Abstract
Bim, a proapoptotic BH3-only member of the Bcl-2 protein family, is required for central and peripheral deletion of T lymphocytes. Mechanisms regulating Bim activity in T cells remain poorly understood. We show that expression of Bim is up-regulated in human T cells after polyclonal or specific T cell receptor triggering. Induction of Bim was affected by the agonistic potency of MHC:peptide ligands. Peptides that failed to induce Bim expression, failed to induce apoptosis in specific T cells, whereas partially agonistic ligands, which trigger death receptor-independent activation-induced cell death (AICD), induced Bim, but were inefficient in up-regulating Bcl-XL. Activation of protein kinase C and calcineurin appeared to be necessary and sufficient for Bim up-regulation after T cell receptor ligation. Immunosuppressive drugs known to prevent T cell deletion in vivo, such as cyclosporin A or FK506, blocked Bim up-regulation and rescued T cells from death receptor-independent AICD, whereas rapamycin, which allows the development of stable immunological tolerance, did not exhibit these activities. These results define a new mode of Bim regulation, strongly implicate Bim as a mediator of AICD, and suggest that Bim up-regulation can be targeted to influence the outcome of specific immune responses.
Keywords: apoptosis, epitope
Death of T lymphocytes in response to repeated antigen-specific stimulation or nonspecific TCR triggering is referred to as activation-induced cell death (AICD) (1). AICD is believed to account for eventual elimination of the vast majority of specific lymphocytes expanding in response to a foreign antigen and to contribute to the development of transplant or peripheral self-tolerance (2, 3).
Signaling through Fas and/or tumor necrosis factor receptor I is usually required for AICD in vitro, whereas in vivo clearance of antigen-specific or superantigen-activated T cells is largely independent of these molecules (4-6). Although death receptors (DRs) contribute to the process (4, 7, 8), deletion of transplant or self-reactive T cells also involves a DR-independent apoptotic program (7, 9, 10), which is blocked by overexpression of antiapoptotic proteins of the Bcl-2 family (11-13), but its molecular nature remains unclear.
Recent studies using gene knockout mice have identified Bim as a major regulator of apoptosis in the lymphoid system. Bim contains one region homologous to Bcl-2, the BH3 domain, and is referred to as a BH3-only proapoptotic member of the Bcl-2 protein family. Bim plays a critical role in both thymic and peripheral deletion of T lymphocytes (4, 14, 15) and is required for B cell death, in response to crosslinking of surface immunoglobulins (16). Bim also triggers apoptosis in neurons, hematopoietic progenitors, and other types of cells, where its activity is regulated both at the level of protein expression and by posttranslational modifications such as phosphorylation (14, 17-19). Three major forms of Bim protein, small (BimS), large (BimL), and extra-large (BimEL), can be generated by alternative mRNA splicing (20, 21). Although the expression of BimL predominates at the protein level, BimS exhibits the highest proapoptotic activity (20). Exactly how Bim performs its proapoptotic function is still debated, but ectopic expression of Bim is sufficient to induce a drop of mitochondria membrane potential and development of apoptosis in a Bak- and Bax-dependent manner (22, 23) in different cells, including T lymphocytes (4). Several studies support a model suggesting that Bim promotes apoptosis through binding to and inhibition of antiapoptotic proteins of the Bcl-2 family (24, 25). Activation of mixed lineage kinases/c-Jun N-terminal kinases (18, 19, 23), as well as inactivation of Ras/mitogen-activated protein kinase (17, 19), extracellular signal-activated kinases (26-28), or phosphatidylinositol 3-kinase/Akt signaling (29-31) pathways have been shown to account for Bim induction or activation in the course of anoikis, or in response to trophic factor deprivation or other apoptosis inducing signals. In contrast, mechanisms regulating Bim activity in T cells remain enigmatic. Bim binds to the DLC1/LC8 chain of the dynein motor complex and may be activated after release from this interaction (32). However, it has been reported that most of Bim expressed in nonapoptotic T cells localizes in mitochondria (25) and, therefore, the precise role of Bim interaction with DLC1/LC8 remains unclear.
Here, we demonstrate that Bim is up-regulated after TCR triggering in human T cells and provide evidence that strongly implicates Bim as a mediator of DR-independent AICD.
Materials and Methods
Cell Lines and Cytotic T Lymphocyte (CTL) Clones. C1R/A11, an Epstein-Barr virus-transformed lymphoblastoid cell line deficient in the expression of endogenous MHC class I alleles and transfected with a pHEBO vector-based HLA A11 expression vector, was maintained in RPMI medium1640 supplemented with 100 μg/ml streptomycin, 100 units/ml penicillin, and 10% FCS (standard medium) with 400 units/ml hygromycin B (33). The generation and characterization of HLA A11-restricted CTL lines and clones derived from Epstein-Barr virus-seropositive donors BK (BK bulk, BK250 and BK289) and EA (EA44-10) and specific to Epstein-Barr virus nuclear antigen 4-derived peptide epitope IVTDFSVIK (IVT) were described (34-36).
