Abstract
Chemokine CCL24 is the second member of eotaxins, a group of eosinophils’ selectively chemoattractants. Via binding to its only receptor CCR3, CCL24 mainly mediates atopic disorders, parasitic infections and systemic diseases. It is well-known that CCR3 is expressed at the maternal-fetal interface; nevertheless whether CCL24 is located there and which role CCL24/CCR3 axis played is unclear. In this article, we assessed the expression of CCL24 and CCR3 in decidual stromal cells (DSCs) and trophoblasts, investigated the effects of DSCs-trophoblasts contact and pregnancy-associated hormones on the expression of CCR3 by DSCs, and last examined the role of trophoblasts-derived CCL24 on the proliferation, cell numbers and apoptosis of DSCs in vitro. We found that trophoblasts secrete chemokine CCL24, whereas DSCs express receptor CCR3. DSCs and trophoblasts co-culture had an raised level of CCL24 in culture supernatants, and the expression of CCR3 on DSCs was also obviously improved. Estrogen, progesterone and hCG up-regulated the expression of CCR3 on DSCs at appropriate concentration. CCL24 increased the proliferation and apoptosis of DSCs, whereas on the whole it promoted the number of DSCs. Thus, we conclude that by secreting CCL24 trophoblasts could promote the growth of DSCs; pregnancy associated environments such as DSCs-trophoblasts contact and hormones increased local CCL24/CCR3, which means a beneficial factor for the process of decidualization in human early pregnancy.
Keywords: CCL24, CCR3, DSCs, maternal-fetal interface, trophoblasts
Introduction
Implantation involves a series of complicate biological programs, which requires the synchronous development of receptive endometrium and functional blastocyst [1]. Meanwhile, the different cytokines, chemokines, growth factors, and adhesion molecules are expressed and involved in that. At maternal-fetal interface, the abnormal dialogue between mother and embryo may impair the biological functions of component cells and result in several pregnancy associated diseases, such as implantation failure, miscarriage, pre-eclampsia, fetal intrauterine growth restriction and preterm birth [2-6].
When embryo attaches to uterine epithelium, the underlying stromal cells start to proliferate, then differentiate into unique decidual tissue and form the implantation chamber. Decidual stromal cells (DSCs) comprise 75 % of total decidual cells in decidua. In the human early pregnancy, DSCs participate in the process of uterine remodeling, maternal immune response, uterine angiogenesis, and early embryonic growth [7-10]. Although the biological behaviors of embryo-derived trophoblasts are intensively investigated, their regulations on DSCs in early pregnancy have not got enough attention.
Eotaxins are broadly regarded as eosinophils’ high selectively chemoattractants. Up to now, three members have been discovered: eotaxin/CCL11, eotaxin 2/CCL24/MPIF-2 (myeloid progenitor inhibitory factor 2) and eotaxin 3/CCL26. CCL24 is constitutively expressed in fibroblasts, epithelial cells, macrophages [11,12]. Unlike CCL11, CCL24 has only one receptor CCR3, which mainly presents on eosinophils and was also found on basophils, monocytes, Th2 lymphocytes, epithelial cells and airway smooth muscles [13]. Except for participating in immune regulation, CCL11 has shown a weak stimulus to keratinocytes and endothelial cells. It has been reported that CCR3 protein was localized to decidual stromal cells, and also invading iEVTs, glandular epithelium, leukocyte subpopulations, endothelial and vascular smooth muscle cells of endometrial blood vessels [14,15].
The present study was undertaken to analyze the expression and possible role of CCL24 at human maternal-fetal interface. Our current results illustrate that CCL24 derived from trophoblasts can promote the survival and growth of DSCs by binding CCR3, which may play an important role in establishing and maintaining physiological pregnancy.
Materials and methods
Tissue collection, and cell isolation and culture
All tissue samples were obtained with informed consent in accordance with the requirements of the Research Ethics Committee in the Obstetrics and Gynecology Hospital, Fudan University Shanghai Medical College, and all subjects have completed an informed consent to collect tissue samples.
Decidual and placental tissues were collected from elective termination of the first-trimester pregnancies (gestational age, 6-8 weeks) for no medical reason. The tissues were put immediately into ice-cold Dulbecco’s modified Eagle’s medium (DMEM high D-glucose; Gibco Grand Island, USA), transported to the laboratory within 30 min after surgery, and washed with Hank’s balanced salt solution for isolation of DSCs and trophoblast cells.
DSCs and trophoblasts were isolated according to our previous procedures [16,17]. These methods supplied >98 % vimentin-positive and cytokeratin-negative DSCs and 95 % purity of trophoblast cells, respectively. The purified DSCs were cultured in Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F12 (DMEM/F12; Gibco, USA) with 10 % bovine serum albumin (BSA; Hyclone, Logan, USA), and trophoblasts were cultured in high glucose DMEM with 20 % BSA at 37°C with 5 % CO2 for subsequent experiments.
