Abstract
Objective: CD4+CD25+ regulatory T (Treg) cells and Th17 cells play important roles in peripheral immunity. Oxidized low-density lipoprotein (ox-LDL) is an instrumental factor in atherogenesis. However, the changes of Th17/Treg cells in patients with acute cerebral Infarction (ACI) and impact on Th17/Treg by ox-LDL are not clear. Here, we examined the balance of Th17/Treg in ACI patients and the effect of ox-LDL on this balance. Materials and methods: The frequencies of Th17 and Treg cells, key transcription factors and relevant cytokines were examined in patients with ACI, Transient ischemic attack (TIA) and controls. The correlations of cytokines, inflammatory biomarkers and ox-LDL in serum to Th17/Treg frequency, and the effects of ox-LDL on Th17/Treg cells in vitro were analyzed. Results: ACI patients have shown a significant increase of Th17 frequency, RORγt expression and Th17 related cytokines (IL-17 and IL-6 ) levels, and a clear decline of Treg frequency, Foxp3 expression, suppressive function and regulatory cytokines (IL-10 and TGF-β1) levels. Furthermore, TIA patients also have notable variation as compared to control group. Serum ox-LDL and inflammatory biomarkers were positively correlated with the frequency of Th17 cells and negatively correlated with the frequency of Treg cells. Treg and Th17 cells from ACI patients were significantly susceptible to ox-LDL-mediated alterations in vitro. Conclusions: Th17/Treg cells were imbalanced in ACI patients, and ox-LDL may contribute to this imbalance and lead to the occurrence of ACI suggesting their pathogenetic role in ACI.
Keywords: Cerebral infarction, transient ischemic attack, atherosclerosis, oxidized low-density lipoprotein, T helper 17, regulatory T cells
Introduction
Atherosclerosis (AS) is a chronic inflammatory disease that involves various immune cells, particularly T lymphocytes, such as CD4+ T-helper cells [1,2]. Acute cerebral Infarction (ACI) is a leading cause of death and the most frequent cause of permanent disability worldwide. ACI occurs as a consequence of plaque erosion or rupture and T lymphocytes play an important role in the process [3]. Transient ischemic attack (TIA) involves the sudden and brief loss of cerebral or ocular function, due to ischemic causes, with complete recovery. AS and plaque rupture is the principal causes of ACI and TIA [4].
CD4+CD25+ regulatory T (Treg) cells and T-helper 17 (Th17) cells are two subsets distinguished from Th1 and Th2 cells. Treg cells expressing the forkhead/winged helix transcription factor (Foxp3) are a fraction of inflammatory-regulated negative cells. Treg cells have important effects on the maintenance of immune tolerance and immune homeostasis by contact-dependent suppression or by the release of anti-inflammatory cytokines, such as interleukin (IL)-10 and transforming growth factor (TGF)-β [5]. Th17 cells expressing retinoic acid-related orphan receptor γt (RORγt) play critical roles in the development of many autoimmune diseases and inflammatory conditions by producing IL-17, tumor necrosis factor (TNF)-α and IL-6 [6]. In other and our previous studies, The Th17/Treg imbalance was critical in the pathogenesis of AS and acute coronary syndrome [7,8]. Yet little is known about the changes of Th17/Treg balance in ACI and TIA patients.
Oxidized low-density lipoprotein (ox-LDL) is a key factor in the occurrence of progressive AS and contributes to plaque rupture and thrombosis through multiple mechanisms [9,10]. The role of ox-LDL in ACI, however, has not been fully established in humans.
In this study, we have explored the balance of Th17/Treg cells in ACI patients, and the effects of ox-LDL on the balance.
Methods
Patient population
The study conformed to protocols approved by the NIH Institutional Review Board, and all patients gave written informed consent before enrollment into this study. This study was cross-sectional and blinded. We examined patients at Anhui provincial hospital who underwent diagnostic catheterisation (42 male and 29 female) between December 2010 and November 2012. Patients were classified into 3 groups: Group 1, atherosclerotic cerebral infarction (ACI) patients (22 males and 15 females; mean age, 61.9 ± 15.8 years). The diagnosis was based on a modification of the TOAST criteria based on the clinical, radiographic, and diagnostic information available [11]. Group 2, transient ischemic attacks (TIA) patients (20 males and 14 females, mean age 59.7 ± 13.6), TIA was defined as a documented neurological deficit lasting < 24 hours without definite radiographic evidence of acute ischemia [12]. Group 3, Control subjects (18 males and 12 females; mean age, 57.6 ± 16.5 years), control subjects were selected on a basis of recent angiography showing normal carotid arteries. Patients with ACI and TIA had a similar extent of carotid atherosclerosis. There were no evident differences between the three groups with regard to age.
No patient was treated with anti-inflammatory drugs and/or immunosuppressive agents. None had subarachnoid haemorrhage, extradural or subdural haemorrhage, brain abscess, surgery, or trauma, thromboembolism, disseminated intravascular coagulation, advanced liver disease, renal failure, malignant disease, other inflammatory disease, chronic-immune-mediated disorders.
Blood samples
We collected 5–10 ml of peripheral blood (PB) from all the patients, in a fasting state, on the morning following admission. The time interval between symptom onset and blood sampling was less than 24 h in all cases. All samples were treated with sodium heparin and examined within 4 h. PB mononuclear cells (PBMCs) were prepared by Ficoll density gradient for analysis of flow cytometry (FCM) and reverse transcription-polymerase chain reaction (RT-PCR). Serum was obtained after centrifugation and stored at −80°C until further use.
Cell separation and flow cytometry
Cell preparation
For the analysis of Th17 cells, PBMCs were suspended at a density of 2.0 × 106 cells/ml in complete culture medium (Gibco BRL, USA). Cultures were stimulated with phorbol myristate acetate (PMA, 25 ng/ml) plus ionomycin (1 μg/ml) for 4 h, in the presence of monensin (1.7 μg/ml, all from Alexis Biochemicals, San Diego, CA, USA). Cells were incubated at 37°C under a 5% CO2 environment. For the analysis of Treg, 100 μl of PBMCs (106) was added to tubes for further staining.