Abs and Reagents. The following Abs were used in the study: polyclonal rabbit anti-Bcl-XL Ab (BD Pharmingen), polyclonal rabbit anti-Bim/BOD Ab (StressGen Biotechnologies), Bcl-2-specific mAb (clone Bcl-2-100, Zymed), anti-FKHRL1 (Fox03) was purchased from Upstate Biotechnology (Lake Placid NY), and phospho-FKHR (Thr-24)/FKHR (Thr-32) was purchased from In Vitro Sweden AB, Stockholm. The CD3-specific Ab OKT3 was purified from cell culture supernatants of the relevant hybridoma (ATCC catalog no. CRL-8001). Cyclosporin A (CsA), ionomycin, 12-O-tetradecanoylphorbol 13-acetate (TPA), and RO32-0432 were purchased from Sigma, FK506 and rapamycin from Calbiochem, recombinant IL-2 from PeproTech (Rocky Hill, NJ), and LY294002 and PD98059 were from Cell Signaling Technology (Beverly, MA).
Peptides synthesized by the Merrifield solid-phase method were purchased from Alta Bioscience (Birmingham, U.K.) and were purified by HPLC on SuperPac Pep-S 5-mm columns (Pharmacia, Uppsala). The peptides were dried by using SpeedVac (Pharmacia) and were dissolved in DMSO at a concentration of 1 × 10-2 M, as determined by Biuret assays.
T Cell Stimulation and Monitoring of Cell Death. C1R/A11 cells, which were unpulsed or pulsed with synthetic peptides at the indicated concentrations, were incubated for 1 h at 37°C, irradiated with 4,000 rad, were extensively washed, and were mixed with 3-4 × 106 CTLs in round-bottom tubes at an effector-:stimulator ratio of 10:1. The mixed cells were then centrifuged (5 min at 300 × g) to help conjugate formation, and were incubated for 1 h at 37°C. Ionomycin, TPA, or the indicated inhibitors of signaling pathways were added as indicated, and cells were cultured at a cell density of 1.5 × 106/ml for the indicated periods of time at 37°C in a CO2 incubator. Recovery of living cells was evaluated by the Trypan blue exclusion method or fluorescence-activated cell sorter analysis as described (36).
Peripheral blood mononuclear cells were isolated from the blood of healthy donors by density gradient centrifugation. Plastic dishes were coated with purified OKT3 Ab (10 μg/ml in PBS). Peripheral blood mononuclear cells were resuspended in complete medium at a cell density of 1 × 106/ml and activated during a 24-h period. Control samples were incubated in dishes coated with total mouse Ig.
Analysis of Protein Expression by Immunoblotting. CTLs incubated with C1R/A11 cells that were unpulsed or pulsed with the indicated synthetic peptides were lysed in electrophoresis sample buffer (1 × 105 cells in 10 μl). Cell lysates were separated by SDS/PAGE. The gels were blotted onto nitrocellulose filters, which were then incubated with the indicated specific Ab diluted 1:1,000 in PBS containing 5% skim milk. After incubation with anti-rabbit horseradish peroxidase-conjugated Abs, the blots were visualized by SuperECL (Amersham Pharmacia Biotech, Little Chalfont, U.K.). Images were acquired on a Fuji Film PhosphorImager by using Las-100Pro imagereader ver 2.1 software and analyzed by using sciencelab'98 image gauge ver 3.4x image analysis software.
Cytometric Analysis of Mitochondrial Transmembrane Potential (ΔΨm). To measure ΔΨm in specific CTLs, BK289 cells were activated with control or peptide-pulsed (1 × 10-7 M) irradiated (4,000 rad) C1R/A11 cells for 24 h, were incubated with DiOC6 (40 nmol/liter in PBS) at 37°C for 30 min (37), and were then analyzed by fluorescence-activated cell sorter analysis by using FACScan and cellquest software (Becton Dickinson). Dead cells were excluded from the analysis by using positivity for propidium iodide staining or were based on forward- and side-scatter characteristics.
Results
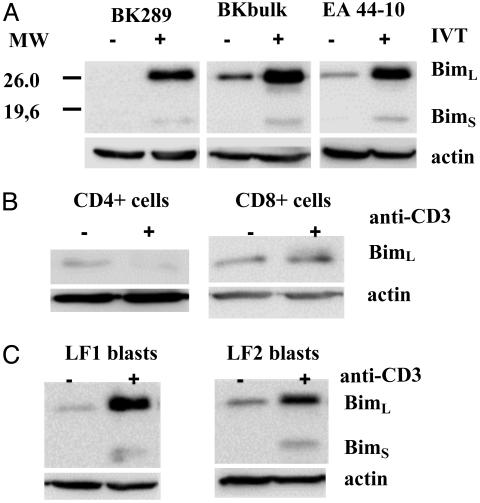
Expression of Bim Is Up-Regulated in Human T Cells in Response to TCR-Triggering. To analyze regulation of Bim activity in T lymphocytes, we monitored expression of Bim in CTL clones and lines specific for the Epstein-Barr virus nuclear antigen 4-derived HLA A11-restricted peptide epitope IVT after coculture with IVT-peptide-pulsed or control C1R/A11 cells. Immunoblotting revealed up-regulation of two forms of Bim protein apparently corresponding to BimL (≈25 kDa) and BimS (≈16 kDa) in specifically activated CTLs (Fig. 1A). Analysis of data from independent experiments demonstrated that the BimL:BimS ratio was stable for a given cell line or clone. CD3 crosslinking led to slight up-regulation of BimL in freshly isolated CD8+ but not in CD4+ T lymphocytes (Fig. 1B) and strong induction of Bim expression in T cell lines established from phytohemagglutinin-activated lymphocytes of healthy blood donors (Fig. 1C). These data showed that the effect of TCR-triggering on Bim expression is more pronounced in chronically activated T cells.
Fig. 1.
