;10:636–646. doi: 10.1038/ni.1728. [DOI] [PubMed] [Google Scholar]
- 14.Cifaldi L, Lo Monaco E, Forloni M, Giorda E, Lorenzi S, Petrini S, Tremante E, Pende D, Locatelli F, Giacomini P, Fruci D. Natural killer cells efficiently reject lymphoma silenced for the endoplasmic reticulum aminopeptidase associated with antigen processing. Cancer Res. 2011;71:1597–1606. doi: 10.1158/0008-5472.CAN-10-3326. [DOI] [PubMed] [Google Scholar]
- 15.Fruci D, Giacomini P, Nicotra MR, Forloni M, Fraioli R, Saveanu L, van Endert P, Natali PG. Altered expression of endoplasmic reticulum aminopeptidases ERAP1 and ERAP2 in transformed non-lymphoid human tissues. J Cell Physiol. 2008;216:742–749. doi: 10.1002/jcp.21454. [DOI] [PubMed] [Google Scholar]
- 16.Hattori A, Kitatani K, Matsumoto H, Miyazawa S, Rogi T, Tsuruoka N, Mizutani S, Natori Y, Tsujimoto M. Characterization of recombinant human adipocyte-derived leucine aminopeptidase expressed in Chinese hamster ovary cells. J Biochem. 2000;128:755–762. doi: 10.1093/oxfordjournals.jbchem.a022812. [DOI] [PubMed] [Google Scholar]
- 17.Shido F, Ito T, Nomura S, Yamamoto E, Sumigama S, Ino K, Itakura A, Hattori A, Tsujimoto M, Mizutani S, Kikkawa F. Endoplasmic reticulum aminopeptidase-1 mediates leukemia inhibitory factor-induced cell surface human leukocyte antigen-G expression in JEG-3 choriocarcinoma cells. Endocrinology. 2006;147:1780–1788. doi: 10.1210/en.2005-1449. [DOI] [PubMed] [Google Scholar]
- 18.Shibata D, Ando H, Iwase A, Nagasaka T, Hattori A, Tsujimoto M, Mizutani S. Distribution of adipocyte-derived leucine aminopeptidase (A-LAP)/ER-aminopeptidase (ERAP)-1 in human uterine endometrium. J Histochem Cytochem. 2004;52:1169–1175. doi: 10.1369/jhc.3A6216.2004. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe Y, Shibata K, Kikkawa F, Kajiyama H, Ino K, Hattori A, Tsujimoto M, Mizutani S. Adipocyte-derived leucine aminopeptidase suppresses angiogenesis in human endometrial carcinoma via renin-angiotensin system. Clin Cancer Res. 2003;9:6497–6503. [PubMed] [Google Scholar]
- 20.Shibata K, Kikkawa F, Mizokami Y, Kajiyama H, Ino K, Nomura S, Mizutani S. Possible involvement of adipocyte-derived leucine aminopeptidase via angiotensin II in endometrial carcinoma. Tumour Biol. 2005;26:9–16. doi: 10.1159/000084181. [DOI] [PubMed] [Google Scholar]
- 21.Kamphausen E, Kellert C, Abbas T, Akkad N, Tenzer S, Pawelec G, Schild H, van Endert P, Seliger B. Distinct molecular mechanisms leading to deficient expression of ER-resident aminopeptidases in melanoma. Cancer Immunol Immunother. 2010;59:1273–1284. doi: 10.1007/s00262-010-0856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goto Y, Tanji H, Hattori A, Tsujimoto M. Glutamine-181 is crucial in the enzymatic activity and substrate specificity of human endoplasmic-reticulum aminopeptidase-1. Biochem J. 2008;416:109–116. doi: 10.1042/BJ20080965. [DOI] [PubMed] [Google Scholar]
- 24.Forloni M, Albini S, Limongi MZ, Cifaldi L, Boldrini R, Nicotra MR, Giannini G, Natali PG, Giacomini P, Fruci D. NF-kappaB, and not MYCN, regulates MHC class I and endoplasmic reticulum aminopeptidases in human neuroblastoma cells. Cancer Res. 2010;70:916–924. doi: 10.1158/0008-5472.CAN-09-2582. [DOI] [PubMed] [Google Scholar]
- 25.Saveanu L, Carroll O, Hassainya Y, van Endert P. Complexity, contradictions, and conundrums: studying post-proteasomal proteolysis in HLA class I antigen presentation. Immunol Rev. 2005;207:42–59. doi: 10.1111/j.0105-2896.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 26.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 27.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 28.Sobin LH, Wittekind C. TNM Classification of Malignant Tumours. Hoboken, New Jersey: John Wiley & Sons; 2002. [Google Scholar]
- 29.Klopocki E, Kristiansen G, Wild PJ, Klaman I, Castanos-Velez E, Singer G, Stohr R, Simon R, Sauter G, Leibiger H, Essers L, Weber B, Hermann K, Rosenthal A, Hartmann A, Dahl E. Loss of SFRP1 is associated with breast cancer progression and poor prognosis in early stage tumors. Int J Oncol. 2004;25:641–649. [PubMed] [Google Scholar]
- 30.Atkins D, Ferrone S, Schmahl GE, Storkel S, Seliger B. Down-regulation of HLA class I antigen processing molecules: an immune escape mechanism of renal cell carcinoma? J Urol. 2004;171:885–889. doi: 10.1097/01.ju.0000094807.95420.fe. [DOI] [PubMed] [Google Scholar]
- 31.Seliger B, Stoehr R, Handke D, Mueller A, Ferrone S, Wullich B, Tannapfel A, Hofstaedter F, Hartmann A. Association of HLA class I antigen abnormalities with disease progression and early recurrence in prostate cancer. Cancer Immunol Immunother. 2010;59:529–540. doi: 10.1007/s00262-009-0769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seliger B, Hohne A, Knuth A, Bernhard H, Ehring B, Tampe R, Huber C. Reduced membrane major histocompatibility complex class I density and stability in a subset of human renal cell carcinomas with low TAP and LMP expression. Clin Cancer Res. 1996;2:1427–1433. [PubMed] [Google Scholar]
- 33.Fruci D, Ferracuti S, Limongi MZ, Cunsolo V, Giorda E, Fraioli R, Sibilio L, Carroll O, Hattori A, van Endert PM, Giacomini P. Expression of endoplasmic reticulum aminopeptidases in EBV-B cell lines from healthy donors and in leukemia/lymphoma, carcinoma, and melanoma cell lines. J Immunol. 2006;176:4869–4879. doi: 10.4049/jimmunol.176.8.4869. [DOI] [PubMed] [Google Scholar]
- 34.Saenz-Lopez P, Gouttefangeas C, Hennenlotter J, Concha A, Maleno I, Ruiz-Cabello F, Cozar JM, Tallada M, Stenzl A, Rammensee HG, Garrido F, Cabrera T. Higher HLA class I expression in renal cell carcinoma than in autologous normal tissue. Tissue Antigens. 2010;75:110–118. doi: 10.1111/j.1399-0039.2009.01409.x. [DOI] [PubMed] [Google Scholar]
- 35.Dovhey SE, Ghosh NS, Wright KL. Loss of interferon-gamma inducibility of TAP1 and LMP2 in a renal cell carcinoma cell line. Cancer Res. 2000;60:5789–5796. [PubMed] [Google Scholar]