Detection of Treg and Th17 cells
For the analysis of Treg cells, cell surface staining was performed by the use of fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (13B8.2 clone; Beckman Coulter-Immunotech, Marseille, France), phycoerythrin (PE)-conjugated anti-CD25 (B1.49.9 clone, Beckman Coulter-Immunotech), PE-cy7-conjugated anti-CD127 (ebioRDR5 clone, eBioscience, San Diego, CA, USA) and appropriate isotype controls for 20 min at room temperature in the dark, followed by washing in phosphate buffered solution (PBS). Cells were fixed and permeabilized with the Fix/Perm reagent (Beckman Coulter-Immunotech), incubated with APC-conjugated anti-Foxp3 (PCH101 clone, eBioscience) and its isotype control antibody, washed with PBS and analyzed by FCM. For Th17 analysis, the cells were incubated with FITC-conjugated anti-CD4 at 4°C for 15 min and then stained with PE-conjugated anti-IL-17A (ebio64DEC17 clone, eBioscience) after fixation and permeabilization according to the manufacturer’s instructions. Stained cells were assessed by FCM using FACS Aria II flow cytometer with BD FACSDiva Software (Becton Dickinson, San Jose, CA, USA). The frequency of Treg (CD4+CD25+CD127low and CD4+CD25+Foxp3+) and Th17 (CD4+IL17+) cells was expressed as a percentage of CD4+ T cells by sequential gating on lymphocytes and CD4+ T cells.
Flow sorting
CD4+CD25+CD127low and CD4+CD25- cells were sorted using the gates (Figure 1) on a Beckman Counter flow cytometry cell-sorter (ALTRA HyPerSort System), after cells were dyed with FITC-conjugated anti-CD4, PE-conjugated anti-CD25, and PE-cy7-conjugated anti-CD127. We obtained a consistent purity of > 90% for both CD4+CD25+CD127low and CD4+CD25- cell fractions.
Figure 1.
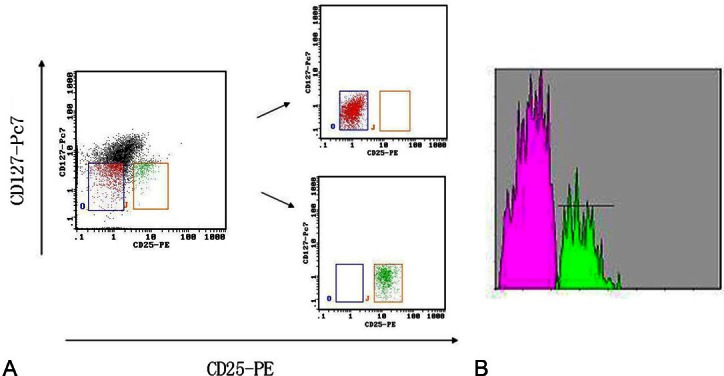
Flow sorting of Tregs and responder T cells. A. The dot plots show the CD4+CD25+CD127low (right box) and CD4+CD25- cells (left box) T cell-gating strategy pre-flow sorting and the subsequent purified subsets, and the purity of CD4+CD25+CD127low and CD4+CD25- T cells isolated were > 90% for each fraction. B. Over 90% of the freshly purified CD4+CD25+CD127low T cells (marked with green)express Foxp3, and over 90% of the freshly purified CD4+CD25- T cells (marked with red) did not express Foxp3.
Functional assay of Treg cells
Freshly purified CD4+CD25+CD127low T cells from controls and from patients with ACI and TIA (n = 5 in each group) were assayed for their suppressive activity in the allogeneic mixed lymphocyte response (MLR) assay. Irradiated (3000 rad) PBMCs from healthy controls were used as allogeneic stimulator cells. CD4+CD25- cells were used as responder cells. CD4+CD25- cells (1.0 × 104 cells per well) were co-cultured with CD4+CD25+CD127low cells (1.0 × 104 cells per well, 1:1 ratio) in the presence of irradiated PBMCs (2.0 × 104 cells per well) in a 96-well flat-bottom plate. Wells without CD4+CD25+CD127low cells (responders and stimulators only) served as positive controls. Wells containing CD4+CD25+CD127low cells and irradiated PBMCs (none-responders) served as baseline controls. All incubations were run in triplicate with a final volume of 150 μl at 37°C and 5% CO2. After 48 h of incubation, 10 μl Cell Proliferation Reagent WST-1 was added to each well. After incubation for a further 4 h, the absorbance of the samples against a background control as blank was measured at 450 nm using 650 nm as a reference wavelength on a Biocell HT1 ELISA microplate reader (Salzburg, Austria). Suppression was expressed as a percentage of the positive control.
RORγt and Foxp3 expression determined by real time-PCR
Total RNA was extracted with TRIzol (Invitrogen, USA) according to the manufacturer’s instructions. The amount and purity of the obtained RNA was determined by measurements of the optical density (OD) at 260 and 280 nm. For real-time PCR, cDNA was synthesized by using random hexamer primers and RNase H-reverse transcriptase (Invitrogen). TaqMan primers and probes for human Foxp3 and RORγt were purchased from Applied Biosystems. The following primer pairs were used: Foxp3: F: 5′-CACGCATGTTTGCCTTCTTCAGA-3′, R: 5′-GTAGGGTTGGAACACCTGCTGGG-3′, and RORγt: F: 5′-GCAATGGAAGTGGTGCTGGTT-3′, R: 5′-AGGATGCTTTGGCGATGAGTC-3′. All reactions were performed using the ABI Prism 7900 Sequence Detection System (Applied Biosystems). For each sample, mRNA expression level was normalized to the level of β-actin housekeeping genes. β-actin was analysed using the following primers: F: 5′-ATCTGGCACCACACCTTC-3′, R: 5′-AGCCAGGTCCAGACGCA-3′.