TCR triggering induces expression of Bim in chronically activated T cells. Bim expression was analyzed in the IVT-specific CTL clones BK289 and EA44-10 or polyclonal CTL culture of donor BK (A), freshly isolated purified CD4+ or CD8+ T lymphocytes (B), or long-term cultures of phytohemagglutinin-induced T blasts of two unrelated donors (LF1 and LF2) by immunoblotting with Bim-specific polyclonal Ab. (A) IVT-specific T cells were incubated with control or IVT peptide-pulsed irradiated (4,000 rad) C1R/A11 cells at the effector:targer ratio 10:1 over night. (B and C) Freshly isolated T cells or phytohemagglutinin blasts were stimulated for 24 h by OKT3 mAb.
Induction of Bim Expression by Peptide Ligands with Different Agonistic Potency. We have previously shown that triggering with IVT-pulsed antigen-presenting cells (APCs) induces Fas-mediated AICD in the IVT-specific CTL clone BK289. In contrast, death of the same clone after activation with an analogue of IVT carrying the F to Y substitution in the position 5 of the peptide [IVTDYSVIK (Y5) peptide] occurs in a Fas/FasL-independent manner and does not involve other DRs (36). Another analogue of IVT carrying the I to A substitution in position 8 (A8 peptide) is comparable to Y5 in stimulating specific CTL lysis of peptide pulsed HLA A11+ target cells but it does not induce death in BK289 CTLs (35).
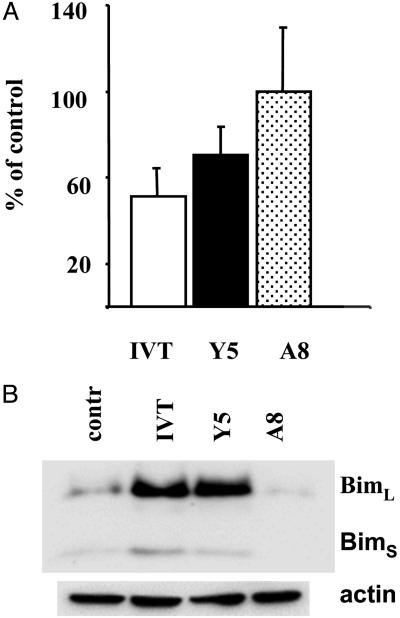
We used this experimental model to study the involvement of Bim in DR-independent AICD. As shown in Fig. 2A, no significant cell loss was observed in BK289 CTLs 24 h after triggering with the A8 peptide. In contrast, the recovery decreased by ≈50% and 30% in CTL cultures stimulated with IVT- or Y5-pulsed APCs, respectively. This correlated with strong Bim up-regulation that was observed after 14 h of activation in IVT- or Y5- but not in A8-stimulated CTLs (Fig. 2B).
Fig. 2.
Partially agonistic peptide ligands that do not trigger apoptosis fail to induce Bim expression in specific CTLs. (A) BK 289 CTLs were triggered with C1R/A11 cells preincubated with the indicated peptides at a final concentration of 1 × 10-7 M. Recovery of viable cells was evaluated by Trypan blue staining or fluorescence-activated cell sorter analysis. The data are expressed as a percentage relative to control samples incubated with APCs without peptide. Mean ± SD of three experiments are shown. (B) Induction of Bim expression in BK289 cells after 18 h of triggering was assessed by immunoblotting.
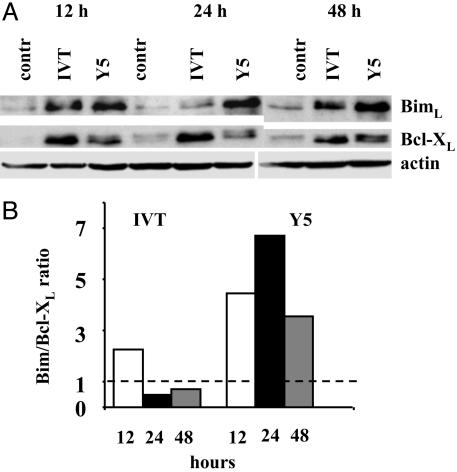
The IVT- and Y5-Mediated Activation Programs in BK289 Cells Differ in the Level and Kinetics of Bim and Bcl-XL Up-Regulation. The apoptotic program mediated by Fas-FasL interactions is completed within 24 h after triggering in IVT-stimulated BK289 cultures. In contrast, the Fas-independent apoptosis triggered by Y5 proceeds with a slower kinetics and reaches its maximum between 24-48 h after triggering (36). Kinetic analysis of Bim expression in BK289 cells demonstrated that IVT induces maximal levels of Bim ≈12-16 h after stimulation (Fig. 3A), whereas Bim expression in Y5-stimulated CTLs reaches higher absolute levels but with a slower kinetics having its maximum between 24-48 h after stimulation, which correlates with the slow kinetics of DR-independent apoptosis in these cells.
Fig. 3.
DR-independent apoptosis in BK289 cells induced by the Y5 peptide is associated with high Bim:Bcl-XL ratios. BK289 CTLs were stimulated by coculture with control, IVT-pulsed, or Y5-pulsed (1 × 10-7 M) C1R/A11 cells. (A) Expression of Bim and Bcl-XL was analyzed by immunoblotting with the relevant specific Ab at the indicated time points. (B) Intensities of Bcl-XL- and Bim-specific bands were measured and expressed in arbitrary units by using las-100pro imagereader ver 2.1 software as described in Materials and Methods. Shown are the ratios between the values obtained for Bim and Bcl-XL at the indicated time points after triggering, after correction for small differences in loading established by comparison of the intensities of actin-specific bands. Data are representative of two experiments.