Immunohistochemistry
Paraffin sections (5 um) of human decidua and villi from the early pregnancy (n=10) were dehydrated in graded ethanol, and incubated with hydrogen peroxide and 1 % bovine serum albumin (BSA)/TBS to block endogenous peroxidase. The samples were then incubated with mouse anti-human CCL24 monoclonal antibody (5 ug/ml, R&D Systems, USA), goat anti-human CCR3 antibody (15 ug/ml, R&D Systems, USA), or mouse/goat IgG isotype overnight at 4°C in a humid chamber. After washing three times with TBS, the sections were overlaid with peroxidase-conjugated goat anti-mouse IgG or peroxidase-conjugated anti-goat IgG (Golden Bridge International, Inc, Beijing, China), and the reaction was developed with 3,3-diaminobenzidine (DAB), and counterstained with hematoxylin. The experiments were repeated five times.
Enzyme-linked immunosorbent assay for determination of CCL24
In order to evaluate the secretion of CCL24, DSCs alone (1×105 cells/well), trophoblasts alone (1×105 cells/well) or the co-culture of these two cells (1×105 of each cells per well) were seed in 24-well plates for 24, 48, 72 and 96 h, respectively. The culture supernatants were then harvested, centrifuged to remove cellular debris, and stored at -80°C until enzyme-linked immunosorbent assay (ELISA) for CCL24 determination. The CCL24 assay (R&D Systems, Abingdon, UK) sensitivity is 2.5 pg/ml.
CCR3 on human DSCs by flow cytometry
DSCs alone (2×105 cells/well), trophoblasts alone (2×105 cells/well) or the co-culture of the two cells (the proportion of DSCs and trophoblast cells was 1:1, 2×105 cells for each cells) (n=13) were seeded in 6-well plates and cultivated for 48 h. These cells were then treated with 0.25 % trypsin only for 30-50 s, and blown off gently and washed with phosphate-buffered saline (PBS). After blocking with 10 % FBS, the recovered cells were mixed with mouse anti-human CCR3-APC monoclonal antibody (Biolegend, USA) or/and CK7-FITC monoclonal antibody (BD, USA) with the isotypic control. After incubation in darkness for 30min at room temperature, the cells were analyzed immediately by a flow cytometer (FACS Calibur, BD). The experiments were repeated five times.
We also investigated the effects of pregnancy-associated hormones (estrogen, progesterone or HCG) on the expression of CCR3 on DSCs by flow cytometry. Briefly, after starvation for 12 h, the DSCs (2×105 cells/well) (FCS of cultured media was the charcoal stripped FCS) were treated, respectively, with 17β-estradiol (10-10 M to 10-7 M, Sigma, USA), progesterone (10-10 M to 10-7 M, Sigma, USA) or HCG (1.25 kU/l to10 kU/l, Sigma, USA) for 48 h with vehicle as control. Each experiment was carried out in triplicate, and repeated five times.
BrdU cell proliferation assay, cell number count and apoptosis assay
BrdU cell proliferation and apoptosis assay were utilized to evaluate the effects of CCL24 on cell proliferation and apoptosis. DSCs were re-suspended in DMEM/F-12 supplemented with 10 % FBS, seeded at a density of 1×104 cells/well in 96-well flat-bottom microplates for BrdU cell proliferation assay, 1×105 cells/well in 24-well flat-bottom microplates for apoptosis assay, and 1×105 cells/well in 12-well flat-bottom microplates for cell number counting. Thereafter, the cells were starved with DMEM containing 1 % FBS for 12 h before treatment. Then DSCs were stimulated with recombinant human CCL24 (0.1, 1, 10, or 100 ng/ml), supernatant of trophoblasts, anti-human CCL24 (0.08, 0.4, or 2 ug/ml) neutralizing antibodies, and CCR3 neutralizing antibodies (0.32, 1.6, or 8 ug/ml) for 48 h with vehicle as negative control.
Before and after treatment, cells in 12-well flat-bottom microplates were counted at a magnification of 200x with microscope (Olympus 1X71, Olympus, Tokyo, Japan). Cells were counted in five predetermined fields. Each experiment was carried out in triplicate, and repeated three times.
The proliferation ability of DSCs was analyzed by BrdU cell proliferation assay (Millipore, USA) according to the manufacturer’s instruction. Each experiment was performed in triplicate, and repeated five times.
Phosphatidylserine externalization was quantified by flow cytometry with a commercially available annexin V-FITC apoptosis detection kit (Invitrogen, USA) according to the manufacturer’s guideline and our previous procedure [18]. The experiments were performed in triplicate, and repeated five times.
Statistical analysis
All values are shown as the mean ± SD. Data were analyzed by t test or one-way analysis of variance with Statistical Package of the Social Sciences software version 11.5. Differences were considered as statistically significant at P<0.05.