Measurement of blood biochemistry
Blood sugar and lipids were determined by the enzymatic method. High sensitive C-reactive protein (hsCRP) was measured by the immunoturbidimetric method. All of the assays were conducted on an Olympus AU2700 biochemical autoanalyzer (Olympus, Japan).
Cytokines, inflammatory biomarker, and ox-LDL in serum
The levels of Interleukin 10 (IL-10), transforming growth factor (TGF)-β1, IL-17, IL-6, lipoprotein-associated phospholipase A2 (LpPLA2) and ox-LDL in serum were examined by the enzyme-linked immunosorbent assay (ELISA) and measured at 450 nm on Biocell HT1 ELISA microplate reader. (IL-17 and IL-10 ELISA kits from Bender MedSystems; IL-6, TGF-β1 and LpPLA2 ELISA kits, both from R&D system, Minneapolis, MN, USA; ox-LDL ELISA kits from Uscnlife, USA). The minimal detectable concentrations were 0.5 pg/ml for IL-17, 1.0 pg/ml for IL-10, 0.7 pg/ml for IL-6, 5 pg/ml for TGF-β1, 0.074 ng/ml for LpPLA2, and 4.5 μg/L for ox-LDL. Intra- and inter-assay coefficients of variation for all ELISA were < 5%. All samples were measured in duplicate.
Effects of ox-LDL on Treg and Th17 cells
Preparation of LDL and ox-LDL
Blood for lipoprotein isolation was collected from health controls after 12 h of fasting. LDL was isolated by the ultracentrifugation of serum as previously described [13]. The LDL oxidation assay was performed as previously described [14], and the lipid hydroperoxide content was then determined using the Fox assay [15].
Ox-LDL induction experiments
To compare the effects of ox-LDL on Treg and Th17 cells from ACI, TIA and controls, PBMCs (n = 5 from each group) were incubated with 1 μg/ml ox-LDL for 48 h in vitro, and the frequency of Treg and Th17 cells was measured, and analysis of Th17 was carried out after stimulation just as before. The suppressive functions of Tregs were assessed in the presence or absence of ox-LDL (1 μg/ml).
Statistical analysis
Values were expressed as the mean ± standard deviation (SD). Data were analysed by using statistical software (SPSS 13.0; LEAD Technologies, Inc., Chicago, IL, USA). Statistical significance for the differences in the groups was assessed by one-way analysis of variance (ANOVA). If significance was found, Bonferroni’s test was performed for post-hoc analysis to detect the differences among groups when equal variance was assumed, while Dunnett’s C test was performed when equal variance was not assumed. Spearman’s correlation was used as a test of correlation between two continuous variables. P < 0.05 was considered to be statistically significant.
Results
Patients and controls
There were no significant differences in age, gender, hypertension, smoking rate, high density lipoprotein-cholesterol (HDL-C) and very low density lipoprotein-cholesterol concentrations (VLDL-C) among the 3 group. However, fasting blood glucose (FBG), total cholesterol (TC) and total triglyceride (TG), low density lipoprotein-cholesterol (LDL-C) levels in the ACI and TIA groups were significantly higher than those in the NCA groups (P < 0.05 and P < 0.01, respectively). There were also no significant differences in BFS, TC, TG, LDL-C concentrations between TIA and ACI group (Table 1).
Table 1.
Patient characteristics
| Item | Control (n = 30) | TIA (n = 34) | ACI (n = 37) |
|---|---|---|---|
| Age | 57.6 ± 16.5 | 59.7±13.6 | 61.9 ± 15.8 |
| Gender (male/female) | 18/12 | 20/14 | 22/15 |
| Hypertension, n (%) | 12 (40) | 15 (44.1) | 18 (48.6) |
| Smoking rate, n (%) | 9 (30) | 11 (32.4) | 13 (35.1) |
| FBG (mmol/L) | 4.97 ± 0.49 | 5.46 ± 0.87* | 5.58 ± 0.91* |
| TC (mmol/L) | 4.32 ± 0.63 | 5.08 ± 0.76▲ | 5.23 ± 0. 87▲ |
| TG (mmol/L) | 1.12 ± 0. 41 | 1.69 ± 0. 58* | 1.87 ± 0. 49* |
| HDL-C (mmol/L) | 1.26 ± 0.18 | 1.04 ± 0.23 | 1.09 ± 0.25 |
| LDL-C (mmol/L) | 2.41 ± 0.36 | 3.08 ± 0.62* | 3.15 ± 0.71* |
| VLDL-C (mmol/L) | 0.48 ± 0.17 | 0.57 ± 0.21 | 0.55 ± 0.19 |
Values are expressed as mean ± SD or number. ACI: acute cerebral infarction; TIA: Transient ischemic attacks; NCA: subjects with normal carotid arteries; FBG: fasting blood glucose; TC: total cholesterol; TG: total triglyceride; HDL-C high-density lipoprotein-cholesterol; LDL-C: low density lipoprotein-cholesterol; VLDL-C: very low density lipoprotein-cholesterol.
P < 0.05 vs. control;
P < 0.01 vs. control.
Decrease of Treg cells and increase of Th17 cells in ACI patients
As shown in Figure 2, the frequencies of Treg (CD4+CD25+Foxp3+/CD4+ T cells) cells were significantly lower in ACI (1.75 ± 0.47%) than in TIA patients (2.67 ± 0.38%) and control subjects (3.89 ± 0.52%) (P < 0.05, P <0.01 respectively). The frequencies of CD4+CD25+Foxp3+ Treg cells in TIA patients were also markedly lower than in control group (P < 0.01).
Figure 2.