The relative levels of antiapoptotic and BH3-only proteins of the Bcl-2 family regulate initiation of apoptosis mediated by Bax-like family members (38). Expression of Bcl-2 does not significantly change in response to either IVT- or Y5-mediated triggering, whereas expression of Bcl-XL is inducible in BK289 cells (36). The Y5 peptide was less efficient than IVT in mediating Bcl-XL up-regulation in BK289 cells at all tested time points after triggering (Fig. 3A). More importantly, the Bim-:Bcl-XL ratio was significantly higher in Y5-stimulated cells and reached its maximum at 24 h after activation (Fig. 3B), which coincides with the peak of cell death in response to Y5-mediated stimulation (36). Notably, Bcl-XL was revealed as a doublet of closely migrating bands in Y5-stimulated BK289 cells, whereas the slower migrating form of Bcl-XL was less apparent after IVT-mediated triggering, suggesting that a larger proportion of Bcl-XL may undergo posttranslational modifications in response to signals induced by partially agonistic, as compared with fully agonistic, peptide ligands.
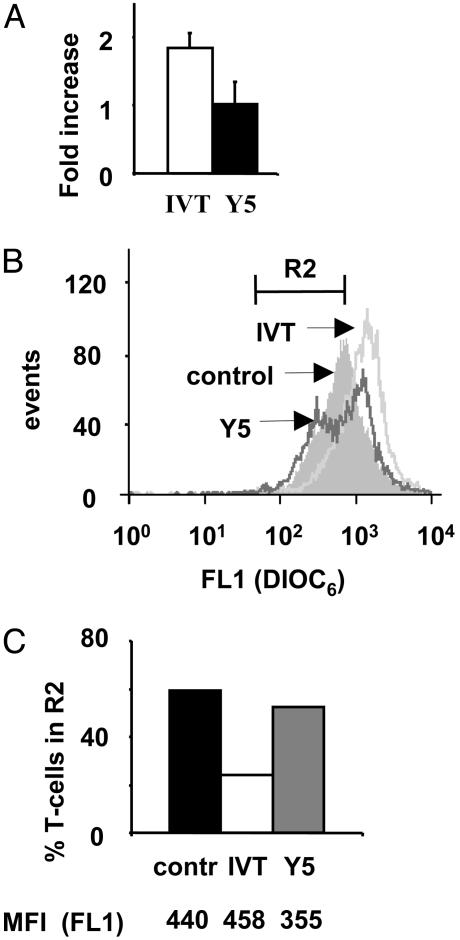
Mitochondria Membrane Potential Is Differentially Regulated in IVT- or Y5-Stimulated CTLs. Relative levels of pro- and antiapoptotic proteins of the Bcl-2 family affect ΔΨm (39). To determine whether the differences in the Bim/Bcl-XL balance observed in IVT- or Y5-stimulated CTLs are translated into differences in the level of ΔΨm, BK289 cells were triggered with peptide-pulsed APCs for 24 h and stained with DiOC6 dye to monitor changes in ΔΨm (37). An approximate 2-fold overall increase and a homogeneous shift toward higher intensity of DiOC6 staining were observed in IVT-stimulated BK289 cells as compared with controls. In contrast, no significant overall increase of ΔΨm was revealed in Y5-stimulated cells and a population of cells with decreased ΔΨm was clearly identifiable (Fig. 4 A and B). The percentage of cells with low ΔΨm in the R2 region (Fig. 4B) decreased by >2-fold in the IVT-stimulated cultures but did not significantly change after Y5 stimulation. However, the mean fluorescence intensity of cells in R2 was lower in Y5-stimulated cultures as compared with controls (Fig. 4 B and C). Collectively, these data support involvement of Bim up-regulation in DR-independent AICD of specific CTLs.
Fig. 4.
Triggering with the IVT or Y5 peptide have opposing effects on mitochondria membrane potential in specific CTLs. BK289 cells were stimulated for 24 h by control or peptide-pulsed C1R/A11 cells and were stained with DiOC6 dye as described above. Dead cells were excluded from the analysis by using propidium iodide staining. (A) Fold increase of intensity of fluorescence in IVT- or Y5-stimulated BK289 cells relative to the control. Mean ± SD of three independent experiments are shown. (B) Representative histogram of DiOC6 staining of control, IVT-, or Y5-stimulated BK289 cells. (C) Percentage of BK289 cells in R2 region shown in B in control, IVT-, or Y5-stimulated cultures. Numbers below the graph represent the mean fluorescence intensity of cells included in R2 region in each sample. Data are representative of three experiments.