Results
The trophoblasts and DSCs express CCR3, whereas only trophoblasts secrete CCL24 in human early pregnancy
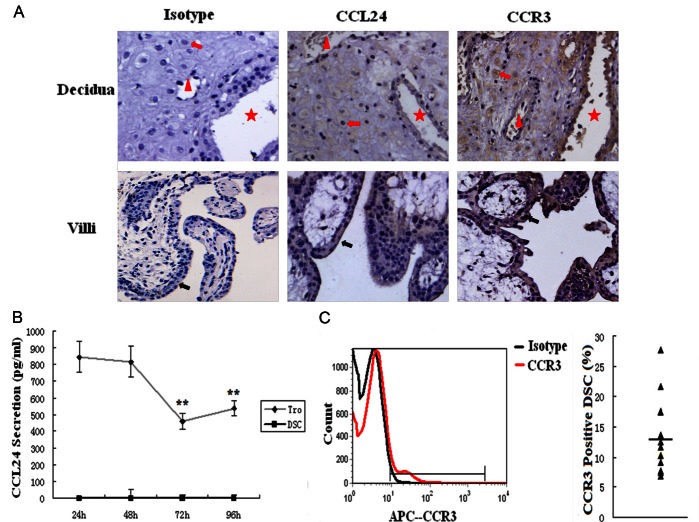
To determine whether CCL24 and CCR3 proteins are expressed and where they locate at human maternal-fetal interface, immunohistochemistry was performed for paraffin-embedded decidua and villi. As shown in Figure 1A, the cytomembrane and cytoplasm of trophoblasts were moderate stained for CCL24 and CCR3 in all villi samples (n=10). However, in decidual samples, epithelial and stromal CCL24 staining was weakly (n=3) or not observed (n=7), which is consistent with other reports [14,15,19]. CCR3 protein was localized in glandular epithelium, endothelial cells of endometrial blood vessels, and DSCs in all the samples (Figure 1A).
Figure 1.
Both trophoblasts and DSCs express CCR3, whereas only trophoblasts secrete CCL24 in human early pregnancy. A. The expression of CCL24 and its receptor CCR3 in villi and decidua was analyzed by immunohistochemistry. Red arrow: DSCs. Red triangle: blood vessel. Red star: epithelial gland. Black arrow: trophoblasts. Original magnification: ×200. The secretion of CCL24 (B) by primary trophoblasts and DSCs were determined by ELISA, and the expression of CCR3 on DSCs (C) by flow cytometry. Tro: trophoblast cells. DSC: decidual stromal cells. Error bars depict the standard deviation of the mean. **P<0.01 compared to the secretion level of 24 h.
To further investigate the secretion of CCL24 by primary trophoblasts and the expression of CCR3 on DSCs, ELISA and flow cytometry were used respectively. The secretion of CCL24 by trophoblasts showed a peak at 24 h, then the secretion decreased along with duration; nevertheless we did not observe the secretion of CCL24 by DSCs at any time point (Figure 1B). As measured by flow cytometry, CCR3 protein could be detected in all the analyzed DSCs. The percentage of CCR3-postive cells varied from 6.8 to 27.6 %, with an average of 12.9 ± 6.3 (Figure 1C).
The interaction between trophoblasts and DSCs stimulates the secretion of CCL24 by trophoblasts and the expression of CCR3 on DSCs
To clarify the effect of the interaction between trophoblasts and DSCs on the expression of CCL24 and CCR3 at the maternal-fetal interface, we constructed a co-culture system in vitro (Figure 2A). DSCs alone (1×105 cells/well), trophoblasts alone (1×105 cells/well) or the coculture of these two cells (1×105 of each cells per well) were seed in 24-well plates. Plates were washed next morning and was recorded as 0 h. After 48 h, we photographed the cultured cells, and then harvested them for flow cytometry. As seen in Figure 2A and Figure 2C, the adhering and proliferation rate of DSCs and trophoblasts were different. In the co-culture, CK7 positive trophoblasts accounted for about 20 % of final cells. Figure 2B shows that the secretion of CCL24 by trophoblasts increased from about 800 pg/ml to 1400 pg/ml, which means a significant difference (P<0.01). Meanwhile, the expression of CCR3 on DSCs also obviously elevated (P<0.01) in co-culture for 48 h (Figure 2D). As a result, our data indicate that the crosstalking between trophoblasts and DSCs can promote CCL24 secretion and CCR3 expression at the maternal-fetal interface.
Figure 2.