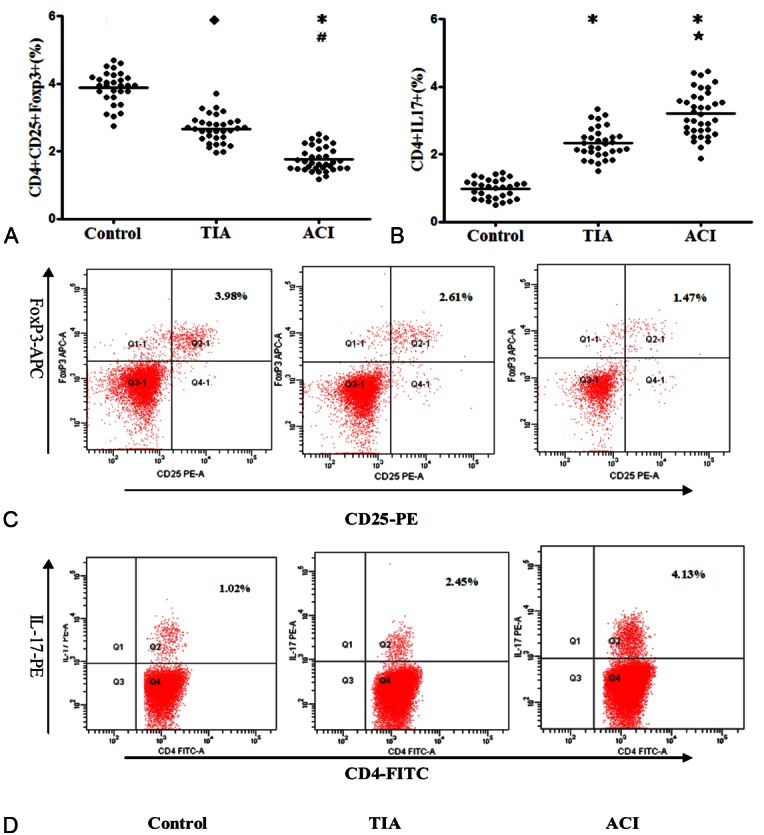
Treg frequencies decreased and Th17 Frequencies increased in patients with ACI. A. Comparison of Treg expression among the 3 groups. B. Comparison of Th17 expression among the 3 groups. ◆P < 0.05 vs. Control; *P < 0.01 vs. Control; #P < 0.05 vs. TIA; ★P < 0.01 vs. TIA. C, D. Representative FACS figures of Treg (C) and Th17 cells (D) from a single patient in each group. The percentage of positive cells is shown in each panel. ACI: acute myocardial infarction; TIA: unstable angina; Control: control subjects.
The frequencies of Th17 (CD4+IL17+/CD4+ T cells) were markedly higher in ACI (3.92 ± 0.64%) than in TIA patients (2.36 ± 0.45%) and control subjects (0.96 ± 0.28%) (both P < 0.01). There was also an obvious difference between the TAI and control groups (P < 0.01; Figure 2).
Expression of Foxp3 and RORγt in PBMCs from ACI
Foxp3 levels in PBMCs were significantly lower in ACI patients than in TIA and control subjects (P < 0.05, P < 0.01 respectively), while RORγt levels were markedly higher in ACI patients than in TIA and control subjects (both P < 0.01). With respect to Foxp3 and RORγt levels, there were also obvious differences between TIA and control groups (P < 0.05; Figure 3).
Figure 3.
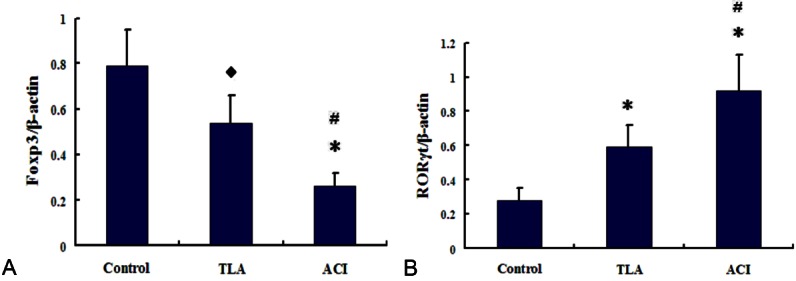
Expression of RORγt and Foxp3 in PBMCs from controls, TIA and ACI patients was determined by real time-polymerase chain reaction (PCR). A. The ratios of RORγt/β-actin mRNA were compared in the 3 groups. B. The ratios of Foxp3/β-actin and RORγt/β-actin for the gray-scale value were compared in 3 groups. ◆P < vs. Control; *P < 0.01 vs. Control; #P < 0.05 vs. TIA.
Decrease in suppression of Tregs from ACI
The function of Treg cells was assessed by inhibition of the proliferation of CD4+CD25- cells in controls, TIA, and ACI patients. CD4+CD25+CD127low cells showed a different suppressive rate: 83.2 ± 4.9%, 62.3 ± 4.1%, and 37.5 ± 2.8%, respectively. Suppressive rates of Treg cells were significantly lower in ACI patients than in TIA patients and controls (both P < 0.01). Suppressive rates of Treg cells were also significantly lower in TIA patients than in control group (P < 0.05; Figure 4).
Figure 4.
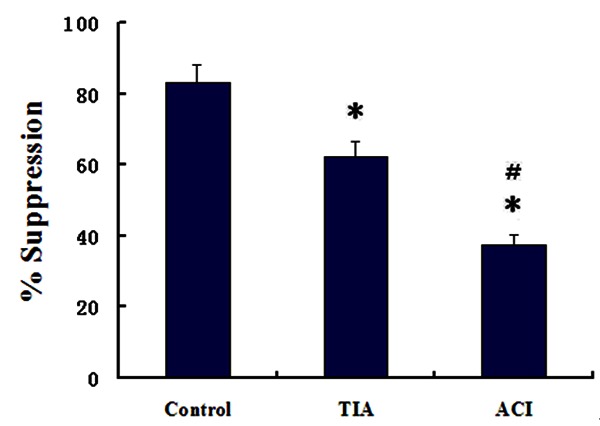
Comparison of the suppressive rate of Treg cells among the controls, TIA and ACI groups (n = 5 in each group). *P < 0.01 vs. Control; #P < 0.05 vs. TIA.