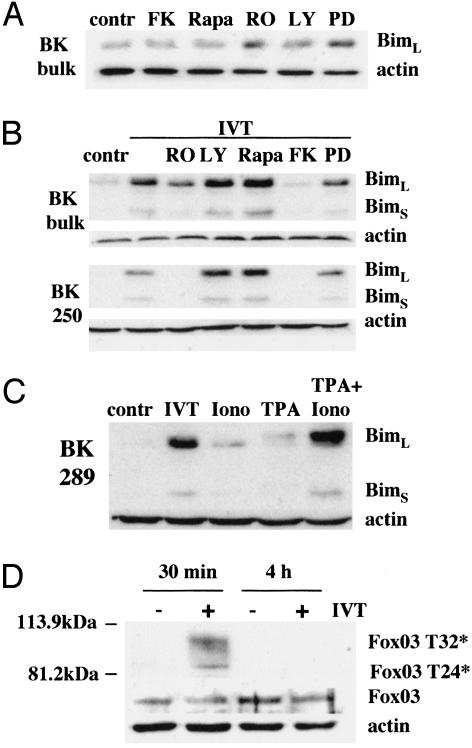
Activation of PKC and Calcineurin Are Necessary for Bim Up-Regulation Induced by TCR Triggering. To characterize signal transduction pathways involved in regulation of Bim expression by TCR triggering we used RO320432, LY294002, rapamycin, FK506, and PD98059, which specifically inhibit PKC, phosphatidylinositol 3-kinase, mTOR kinase, calcineurin, and MEK1, respectively. At concentrations that block the proliferation of IVT-specific CTLs in response to peptide-pulsed APCs (data not shown), the inhibitors of PKC or MEK1 induced a slight but reproducible up-regulation of Bim expression in nontriggered CTLs cultured for 24 h in the absence of exogenous IL-2, whereas the other inhibitors had no effect (Fig. 5A and data not shown). In contrast, up-regulation of both BimL and BimS induced by specific activation of IVT-specific CTLs was completely blocked by inhibition of calcineurin (FK506), whereas inhibition of PKC (RO320432) resulted in either very significant (BK polyclonal CTL line) or complete (CTL clone BK250) inhibition of TCR-induced Bim up-regulation (Fig. 5B). Consistent with the requirement of both PKC activation and Ca2+ influx, Bim expression was significantly induced by the combination of ionomycin and TPA and was only slightly up-regulated by each reagent (Fig. 5C). In the presence of TPA, BimL acquired lower electrophoretic mobility, most likely due to excessive PKC activation leading to posttranslational modifications of Bim.
Fig. 5.
Induction of Bim by TCR triggering requires both calcineurin and PKC activation and is not associated with Fox03 dephosphorylation. (A) The effect of the indicated inhibitors of signaling pathways on Bim expression in the nonstimulated polyclonal CTL culture BK bulk. T cells were incubated for 24 h with the inhibitors taken at concentrations sufficient to block proliferation of IVT-specific CTLs induced by peptide-pulsed APCs. The following concentrations were used: RO320432 (RO), 1 μM; LY294002 (LY), 20 μM; rapamycin (Rapa), 50 ng/ml; FK506 (FK), 1 μM; and PD98059 (PD), 0.9 nM. (B) Effect of the indicated inhibitors on activation-induced Bim up-regulation was tested by immunoblotting in the BK bulk CTLs and CTL clone BK250 at 24 h after activation with IVT-pulsed C1R/A11 cells. (C) BK289 CTLs were cultured with C1R/A11 cells without any exogenous peptide (control), with IVT-pulsed C1R/A11 cells (IVT), or in the presence of ionomycin or TPA alone or in combination. Bim expression was analyzed by immunoblotting. (D) The levels of phosphorylated Fox03 and total Fox expression were analyzed at the indicated time points after specific activation in total cell lysates of BK250 CTLs.
Inactivation of the PKB/Akt signaling pathway culminating in dephosphorylation of the forkhead transcription factor Fox03 has been implicated in Bim up-regulation after lymphokine deprivation of T cells (29-31). The level of total Fox03 did not change in IVT-stimulated CTLs (Fig. 5D). Immunoblotting with Abs recognizing Fox03 phosphorylated at Thr-32 and/or Thr-24 revealed that Fox03 is dephosphorylated at these sites in nontriggered IVT-specific CTLs. Specific stimulation of CTLs resulted in transient Fox03 phosphorylation that could be observed 30 min after T cell activation but was again undetectable 4 h after triggering.
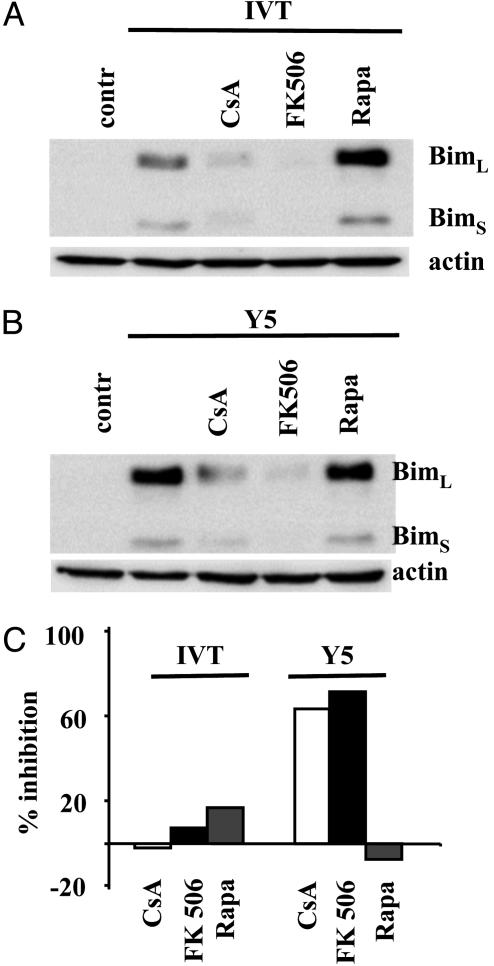
Effect of Immunosuppressive Drugs on TCR-Induced Bim Up-Regulation. Deletion of transplant-specific T cells after costimulatory blockade occurs in the presence of rapamycin, but is blocked by CsA (9, 11). To test whether the different effects exerted by CsA and rapamycin on in vivo deletion of alloreactive T cells correlate with their different capacity to block up-regulation of Bim after TCR triggering, BK 289 cells were activated by IVT- or Y5 peptide-loaded APCs in the presence or absence of these compounds. To exclude that effects of CsA may be due to the known interference of CsA with the mitochondria permeability transition pore the cells were treated also with FK506, which inhibits calcineurin activation, but does not directly affect mitochondria (40, 41). Up-regulation of Bim expression was blocked in the presence of CsA and FK506. In contrast, rapamycin did not prevent Bim induction after Y5-mediated triggering and up-regulated, at least in some experiments, the level of Bim expression in IVT-stimulated BK289 cells (Fig. 6 A and B). In agreement with our previously published data, loss of BK289 cells induced by the Y5-mediated triggering was strongly inhibited in the presence of CsA or FK506, whereas these reagents did not affect the level of IVT-mediated cell death. The Y5 peptide weakly induces Fas-dependent apoptosis in BK289 cells and in combination of CsA with FasL-blocking Abs, but not the blocking Abs alone, completely rescued BK289 cells from Y5-induced cell death (ref. 36 and data not shown). Rapamycin did not significantly affect cell death induced by either the IVT or Y5 peptide (Fig. 6C).