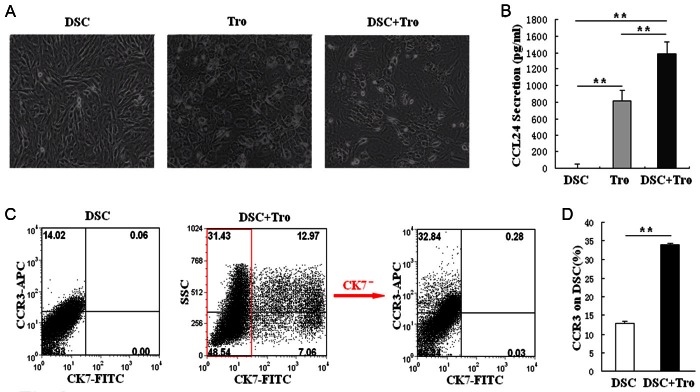
The interaction between trophoblasts and DSCs stimulates the secretion of CCL24 by trophoblasts and the expression of CCR3 on DSCs. We co-cultured trophoblasts (1×105 cell/well) and DSCs (1×105 cell/well) for 48 h (A), then analyzed CCL24 secretion by ELISA (B), and detected CCR3 expression on DSCs by flow cytometry (C, D). Original magnification: ×200. Tro: trophoblast cells. DSC: decidual stromal cells; DSC+Tro: the co-culture of DSCs and trophoblasts. Error bars depict the standard deviation of the mean. (**P<0.01).
Pregnancy-associated hormones up-regulate the expression of CCR3 on DSCs
In human early pregnancy, the most obvious hormonal changes are estrogen, progestin, and hCG. The pregnancy-associated hormones have been identified to regulate the expression of chemokine receptors in vitro [20]. In order to evaluate whether they regulate the CCR3 expression on DSCs, we treated DSCs with 17β-estradiol (10-10 M to 10-7 M), progesterone (10-10 M to 10-7 M) or hCG (1.25 kU/l to 10 kU/l) for 48 h. We found hCG could promote the expression of CCR3 on DSCs, especially at the concentration of 1.25 kU/l (P<0.01). However, with the concentration increases, the promotion function decreases (Figure 3A). As shown, both 17β-estradiol (P<0.01) (Figure 3B) and progesterone (P<0.01) (Figure 3C) increased the expression of CCR3 on DSC. Our observations suggest that the lower HCG, higher 17β-estradiol and progesterone can promote the expression of CCR3 on DSC in human early pregnancy.
Figure 3.
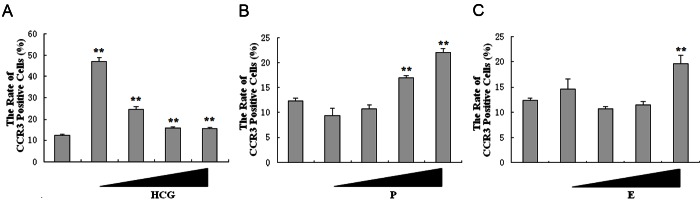
Pregnancy-associated hormones up-regulate the expression of CCR3 on DSCs. DSCs were treated respectively with hCG (1.25 kU/l to10 kU/l) (A), progesterone (10-10 M to 10-7 M) (B) or 17β-estradiol (10-10 M to 10-7 M) (C) for 48 h, with vehicle as control. Flow cytometry was performed to analyze the expression of CCR3 on DSCs. Error bars depict the standard deviation of the mean. **P<0.01 compared to the vehicle control.
Trophoblasts-derived CCL24 promotes the proliferation, growth and apoptosis of DSCs
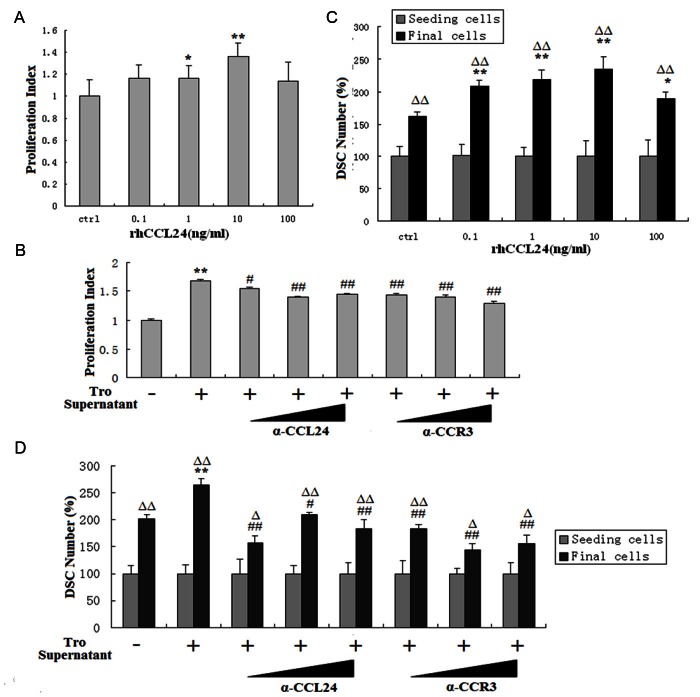
In order to evaluate whether CCL24 regulates the growth and apoptosis of DSCs, DSCs were analyzed for apoptosis, proliferation and cell numbers after treatment with recombinant human CCL24 or trophoblasts supernatant. As shown, CCL24 could stimulate the apoptosis of DSCs. This effect was decreased along with the increase of CCL24 concentration (P<0.05 or P<0.01) (Figure 4A and 4B). Meanwhile, trophoblasts supernatant promoted DSCs apoptosis too. Blocking CCL24 or CCR3 by anti-CCL24 or anti-CCR3 neutralizing antibodies could apparently abolish the effect induced by the supernatant of trophoblasts (P<0.05 or P<0.01) (Figure 4C).