Correlation of Treg and Th17 cells with the levels of cytokines and inflammatory biomarkers
Changes of serum cytokines and inflammatory biomarkers in ACI patients
The levels of IL-10 and TGF-β1 were significantly lower in ACI patients than in TIA patients and controls (all P < 0.01). The levels of IL-17 and IL-6 were markedly higher in the ACI patients than in TIA patients and controls (P < 0.01, P < 0.05 respectively). Similarly, the concentrations of hsCRP and LpPLA2 were significantly increased in ACI patients than in TIA patients and control subjects (P < 0.01, P < 0.05 respectively). Moreover, a decrease in the levels of IL-10 and an increase in the levels of IL17, hsCRP and LpPLA2 were significant for TIA patients than for control group (P < 0.01, P < 0.05 respectively; Table 2).
Table 2.
Serum levels of cytokines, inflammatory biomarkers and ox-LDL in the three groups
| Control (n = 30) | TIA (n = 34) | ACI (n = 37) | |
|---|---|---|---|
| IL-10 (pg/mL) | 27.20 ± 5.46 | 18.90 ± 3.82# | 7.98 ± 1.84▲,* |
| TGF-β1 (pg/mL) | 453.60 ± 128.72 | 372.50 ± 109.34# | 243.73 ± 89.52▲,* |
| IL-17 (pg/mL) | 16.40 ± 3.81 | 30.14 ± 7.09▲ | 54.87 ± 12.13▲,* |
| IL-6 (pg/mL) | 4.24 ± 2.13 | 8.31 ± 3.64# | 15.43 ± 5.91▲,* |
| hsCRP (mg/L) | 1.49 ± 0.78 | 5.60 ± 2.03▲ | 9.73 ± 4.58▲,◆ |
| LpPLA2 (ng/mL) | 232.80 ± 47.90 | 317.10 ± 71.50# | 385.40 ± 92.60▲,◆ |
| OxLDL (µg/L) | 308.70 ± 35.20 | 363.10 ± 43.80# | 436.10 ± 57.40▲,* |
P < 0.01 vs. Control;
P < 0.05 vs. Control;
P < 0.01 vs. TIA;
P < 0.05 vs. TIA.
Correlation of Treg and Th17 cells to the levels of cytokines
For the 4 groups, serum TGF-β1 and IL-10 levels were strongly correlated with the frequency of CD4+CD25+Foxp3+ Treg cells (P < 0.01 and r = 0.823, 0.786, respectively), and were inversely correlated with the frequency of Th17 cells (P < 0.01 and r = -0.857, -0.805, respectively). In contrast, serum IL-17 and IL-6 levels were inversely correlated with the frequency of Treg cells (P < 0.01 and r = -0.842, -0.819 respectively), and positively correlated with the frequency of Th17 cells (P < 0.01 and r = 0.875, 0.834, respectively).
Correlation of Treg and Th17 cells to the levels of inflammatory biomarkers
The inflammatory markers hsCRP and LpPLA2 were significant correlated with Treg and Th17 cells. hsCRP serum concentrations were negatively correlated with the frequency of Treg cells (P < 0.01 and r = -0.735), and positively correlated with the frequency of Th17 cells (P < 0.01 and r = 0.764). Similar results were observed for the correlation of serum LpPLA2 levels to Treg and Th17 cells (LpPLA2 and Treg levels, P < 0.01 and r = -0.718; and LpPLA2 and Th17 levels, P < 0.01 and r = 0.741).
Role of ox-LDL in the balance of Th17/Treg cells
Correlation of serum ox-LDL levels to Th17/Treg cells in ACI patients
The concentration of ox-LDL increased more significantly in the ACI patients than in TIA patients and control subjects (P < 0.05, P < 0.01 respectively). There was also an obvious difference between the TIA and control groups (P < 0.05). In addition, ox-LDL concentrations in serum were negatively correlated with the frequency of Treg cells (P < 0.01 and r = -0.776), and positively correlated with the frequency of Th17 cells (P < 0.01 and r = 0.793). Furthermore, There was an inverse correlation between the concentrations of ox-LDL and Treg-related cytokines (TGF-β1, IL-10) in serum (P < 0.01 and r = -0.815, -0.759). There were also positive correlations between the serum ox-LDL concentration and serum Th17-related cytokines (IL17 and IL-6), hsCRP, and LpPLA2 concentrations (P < 0.01 and r = 0.843, and 0.829, 0.794, and 0.861, respectively).
Effects of ox-LDL on Treg and Th17 cells in vitro
After incubated with 1.0 μg/ml ox-LDL for 48h in vitro, we found that the frequency of Treg cells was decreased while the frequency of Th17 cells was elevated in all the 3 groups. We further investigated the sensitivity of the ox-LDL-mediated decrease of Treg cells, compromise of function and increase of Th17 cells in the ACI, TIA, and control groups. There were significant changes in the number of Treg and Th17 cells by ox-LDL in patients with ACI (approximately 45% reduction of Treg and 53% increase of Th17 cells) than in TIA (about 31% reduction of Treg and 36% increase of Th17 cells) and control subjects (approximately 18% reduction of Treg and 24% increase of Th17 cells, Figure 5A and 5B). However, there was no difference in the numbers of CD4+ cells in cultured PBMCs derived from ACI and TIA patients and control subjects treated with ox-LDL.
Figure 5.