Fig. 6.
CsA and FK506, but not rapamycin, block Bim up-regulation and DR-independent AICD. BK289 CTLs were triggered in the presence or in the absence of the indicated drugs with C1R/A11 cells that were prepulsed with either the IVT or Y5 peptide in the presence or absence of the indicated drugs. (A and B) Bim expression in BK289 cells after 24 h of activation. (C) Cell recovery was monitored 48 h after triggering as described above. The data are presented as percentage of inhibition of cell death observed in BK289 cultures in the presence of the indicated drugs. Mean values of five independent experiments are shown.
Discussion
Knowledge of mechanisms regulating Bim activity in lymphocytes may be critical for our understanding of immune physiology and pathogenesis. In this study, we show that TCR triggering up-regulates the expression of two major isoforms of Bim, BimL and BimS, in human T cells. Studies using IVT-specific CTLs clones, which are activated with variable efficiency by Y5 peptide-pulsed targets (42), revealed that agonistic potency of the epitope directly correlates with the stability of interaction between the MHC:peptide complex and a given TCR. In BK289 CTLs, the Y5 peptide fails to induce IL-2 production and cell proliferation, whereas it efficiently triggers secretion of tumor necrosis factor α and IFNγ (42). This selective activation is sufficient to induce up-regulation of Bim. Less potent altered peptide ligands, represented in this study by the A8 peptide, fail to induce Bim expression and AICD in BK289 cells. However, expression of Bim is not always sufficient to activate a DR-independent apoptotic program, as illustrated by IVT-mediated activation of these CTLs, which die exclusively through Fas-mediated apoptosis, despite up-regulation of Bim. Our data suggest that the proapoptotic activity of Bim is neutralized in IVT-stimulated cells by the concomitant up-regulation of Bcl-XL that does not occur after Y5-mediated triggering. This conclusion is supported by the demonstration that Y5-stimulated cells exhibit significantly higher Bim:Bcl-XL ratios at different time points after triggering and lower mitochondria membrane potential, as compared with IVT-stimulated CTLs (Figs. 4 and 5). Moreover, in Y5-stimulated BK289 cells, Bcl-XL appears to undergo posttranslational modifications that are less pronounced after IVT-mediated triggering (Fig. 4). At least some posttranslational modifications are known to inhibit the antiapoptotic activity of Bcl-XL and may contribute to its failure to neutralize Bim in Y5-stimulated cells (43). Overall, our data are consistent with the notion that relative levels of pro- and antiapoptotic proteins of the Bcl-2 family regulate initiation of DR-independent programs of apoptosis.
Induction of Bim by partially agonistic altered peptide ligands demonstrated in this study may represent a new mechanism of immune escape by antigenic variation. Conceivably, some of the variants of CTL peptide epitopes arising during replication of genetically unstable microorganisms; e.g., HIV, can act in a manner similar to the Y5 peptide and actively suppress specific CTL responses through selective induction of DR-independent AICD.
Experiments with pharmacological inhibitors of various signaling pathways demonstrated that TCR-induced Bim up-regulation requires activation of PKC and calcineurin. In agreement, a combination of chemical inducers of PKC and Ca2+ influx was sufficient to cause strong up-regulation of Bim in T cells. Inhibition of PKB/Akt activity after lymphokine withdrawal has been previously shown to induce Bim up-regulation in T cells resulting from dephosphorylation of Fox03 transcription factor, which directly activates the Bim promoter (29-31). Our data indicate that TCR-induced up-regulation of Bim involves other signaling pathways. Although TCR triggering induced transient phosphorylation of Fox03, the overall levels of the transcription factor were unchanged and it was constitutively dephosphorylated in T cells before TCR triggering. These data define another signal and set of regulatory pathways involved in up-regulation of Bim expression.