Figure 4.
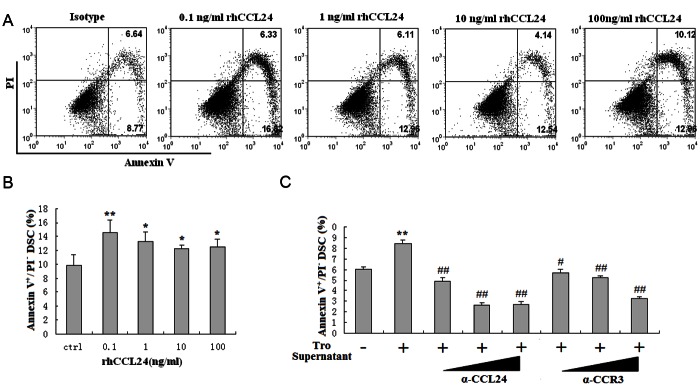
Trophoblasts-derived CCL24 promotes apoptosis of DSCs. DSCs were incubated with recombinant human CCL24 (A, B), trophoblasts supernatant and anti-CCL24 (0.08, 0.4, or 2 ug/ml) neutralizing antibodies, or anti-CCR3 neutralizing antibodies (0.32, 1.6, or 8 ug/ml) (C) for 48h, with vehicle as control. Thereafter, apoptosis of DSCs was detected by Annexin V-FITC apoptosis assay. Tro supernatant: the supernatant of trophoblast cells; α-CCL24: anti-human CCL24 neutralizing antibodies; α-CCR3: the neutralizing antibodies to human CCR3. Error bars depict the standard deviation of the mean. *P<0.05, **P<0.01 compared to the vehicle control. #P<0.05, ##P<0.01 compared to treatment with trophoblasts supernatant.
Interestingly, CCL24 also promoted the proliferation (P<0.05 or P<0.01) (Figure 5A) and increased cell numbers (P<0.01) (Figure 5C) of DSCs, especially at the concentration of 1 and 10 ng/ml. The supernatant of trophoblast cells showed the same stimulating effects, and these effects also could be inhibited partly by anti-CCL24 or anti-CCR3 antibody. Our observations suggest that the trophoblasts-derived CCL24 promotes the proliferation as well as the apoptosis of DSCs, which leads to a growth acceleration of DSCs at human maternal-fetal interface.
Figure 5.
The stimulatory effect of trophoblasts on DSCs proliferation and growth is dependent on CCL24. To detect the effect of CCL24 on the proliferation (A, B) and cell number of (C, D) DSCs, DSCs were incubated with recombinant human CCL24 for 48 h for BrdU proliferation assay and cell number count assay, then both experiments were repeated with adding trophoblasts supernatant and anti-human CCL24/CCR3 neutralizing antibodies. Tro supernatant: the supernatant of trophoblast cells; α-CCL24: anti-human CCL24 neutralizing antibodies; α-CCR3: the neutralizing antibodies for human CCR3. Seeding cells: the standardized cell number of seeding DSCs; Final cells: the relative cell number after treatment with CCL24 for 48 h. Error bars depict the standard deviation of the mean. *P<0.05, **P<0.01 compared to the vehicle control. #P<0.05, ##P<0.01 compared to treatment with trophoblasts supernatant. ΔP<0.05, ΔΔP<0.01 compared to seeding cells in the same group.
Discussion
Decidualization is a hormone-induced remodeling/differentiation process of uterus. Characterized by decidual stromal cells (DSCs) proliferation and differentiation, decidualization is essential for embryo implantation and placentation [21]. After embryo-derived extravillous cytotrophoblast cells (EVTs) penetrate into inner decidua, they locate closely to large numbers of DSCs. DSCs, which comprising 75 % in decidua, are the major cellular component at the maternal-fetal interface. DSCs play vital roles in the interface, including the production of cytokines and extracellular matrix (ECM), antigen presentation, immune regulation, mediator for EVT invasion and hemostatic protection during trophoblasts invasion [22-25].
It is generally accepted that there is a complicate hormone-cytokine network at the maternal-fetal interface [26-28], where chemokines and their receptors present distinctive biological functions. Via respective chemokines/chemokine receptors, trophoblasts and DSCs modulate their own biological behaviors, and also interact with each other by correspondent chemokines and receptors. Therefore, trophoblasts and DSCs build a multiple connection, and participate in the complicate maternal-fetal dialogue. Our previous studies have found the integrative roles of chemokines in the relationship coordination between trophoblasts and decidual cells, such as CXCL12/CXCR4 [25,28,29] and CCL2/CCR2 [9,10].