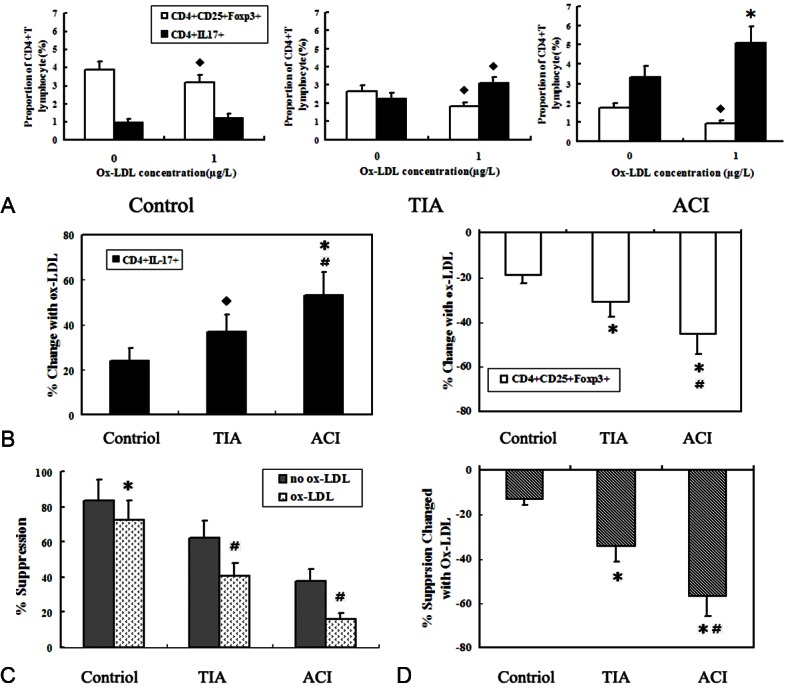
Effects of Ox-LDL on Treg and Th17 cells from the control, TIA and ACI groups in vitro. A. Co-culture assay was performed as described in the Methods section, PBMCs from the Control, TIA and ACI groups were incubated with 1.0 μg/ml ox-LDL for 48 h. ◆P < 0.05 vs. ox-LDL-untreated group; *P < 0.01 vs. ox-LDL-untreated group. B. Average change of Treg and Th17 numbers in the 3 groups after a 48 h co-culture with 1.0 μg/ml ox-LDL. *P < 0.01 vs. control; ◆P < 0.05 vs. control; #P < 0.05 vs. TIA. C. Treg cells from the 3 groups are compromised in their suppressive properties after a 48 h co-culture with 1.0 μg/ml ox-LDL. *P < 0.05 vs no-ox-LDL group; #P < 0.01 vs. no-ox-LDL group. D. Average attenuation of the suppressive function of Treg cells in the 3 groups when exposed to ox-LDL. *P < 0.01 vs control; #P < 0.01 vs. TIA.
Moreover, the suppressive functions of Treg cells from all the 3 groups were compromised after exposure to ox-LDL. Incubation of Treg cells with 1 μg /ml ox-LDL, resulted in a significant attenuation of their ability to suppress the proliferation of CD4+CD25+ cells (P < 0.05 for control and P < 0.01 for TIA and ACI). It is noteworthy that attenuation of the suppressive properties of Treg cells in ACI patients was also more remarkably than in TIA patients and health controls (both P < 0.01). There were also significant differences of Treg function between TIA and control group (P < 0.01; Figure 5C and 5D).
Discussion
Inflammation is increasingly recognized to play an important role in atherosclerosis and stroke [16]. The imbalance of Th17/Treg cells is closely related to inflammation and the onset of AS [7]. Ox-LDL is primarily present in atherosclerotic lesions but not in normal arteries and are associated with plaque vulnerability [17]. In the present study, ACI patients had a significant increase in peripheral Th17 cells, Th17-related cytokines (IL-17, IL-6) and transcription factor (RORγt) levels and a significant decline of Treg numbers, functions, Treg-related cytokines (IL-10, TGF-β1) and Foxp3 levels when compared with the TIA and control groups. In addition, there were also markedly differences between TIA and control groups. Significant positive and negative correlations were noted between Th17 cells, Treg cells, and serum ox-LDL, hsCRP, LpPLA2 levels. After incubation of PBMCs from the 3 groups with ox-LDL, ox-LDL induced a more significant alteration of Treg and Th17 cells from ACI patients than in the TIA and NCA groups. These data indicate that the Th17/Treg imbalance exists in ACI patients, and that ox-LDL may contribute to this imbalance, which leads to thrombosis and pathogenesis of ACI.
It has found that numbers of Treg cells were significantly reduced in atherosclerotic mice, and the adoptive transfer of Treg cells can greatly reduce plaque size [18]. Depletion of Treg cells with an anti-CD25 antibody also enhances atherosclerosis in apoE-/- mice, indicating Treg cells have anti-atherosclerotic effect [19]. Low levels of Treg cells are observed in all developmental stages of human plaques, highlighting the relevance of these cells in human atherosclerosis [20].
However, the exact mechanisms mediating the antiatherogenic properties of Treg cells have not been elucidated. Treg cells may suppress proatherogenic immune responses partly by secretion of anti-inflammatory cytokines IL-10 and TGF-β1. IL-10 can antagonises the Th1 response by inhibiting the production of IFN-γ and antigen presentation, while TGF-β1 can promote Foxp3 expression, induce differentiation of Treg cells, and act as one of the effective factors of Treg cells [21]. Treg cells dysfunction has been found in several kidney disease animal models, and experimental models also revealed an impaired Treg-regulated immune mechanism in atherosclerosis [22].
Since Treg cells are able to dampen tissue immune responses, their low numbers and decreased function in plaques could be responsible for the ongoing inflammation that characterises AS and ACI. It has been reported that Treg cells are major cerebroprotective modulators of postischemic inflammatory brain damage and IL-10 signaling is essential for their immunomodulatory effect [23]. Additionally, the frequency of Treg cells was found to increase in peripheral blood following human stroke, and Treg cells are considered to contribute to repair and recovery from stroke [24]. However, Treg cells were also reported to be strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature [25].
In our study, Treg number and Foxp3 expression were significantly decreased in ACI patients. Treg cells from ACI patients also declined the inhibition of responder CD4+CD25- T-cell proliferation when compared with Treg cells from TIA and control subjects. The levels of serum IL-10 and TGF-β1 were reduced in the ACI group and positively correlated with the levels of Treg cells; they were negatively correlated with ox-LDL and inflammatory markers levels in all 3 groups. These correlations further support the hypothesis that Treg-related cytokines may play a protective role against the onset of ACI.