PKC appears to play a dual role in Bim regulation in T cells. Inhibition of PKC activity in nontriggered T cells resulted in up-regulation of Bim expression, most likely due to the effect of PKC on the activity of phosphatidylinositol 3-kinase and PKB/Akt, because addition of exogenous IL-2, which activates these kinases downstream of PKC, inhibited the up-regulation of Bim induced by a PKC inhibitor (data not shown). The opposite effect of PKC inhibition on induction of Bim by TCR triggering indicates that PKC can positively regulate Bim transcription/translation but the molecular mechanism of this activity remains to be determined.
Both CsA and FK506, which block Ca2+-dependent activation of calcineurin, prevent TCR-induced Bim up-regulation. This previously unknown effect of the immunosuppressive drugs correlates with their ability to prevent deletion of transplant reactive T cells and establishment of stable tolerance (9, 11). In contrast, rapamycin, which allows stable graft acceptance, does not affect AICD and does not block induction of Bim. These data are consistent with an important role of Bim up-regulation in T-cell deletion and define Bim as a target gene for approaches in transplantation and induction of tolerance. Conversely, vaccination protocols may benefit from blocking Bim up-regulation induced by specific TCR triggering.
Acknowledgments
This work was supported by grants awarded by the Swedish Cancer Society, the Swedish Pediatric Cancer Foundation, the Swedish Foundation of Strategic Research, the Petrus and Augusta Hedlund Foundation, and the Karolinska Institute.
Abbreviations: AICD, activation-induced cell death; APC, antigen-presenting cell; A8, IVTDFSVAK; CTL, cytotoxic T lymphocyte; CsA, cyclosporin A; DR, death receptor, IVT, IVTDFSVIK; TCR, T cell receptor; Y5, IVTDYSVIK; TPA, 12-O-tetradecanoylphorbol 13-acetate.
References
- 1.Green, D. R., Droin, N. & Pinkoski, M. (2003) Immunol. Rev. 193, 70-81. [DOI] [PubMed] [Google Scholar]
- 2.Li, X. C., Strom, T. B., Turka, L. A. & Wells, A. D. (2001) Immunity 14, 407-416. [DOI] [PubMed] [Google Scholar]
- 3.Lenardo, M., Ka-Ming Chan, F., Hornung, F., McFarland, H., Siegel, R., Wang, J. & Zheng, L. (1999) Annu. Rev. Immunol. 17, 221-253. [DOI] [PubMed] [Google Scholar]
- 4.Hildeman, D. A., Zhu, Y., Mitchell, T. C., Bouillet, P., Strasser, A., Kappler, J. & Marrack, P. (2002) Immunity 16, 759-767. [DOI] [PubMed] [Google Scholar]
- 5.Nicolo, C., Tomassini, B., Rippo, M. R. & Testi, R. (2001) Immunobiology 97, 1803-1808. [DOI] [PubMed] [Google Scholar]
- 6.Reich, A., Körner, H., Sedgwick, J. D. & Pircher, H. (2000) Eur. J. Immunol. 30, 678-682. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen, L. T., McKall-Faienza, K., Zakarian, A., Speiser, D. E., Mak, T. W. & Ohashi, P. S. (2000) Eur. J. Immunol. 30, 683-688. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein, D. R., Thomas, J. M., Kirklin, J. K. & George, J. F. (2001) J. Heart Lung Transplant. 20, 1132-1135. [DOI] [PubMed] [Google Scholar]
- 9.Li, X. C., Li, Y., Dodge, I., Wells, A. D., Zheng, X. X., Turka, L. A. & Strom, T. B. (1999) J. Immunol. 163, 2500-2507. [PubMed] [Google Scholar]
- 10.Wagener, M. E., Konieczny, B. T., Dai, Z., Ring, G. H. & Lakkis, F. G. (2000) Transplantation 69, 2428-2432. [DOI] [PubMed] [Google Scholar]
- 11.Wells, A. D., Li, X. C., Li, Y., Walsh, M. C., Zheng, X. X., Wu, Z., Nunez, G., Tang, A., Sayegh, M., Hancock, W. W., et al. (1999) Nat. Med. 5, 1303-1307. [DOI] [PubMed] [Google Scholar]
- 12.Wekerle, T., Kurtz, J., Sayegh, M., Ito, H., Wells, A., Bensinger, S., Shaffer, J., Turka, L. & Sykes, M. (2001) J. Immunol. 166, 2311-2316. [DOI] [PubMed] [Google Scholar]
- 13.Wekerle, T., Kurtz, J., Bigenzahn, S., Takeuchi, Y. & Sykes, M. (2002) Curr. Opin. Immunol. 14, 592-600. [DOI] [PubMed] [Google Scholar]
- 14.Bouillet, P., Metcalf, D., Huang, D. C. S., Tarlington, D. M., Kay, T. W. H., Köntgen, F., Adams, J. M. & Strasser, A. (1999) Science 286, 1735-1738. [DOI] [PubMed] [Google Scholar]
- 15.Bouillet, P., Purton, J. F., Godfrey, D. I., Zhang, L. C., Coultas, L., Puthalakath, H., Pellegrini, M., Cory, S., Adams, J. M. & Strasser, A. (2002) Nature 415, 922-926. [DOI] [PubMed] [Google Scholar]