Analogous to other study [14,15,19], our work has demonstrated that trophoblasts and DSCs express CCR3, while trophoblasts but not DSCs secrete chemokine CCL24. To our knowledge, the secretion and function of chemokine CCL24 are first described at the maternal-fetal interface. Chemokine CCL24 secreted by trophoblasts further stimulates the growth of DSCs through binding to its receptor CCR3 in a paracrine manner. Moreover, when co-culturing DSCs with trophoblasts, the secretion of CCL24 by trophoblasts elevates significantly, and the expression of CCR3 on DSC shows a same raise. Meanwhile, pregnancy-associated hormones such as 17β-estradiol, progesterone, and hCG can also up-regulate CCR3 expression on DSCs, which is in accord with a mast cell study [20]. In view of these results, we conclude that trophoblasts secrete chemokine CCL24, DSCs express its receptor CCR3, while at the interface of embryo and decidua the coexistence of trophoblasts and DSCs, and the hormonal microenvironment promotes the CCL24/CCR3 signal. In addition, we found that lower concentration of HCG has more effects on raising CCR3 at certain concentration range. Physically, 4 weeks of gestation has about 1.25 kU/l HCG in blood. The period is just consistent with the maximum extent of decidualization, which implies CCL24/CCR3 axis my be a possible mechanism for HCG promoting decidualization.
Eotaxin receptor, CCR3, belongs to the seven transmembrane spanning receptors, is highly expressed on eosinophils [30] and basophils [31]. Besides, it is also detected on Th1 and Th2 cells [32-34], especially on Th2 cytokines-associated lymphocytes [32]. Although CCR3 does not present on circulating T cells, its expression could be induced on lymphocytes by IL-2 and IL-4 stimulation [35]. It has been reported that the maternal-fetal interface exhibits Th2 bias during human early pregnancy, characterized by IL-4, IL-5, and IL-10 secretion [36]. Therefore, based on these reports, we propose that trophoblasts-derived CCL24 may induce Th2 bias in a paracrine manner, and Th2 type cytokines at the maternal-fetal interface may further stimulate the expression of CCR3, which finally forms a regulatory loop for maintaining maternal-fetal relationship.
One recent study demonstrates that via ligating with receptor CXCR4, trophoblast-derived CXCL12 acts as a stimulator for DSCs proliferation during human first-trimester pregnancy [37]. Similarly, our results reveal that CCL24 also promotes the proliferation of DSCs, and 10 ng/ml of rhCCL24 has the maximum effect. We also found that rhCCL24 promotes the apoptosis of DSCs in a dose-dependent manner, with 10 ng/ml having the a significant impact. On balance, the concentration of 10 ng/ml of rhCCL24 should have the most obvious effect on elevating the number of DSCs, which is verified in our data.
From our immunohistochemistry study and flow cytometry analysis, trophoblasts also express receptor CCR3, it suggests trophoblasts-derived CCL24 may also modulate the function of trophoblasts in an autocrine manner. Tachimoto et al found that CCL24 chemotactic activity was exerted through the modulation of adhesion molecules. CCR3-active chemokines including CCL24 stimulate eosinophils to detach from endothelial cells by down-regulation of VCAM-1, and then via binding to CCR3, they promote eosinophils migrating into the tissue [38]. As we know, normal placentation requires controlled invasion of trophoblasts into the maternal uterine wall. Therefore, our current results set us to propose that CCL24 secreted by trophoblasts may stimulate the migration and invasion of themselves in an autocrine manner, and play an important role in the early placentation.
In conclusion, our findings suggest that the up-regulation of trophoblast-derived CCL24 and the expression of CCR3 on DSCs can be induced by pregnancy-associated hormones or trophoblasts and DSCs co-culture; the increased CCL24/CCR3 signal then promotes the survival and growth of DSCs in a paracrine manner, which further benefits the decidualization and placentation in human early pregnancy.
Acknowledgements
This study is supported by the Major International Joint Research Project of National Natural Science Foundation of China NSFC 30910103909, NSFC 31270969, the Key Project of Shanghai Basic Research (12JC1401600), the Program for Outstanding Medical Academic Leader of Shanghai to DJ Li; NSFC 31101064, the Research Program of Shanghai Health Bureau (2011Y080), the Ministry of Education Research Fund for Doctoral Program (20110071120092) to MQ Li; Creative Foundation for Graduate Students of Fudan University to H Li.