Th17 cells are characterised by their ability to produce specific cytokines such as IL-17, IL-6 [26]. IL-17 is a pro-inflammatory cytokine, which targets various cell types and induces additional cytokines (such as TNF-α, IL-1, IL-6), and chemokines (including CXCL1, CXCL2, CCL7) that are associated with inflammation. IL-6 is a pleiotropic cytokine that mainly signals by membrane (neutrophil and lymphocyte) or soluble (endothelial cell) IL-6 receptors. IL-6 is now known to influence T cell development: in the presence of IL-6 and TGF-ß, naive T cells develop into Th17 cells. IL-6 may lead to the releases of CRP, fibrinogen, and cell adhesion molecules, and is an important inflammatory marker for ACI [27].
In murine models it has been demonstrated that mice with decreased IL-17 levels develop fewer lesions; increases in the levels of IL-17 may enhance early lesion formation, suggesting a potential role for Th17 cells in the promotion of atherogenesis [28]. In human lesions, Th17 may participate in the inflammatory process of plaque repture [29]. So Th17 has been hypothesised to play a role in the development and complications of atherosclerosis.
We found that ACI patients, for the first time, exhibited a marked increase in Th17 number, RORγt expression and IL-17/IL-6 levels when compared with TIA and NCA subjects. The levels of serum IL-17/IL-6 were positively correlated with the Th17 levels, and also positively correlated with ox-LDL, hsCRP, LpPLA2 levels in all 3 groups, which demonstrated that Th17-related cytokines are associated with the development of ACI.
A TIA, known as a “ministroke,” elicits focal neurological deficits similar to an ACI but has been defined as lasting < 24 h [12]. TIAs are strong predictors of short-term risk for a full ischemic stroke, cardiovascular events, and death. In our study, the frequency of CD4+CD25+FoxP3+ Treg cells, Foxp3 expression and IL-10/TGF-β1 levels was significantly lower in TIA patients than in controls. The suppressive function of Treg cells was more hampered in TIA patients than in controls. Moreover, TIA patients exhibited a significant increase in Th17 number, RORγt expression and IL-17/IL-6 levels as compared with control group. These data suggested that imbalance of Tregs/Th17 not only occurred in ACI, but also in TIA.
Treg cells regulate the action of effector T cells, mediate tolerance, and prevent tissue damage, while Th17 cells are the major cell type involved in orchestrating tissue inflammation and autoimmunity. Peripheral Treg and Th17 cells hold certain balance in normal subjects and may change in various diseases, suggesting the balance plays an important role in the control of autoimmunity and inflammation. Furthermore, the relationship between them has recently been determined at the molecular level, where Foxp3 and RORγt were shown to physically associate with each other and inhibit each other’s functions [30]. Our study demonstrated that the balance of Th17/Treg cells was disrupted in ACI and TIA patients.
Ox-LDL plays an important role in the promotion of atherosclerosis initiation, progression and, possibly, plaque destabilisation [10]. Fang et al [31] results indicated that ox-LDL were possible risk factors for carotid plaque, and the ox-LDL level was associated with carotid plaque vulnerability. The effects of ox-LDL on atherosclerosis are associated with the reduced frequency and compromised suppressive function of Treg cells [19]. In our study, ox-LDL serum concentrations were negatively correlated with the frequency of Treg cells, and positively correlated with the frequency of Th17 cells. Moreover, Treg and Th17 cells from ACI patients were more susceptible to the influence of ox-LDL when compared to those in TIA and control subjects. Therefore, elevated sensitivity to ox-LDL results in the aggravation of the Th17/Treg imbalance, which promotes atherosclerotic plaque formation/destabilisation in ACI.
Both hsCRP and Lp-PLA2 are key inflammatory biomarkers of ACI. HsCRP, an acute phase protein, have been used as non-specific measures of vascular inflammation. Lp-PLA2 is an enzyme derived from leukocytes, particularly macrophages, which is involved in metabolism of LDL to the pro-inflammatory mediators [32]. Lp-PLA2 has also emerged as an independent inflammatory marker of cardiovascular risk and predictor of ischemic stroke events. In this study, Th17/Treg frequencies were significantly correlated with the levels of hsCRP and Lp-PLA2, which also seemed to be linked to the degree of carotid damage and plaque destabilisation.
The reasons for ox-LDL effect on Th17/Treg cells are not clear. Ox-LDL-mediated cell apoptosis and proliferation could have been partially responsible for the change in the balance of Th17/Treg cells. Ox-LDL might induce apoptotic signaling pathways [33], resulting in the increase of apoptotic Treg cells and cell proliferation [34], resulting in the amplification of Th17 cells. These pathways may alter the fragile balance between Treg and Th17 cells, which increases the risk of plaque rupture and ACI. Therefore, further studies needs to demonstrate the mechanisms of ox-LDL effects on Th17/Treg cells. The control of ox-LDL levels would be beneficial to maintain plaque stability and prevent ACI occurrence.
In summary, our findings show that a numeral and functional imbalance of the Th17/Treg cells exists in patients with ACI, suggesting a potential role for the imbalance of these cells in the onset of ACI. Ox-LDL may contribute to plaque destabilisation and rupture by its effects on this balance. The imbalance of Th17/Treg cells appears to be a novel target for research on the pathogenesis of ACI and the treatment of ACI.
Acknowledgments
This study was supported by Natural Science Youth Foundation of Anhui province of China (No. 11040606Q08), Natural Science Key Foundation of Anhui Universities of China (No. KJ2011A166), and Anhui Medical University Research Foundation (No. 2010XKJ083).