- 16.Enders, A., Bouillet, P., Puthalakath, H., Xu, Y., Tarlinton, D. M. & Strasser, A. (2003) J. Exp. Med. 198, 1119-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinjyo, T., Kuribara, R., Inukai, T., Hosoi, H., Kinoshita, T., Miyajima, A., Houghton, P. J., Look, A. T., Ozawa, K. & Inaba, T. (2001) Mol. Cell. Biol. 21, 854-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitfield, J., Neame, S. J., Paquet, L., Bernard, O. & Ham, J. (2001) Neuron 29, 629-643. [DOI] [PubMed] [Google Scholar]
- 19.Putcha, G. V., Moulder, K. L., Golden, J. P., Bouillet, P., Adams, J. A., Strasser, A. & Johnson, E. M. (2001) Neuron 29, 615-628. [DOI] [PubMed] [Google Scholar]
- 20.O'Connor, L., Strasser, A., O'Reilly, L. A., Hausmann, G., Adams, J. M., Cory, S. & Huang, D. C. (1998) EMBO J. 17, 384-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Reilly, L. A., Cullen, L., Visvader, J., Lindeman, G. J., Print, C., Bath, M. L., Huang, D. C. & Strasser, A. (2000) Am. J. Pathol. 157, 449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Putcha, G. V., Harris, C. A., Moulder, K. L., Easton, R. M., Thompson, C. B. & Johnson, E. M., Jr. (2002) J. Cell Biol. 157, 441-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris, C. A. & Johnson, E. M., Jr. (2001) J. Biol. Chem. 276, 37754-37760. [DOI] [PubMed] [Google Scholar]
- 24.Puthalakath, H. & Strasser, A. (2002) Cell Death Differ. 9, 505-512. [DOI] [PubMed] [Google Scholar]
- 25.Hildeman, D. A., Zhu, Y., Mitchell, T. C., Kappler, J. & Marrack, P. (2002) Curr. Opin. Immunol. 14, 354-359. [DOI] [PubMed] [Google Scholar]
- 26.Reginato, M. J., Mills, K. R., Paulus, J. K., Lynch, D. K., Sgroi, D. C., Debnath, J., Muthuswamy, S. K. & Brugge, J. S. (2003) Nat. Cell Biol. 5, 733-740. [DOI] [PubMed] [Google Scholar]
- 27.Weston, C. R., Balmanno, K., Chalmers, C., Hadfield, K., Molton, S. A., Ley, R., Wagner, E. F. & Cook, S. J. (2003) Oncogene 22, 1281-1293. [DOI] [PubMed] [Google Scholar]
- 28.Foncea, R., Galvez, A., Perez, V., Morales, M. P., Calixto, A., Melendez, J., Gonzalez-Jara, F., Diaz-Araya, G., Sapag-Hagar, M., Sugden, P. H., et al. (2000) Biochem. Biophys. Res. Commun. 273, 736-744. [DOI] [PubMed] [Google Scholar]
- 29.Dijkers, P. F., Medema, R. H., Lammers, J. W., Koenderman, L. & Coffer, P. J. (2000) Curr. Biol. 10, 1201-1204. [DOI] [PubMed] [Google Scholar]
- 30.Dijkers, P. F., Birkenkamp, K. U., Lam, E. W., Thomas, N. S., Lammers, J. W., Koenderman, L. & Coffer, P. J. (2002) J. Cell Biol. 156, 531-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl, M., Dijkers, P. F., Kops, G. J., Lens, S. M., Coffer, P. J., Burgering, B. M. & Medema, R. H. (2002) J. Immunol. 168, 5024-5031. [DOI] [PubMed] [Google Scholar]
- 32.Puthalakath, H., Huang, D. C., O'Reilly, L. A., King, S. M. & Strasser, A. (1999) Mol. Cell 3, 287-296. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Q.-J., Gavioli, R., Klein, G. & Masucci, M. G. (1993) Proc. Natl. Acad. Sci. USA 90, 2217-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitsky, V., de Campos-Lima, P.-O., Frisan, T. & Masucci, M. G. (1998) J. Immunol. 161, 594-601. [PubMed] [Google Scholar]
- 35.Wei, C.-H., Beeson, C., Masucci, M. G. & Levitsky, V. (1999) J. Immunol. 163, 2601-2609. [PubMed] [Google Scholar]
- 36.Wei, C.-H., Yagita, H., Masucci, M. G. & Levitsky, V. (2001) J. Immunol. 166, 989-995. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti, P., Castedo, M., Susin, S. A., Zamzami, N., Hirsch, T., Macho, A., Haeffner, A., Hirsch, F., Geuskens, M. & Kroemer, G. (1996) J. Exp. Med. 184, 1155-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng, E. H., Wei, M. C., Weiler, S., Flavell, R. A., Mak, T. W., Lindsten, T. & Korsmeyer, S. J. (2001) Mol. Cell 8, 705-711. [DOI] [PubMed] [Google Scholar]
- 39.Kroemer, G. (1999) Biochem. Soc. Symp. 66, 1-15. [DOI] [PubMed] [Google Scholar]
- 40.Connern, C. P. & Halestrap, A. P. (1994) Biochem. J. 302, 321-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffiths, E. J. & Halestrap, A. P. (1993) J. Mol. Cell. Cardiol. 25, 1461-1469. [DOI] [PubMed] [Google Scholar]
- 42.Wei, C.-H., Uhlin, M., Masucci, M. G. & Levitsky, V. (2002) Hum. Immunol. 63, 821-833. [DOI] [PubMed] [Google Scholar]
- 43.Deverman, B. E., Cook, B. L., Manson, S. R., Niederhoff, R. A., Langer, E. M., Rosova, I., Kulans, L. A., Fu, X., Weinberg, J. S., Heinecke, J. W., et al. (2002) Cell 111, 51-62. [DOI] [PubMed] [Google Scholar]