References
- 1.Carson DD, Bagchi I, Dey SK, Enders AC, Fazleabas AT, Lessey BA, Yoshinaga K. Embryo implantation. Dev Biol. 2000;223:217–237. doi: 10.1006/dbio.2000.9767. [DOI] [PubMed] [Google Scholar]
- 2.Salker M, Teklenburg G, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Macklon NS, Brosens JJ. Natural selection of human embryos: impaired decidualization of endometrium disables embryo-maternal interactions and causes recurrent pregnancy loss. PLoS One. 2010;5:e10287. doi: 10.1371/journal.pone.0010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teklenburg G, Salker M, Molokhia M, Lavery S, Trew G, Aojanepong T, Mardon HJ, Lokugamage AU, Rai R, Landles C, Roelen BA, Quenby S, Kuijk EW, Kavelaars A, Heijnen CJ, Regan L, Brosens JJ, Macklon NS. Natural selection of human embryos: decidualizing endometrial stromal cells serve as sensors of embryo quality upon implantation. PLoS One. 2010;5:e10258. doi: 10.1371/journal.pone.0010258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukui A, Funamizu A, Yokota M, Yamada K, Nakamua R, Fukuhara R, Kimura H, Mizunuma H. Uterine and circulating natural killer cells and their roles in women with recurrent pregnancy loss, implantation failure and preeclampsia. J Reprod Immunol. 2011;90:105–110. doi: 10.1016/j.jri.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Norris W, Nevers T, Sharma S, Kalkunte S. Review: hCG, preeclampsia and regulatory T cells. Placenta. 2011;32(Suppl 2):S182–S185. doi: 10.1016/j.placenta.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimatrai M, Oliver C, Abadia-Molina AC, Garcia-Pacheco JM, Olivares EG. Contractile activity of human decidual stromal cells. J Clin Endocrinol Metab. 2003;88:844–849. doi: 10.1210/jc.2002-021224. [DOI] [PubMed] [Google Scholar]
- 7.Dudley DJ, Trautman MS, Mitchell MD. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J Clin Endocrinol Metab. 1993;76:404–410. doi: 10.1210/jcem.76.2.8432783. [DOI] [PubMed] [Google Scholar]
- 8.Jones RL, Hannan NJ, Kaitu’U TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–6167. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 9.He YY, Du MR, Guo PF, He XJ, Zhou WH, Zhu XY, Li DJ. Regulation of C-C motif chemokine ligand 2 and its receptor in human decidual stromal cells by pregnancy-associated hormones in early gestation. Hum Reprod. 2007;22:2733–2742. doi: 10.1093/humrep/dem208. [DOI] [PubMed] [Google Scholar]
- 10.Wu HX, Jin LP, Xu B, Liang SS, Li DJ. Th17 cells are recruited by DSC-secreted CCL2 into deciduas and herein improve growth and invasiveness of trophoblast cells through secreting IL-17 in human first-trimester pregnancy. American Journal of Reproductive Immunology. 2010;64(Suppl 1):25. [Google Scholar]
- 11.Ying S, Meng Q, Zeibecoglou K, Robinson DS, Macfarlane A, Humbert M, Kay AB. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J Immunol. 1999;163:6321–6329. [PubMed] [Google Scholar]
- 12.Jahnsen FL, Haye R, Gran E, Brandtzaeg P, Johansen FE. Glucocorticosteroids inhibit mRNA expression for eotaxin, eotaxin-2, and monocyte-chemotactic protein-4 in human airway inflammation with eosinophilia. J Immunol. 1999;163:1545–1551. [PubMed] [Google Scholar]
- 13.Forssmann U, Uguccioni M, Loetscher P, Dahinden CA, Langen H, Thelen M, Baggiolini M. Eotaxin-2, a novel CC chemokine that is selective for the chemokine receptor CCR3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185:2171–2176. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Lathbury LJ, Salamonsen LA. Expression of the chemokine eotaxin and its receptor, CCR3, in human endometrium. Biol Reprod. 2000;62:404–411. doi: 10.1095/biolreprod62.2.404. [DOI] [PubMed] [Google Scholar]
- 15.Hannan NJ, Jones RL, White CA, Salamonsen LA. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod. 2006;74:896–904. doi: 10.1095/biolreprod.105.045518. [DOI] [PubMed] [Google Scholar]
- 16.Li MQ, Hou XF, Shao J, Tang CL, Li DJ. The DSCs-expressed CD82 controls the invasiveness of trophoblast cells via integrinbeta1/MAPK/MAPK3/1 signaling pathway in human first-trimester pregnancy. Biol Reprod. 2010;82:968–979. doi: 10.1095/biolreprod.109.080739. [DOI] [PubMed] [Google Scholar]
- 17.Meng YH, Shao J, Li H, Hou YL, Tang CL, Du MR, Li MQ, Li DJ. CsA improves the trophoblasts invasiveness through strengthening the cross-talk of trophoblasts and decidual stromal cells mediated by CXCL12 and CD82 in early pregnancy. Int J Clin Exp Pathol. 2012;5:299–307. [PMC free article] [PubMed] [Google Scholar]