References
- 1.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 3.Haeusler KG, Schmidt WU, Foehring F, Meisel C, Guenther C, Brunecker P, Kunze C, Helms T, Dirnagl U, Volk HD, Villringer A. Immune responses after acute ischemic stroke or myocardial infarction. Int J Cardiol. 2012;155:372–377. doi: 10.1016/j.ijcard.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Parmar JP, Rogers WJ, Mugler JP 3rd, Baskurt E, Altes TA, Nandalur KR, Stukenborg GJ, Phillips CD, Hagspiel KD, Matsumoto AH, Dake MD, Kramer CM. Magnetic resonance imaging of carotid atherosclerotic plaque in clinically suspected acute transient ischemic attack and acute ischemic stroke. Circulation. 2010;122:2031–2038. doi: 10.1161/CIRCULATIONAHA.109.866053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman WA, Cooper KD, McCormick TS. Regulation generation: the suppressive functions of human regulatory T cells. Crit Rev Immunol. 2012;32:65–79. doi: 10.1615/critrevimmunol.v32.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdaca G, Colombo BM, Puppo F. The role of Th17 lymphocytes in the autoimmune and chronic inflammatory diseases. Intern Emerg Med. 2011;6:487–495. doi: 10.1007/s11739-011-0517-7. [DOI] [PubMed] [Google Scholar]
- 7.Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, Zhou ZH, Liao MY, Yao R, Yu X, Wang D, Cheng Y, Liao YH, Cheng X. The Th17/Treg functional imbalance during atherogenesis in ApoE(-/-) mice. Cytokine. 2010;49:185–193. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Qing Li, Yi Wang, Ke Chen, Qing Zhou, Wei Wei, Yiping Wang, Yuan Wang. The role of oxidized low-density lipoprotein in breaking peripheral Th17/Treg balance in patients with acute coronary syndrome. Biochem Biophys Res Commun. 2010;394:836–842. doi: 10.1016/j.bbrc.2010.03.090. [DOI] [PubMed] [Google Scholar]
- 9.Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitra S, Deshmukh A, Sachdeva R, Lu J, Mehta JL. Oxidized low-density lipoprotein and atherosclerosis implications in antioxidant therapy. Am J Med Sci. 2011;342:135–142. doi: 10.1097/MAJ.0b013e318224a147. [DOI] [PubMed] [Google Scholar]
- 11.Fure B, Wyller TB, Thommessen B. TOAST criteria applied in acute ischemic stroke. Acta Neurol Scand. 2005;112:254–258. doi: 10.1111/j.1600-0404.2005.00470.x. [DOI] [PubMed] [Google Scholar]
- 12.Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, Hatsukami TS, Higashida RT, Johnston SC, Kidwell CS, Lutsep HL, Miller E, Sacco RL American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease: the American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- 13.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, Cheroutre H, Faull KF, Berliner JA, Witztum JL, Lusis AJ. Combined serum paraoxonase knockout/apolipoprotein E knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 14.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, Lusis AJ, Shih DM. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 15.Jiang ZY, Hunt JV, Wolff SP. Ferrousion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 16.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 17.Nishi K, Itabe H, Uno M, Kitazato KT, Horiguchi H, Shinno K, Nagahiro S. Oxidized LDL in carotid plaques and plasma associates with plaque instability. Arterioscler Thromb Vasc Biol. 2002;22:1649–1654. doi: 10.1161/01.atv.0000033829.14012.18. [DOI] [PubMed] [Google Scholar]
- 18.Mor A, Planer D, Luboshits G, Afek A, Metzger S, Chajek-Shaul T, Keren G, George J. Role of naturally occurring CD4+CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 19.Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 20.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25 naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotsman I, Grabie N, Gupta R, Dacosta R, MacConmara M, Lederer J, Sukhova G, Witztum JL, Sharpe AH, Lichtman AH. Impaired regulatory T-cell response and enhanced atherosclerosis in the absence of inducible costimulatory molecule. Circulation. 2006;114:2047–2055. doi: 10.1161/CIRCULATIONAHA.106.633263. [DOI] [PubMed] [Google Scholar]
- 23.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, Read SJ, Henderson RD, Hull R, O’Sullivan JD, McCombe PA, Greer JM. Frequency and function of regulatory T cells after ischaemic stroke in humans. J Neuroimmunol. 2012;243:89–94. doi: 10.1016/j.jneuroim.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Göbel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louten J, Boniface K, de Waal Malefyt R. Development and function of TH17 cells in health and disease. J Allergy Clin Immunol. 2009;123:1004–1011. doi: 10.1016/j.jaci.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Pola R. Inflammatory markers for ischaemic stroke. Thromb Haemost. 2009;101:800–1. [PubMed] [Google Scholar]
- 28.Song L, Schindler C. IL-6 and the acute phase response in murine atherosclerosis. Atherosclerosis. 2004;177:43–51. doi: 10.1016/j.atherosclerosis.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Hashmi S, Zeng QT. Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron Artery Dis. 2006;17:699–706. doi: 10.1097/01.mca.0000236288.94553.b4. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang R, Zhang N, Wang C, Zhao X, Liu L, Wang Y, Xu J, Wang X, Liu Z, Wang Y. Relations between plasma ox-LDL and carotid plaque among Chinese Han ethnic group. Neurol Res. 2011;33:460–466. doi: 10.1179/016164111X13007856083927. [DOI] [PubMed] [Google Scholar]
- 32.Saenger AK, Christenson RH. Stroke biomarkers: progress and challenges for diagnosis, prognosis, differentiation, and treatment. Clin Chem. 2010;56:21–33. doi: 10.1373/clinchem.2009.133801. [DOI] [PubMed] [Google Scholar]
- 33.Salvayre R, Auge N, Benoist H, Negre-Salvayre A. Negre-Salvayre A. Oxidized low-density lipoprotein induced apoptosis. Biochim Biophys Acta. 2002;1585:213–221. doi: 10.1016/s1388-1981(02)00343-8. [DOI] [PubMed] [Google Scholar]
- 34.Zettler ME, Prociuk MA, Austria JA, Massaeli H, Zhong G, Pierce GN. OxLDL stimulates cell proliferation through a general induction of cell cycle proteins. Am J Physiol Heart Circ Physiol. 2003;284:H644–H653. doi: 10.1152/ajpheart.00494.2001. [DOI] [PubMed] [Google Scholar]