- 18.Li MQ, Luo XZ, Meng YH, Mei J, Zhu XY, Jin LP, Li DJ. CXCL8 enhances proliferation and growth and reduces apoptosis in endometrial stromal cells in an autocrine manner via a CXCR1-triggered PTEN/AKT signal pathway. Hum Reprod. 2012;27:2107–2116. doi: 10.1093/humrep/des132. [DOI] [PubMed] [Google Scholar]
- 19.Red-Horse K, Drake PM, Fisher SJ. Human pregnancy: the role of chemokine networks at the fetal-maternal interface. Expert Rev Mol Med. 2004;6:1–14. doi: 10.1017/S1462399404007720. [DOI] [PubMed] [Google Scholar]
- 20.Jensen F, Woudwyk M, Teles A, Woidacki K, Taran F, Costa S, Malfertheiner SF, Zenclussen AC. Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS One. 2010;5:e14409. doi: 10.1371/journal.pone.0014409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Logan PC, Steiner M, Ponnampalam AP, Mitchell MD. Cell cycle regulation of human endometrial stromal cells during decidualization. Reprod Sci. 2012;19:883–894. doi: 10.1177/1933719112438447. [DOI] [PubMed] [Google Scholar]
- 22.Strowitzki T, Germeyer A, Popovici R, von Wolff M. The human endometrium as a fertility-determining factor. Hum Reprod Update. 2006;12:617–630. doi: 10.1093/humupd/dml033. [DOI] [PubMed] [Google Scholar]
- 23.Lockwood CJ, Krikun G, Hickey M, Huang SJ, Schatz F. Decidualized human endometrial stromal cells mediate hemostasis, angiogenesis, and abnormal uterine bleeding. Reprod Sci. 2009;16:162–170. doi: 10.1177/1933719108325758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril. 2011;95:1278–1283. doi: 10.1016/j.fertnstert.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 25.Ren L, Liu YQ, Zhou WH, Zhang YZ. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod. 2012;27:366–374. doi: 10.1093/humrep/der395. [DOI] [PubMed] [Google Scholar]
- 26.Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update. 2005;11:613–630. doi: 10.1093/humupd/dmi023. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez F, Pellicer A, Simon C. The chemokine connection: hormonal and embryonic regulation at the human maternal-embryonic interface--a review. Placenta. 2003;24(Suppl B):S48–S55. doi: 10.1016/s0143-4004(03)00134-6. [DOI] [PubMed] [Google Scholar]
- 28.Zhou WH, Du MR, Dong L, Yu J, Li DJ. Chemokine CXCL12 promotes the cross-talk between trophoblasts and decidual stromal cells in human first-trimester pregnancy. Hum Reprod. 2008;23:2669–2679. doi: 10.1093/humrep/den308. [DOI] [PubMed] [Google Scholar]
- 29.Li MQ, Tang CL, Du MR, Fan DX, Zhao HB, Xu B, Li DJ. CXCL12 controls over-invasion of trophoblasts via upregulating CD82 expression in DSCs at maternal-fetal interface of human early pregnancy in a paracrine manner. Int J Clin Exp Pathol. 2011;4:276–286. [PMC free article] [PubMed] [Google Scholar]
- 30.Daugherty BL, Siciliano SJ, DeMartino JA, Malkowitz L, Sirotina A, Springer MS. Cloning, expression, and characterization of the human eosinophil eotaxin receptor. J Exp Med. 1996;183:2349–2354. doi: 10.1084/jem.183.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, Rao P, Ponath PD, Baggiolini M, Dahinden CA. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–1143. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nanki T, Lipsky PE. Lack of correlation between chemokine receptor and T(h)1/T(h)2 cytokine expression by individual memory T cells. Int Immunol. 2000;12:1659–1667. doi: 10.1093/intimm/12.12.1659. [DOI] [PubMed] [Google Scholar]
- 34.Romagnani P, De Paulis A, Beltrame C, Annunziato F, Dente V, Maggi E, Romagnani S, Marone G. Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. Am J Pathol. 1999;155:1195–1204. doi: 10.1016/S0002-9440(10)65222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jinquan T, Quan S, Feili G, Larsen CG, Thestrup-Pedersen K. Eotaxin activates T cells to chemotaxis and adhesion only if induced to express CCR3 by IL-2 together with IL-4. J Immunol. 1999;162:4285–4292. [PubMed] [Google Scholar]
- 36.Guo PF, Du MR, Wu HX, Lin Y, Jin LP, Li DJ. Thymic stromal lymphopoietin from trophoblasts induces dendritic cell-mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood. 2010;116:2061–2069. doi: 10.1182/blood-2009-11-252940. [DOI] [PubMed] [Google Scholar]
- 37.Ren L, Liu YQ, Zhou WH, Zhang YZ. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod. 2012;27:366–374. doi: 10.1093/humrep/der395. [DOI] [PubMed] [Google Scholar]
- 38.Tachimoto H, Burdick MM, Hudson SA, Kikuchi M, Konstantopoulos K, Bochner BS. CCR3-active chemokines promote rapid detachment of eosinophils from VCAM-1 in vitro. J Immunol. 2000;165:2748–2754. doi: 10.4049/jimmunol.165.5.2748. [DOI] [PubMed] [Google Scholar]