Abstract
Argonaute subfamily proteins are involved in human organ growth and development. Recent studies found its association with human breast cancer, however, its expression profile and its prognostic value in clear cell renal cancer (ccRCC) have not been investigated. Methods: Expression of the Argonaute proteins were assessed by immunohistochemistry (IHC) in tissue microarrays (TMA), containing paired tumor tissue and adjacent non-cancer tissue from 176 patients who had undergone surgery in hospital for histologically proven ccRCC. Prognostic value and correlation with other clinico-pathologic factors were evaluated in two classifications. Results: Data showed a significant higher expression of Argonaute 1 and Argonaute 2 present in neoplastic tissues compared with that in adjacent tissue; A significant correlation existed between the higher expression of Argonaute 1 protein with the T stage, lymph node metastasis and clinical TNM (cTNM); Survival analysis by Kaplan-Meier survival curve and log-rank test demonstrated that elevated Argonaute 1 and Argonaute 2 expression in cancer tissue predicted poorer overall survival (OS) compared with group in lower expression (36.3% VS 67.1%; 37.3% VS 53.9%; respectively). Notably, multivariate analyses by Cox’s proportional hazard model revealed that expression of Argonaute 2 was an independent prognostic factor in renal cancer. Conclusions: In summary, our present study clarify that the aberrant expression of Argonaute in human RCC is possibly involved with tumorigenesis and development, and the Argonaute protein could act as a potential biomarker for prognosis assessment of renal cancer. Related mechanism is worthy of further investigation.
Keywords: Renal cancer, Argonaute protein, immunohistochemistry, tissue microarray
Introduction
Renal cell carcinoma (RCC) is the most lethal urologic tumor and the sixth leading cause of cancer deaths in Western countries. Each year, around 200,000 patients are diagnosed with this malignancy resulting in approximately 100,000 deaths, and its incidence is increasing steadily in recent years [1,2]. RCC is represented by 80% by clear cell RCC (ccRCC), originating from the renal proximal tubule [3]. The curative effect of surgical resection varied differently in patients population: the 5-year survival rate in stage I, II, III and IV is about 93%, 65%, 53% and 27% respectively [4]. So it is clear that early diagnosis and medical intervention seems vital in decreasing mortality and promoting total quality of life, novel molecular markers about kidney cancer that can help individually evaluate risk of patient outcome and predict the prognosis are urgently required, as well as the prediction of therapy effect and advocating personalized treatment.
Argonaute protein (AGO protein) is one of the most indispensible components in the RNA induced silencing complex (RISC), which is responsible for the gene silencing phenomenon known as RNA interference (RNAi) [5]. These proteins bind different classes of small noncoding RNAs, including microRNAs (miRNAs), small interfering RNAs (siRNAs) and PIWI interacting RNAs (piRNAs), then small RNAs guide Argonaute proteins to their specific target through sequence complementarity, which typically leads to silencing of the target. Previous studies have indicated that these proteins are also partially responsible for a series of biological processes such as cell proliferation, differentiation and apoptosis [6]. In fact, it is well accepted that Argonaute proteins have shown that some are endonucleases referred as “slicers” [7]. Recent structural analysis of the Ago protein has revealed a third functionally important domain which located between the former two domains, called the MID domain. This domain can bind the characteristic 5’ phosphates of small RNAs, then anchors these small RNAs onto the Ago protein [8,9]. Moreover, some other studies have reported that this newly-found domain may be a key role in some protein-protein interactions [10]. This protein family was first identified in plants, classified by the presence of PAZ and PIWI domains [11]. In mammals there are eight Argonaute proteins which can be divided into the Ago subfamily and the PIWI subfamily [12,13]. Ago subfamily is ubiquitously expressed in many organisms such as animals, plants, and fission yeast and can be divided into AGO1, AGO2, AGO3 and AGO4. Proteins of this subfamily could interact and bind with siRNA and miRNA to activate gene silencing pathway, which regulate gene expression in the transcription and post-transcription steps. For the past few years, the Argonaute family has drawn more and more attention as a potential factor related to tumorigenesis in colonic cancer study, as well as in prostate cancer [14,15]. Therefore this research is concentrated to investigate the expression of the human Ago subfamily proteins by Immunohistochemistry (IHC) and identify their potential roles in tumor occurrence, development and prognosis for patients with kidney cancer.
Mateirals and methods
Patients
A total of 176 patients who underwent surgery at hospitals for histologically proven renal cell carcinoma (ccRCC) that cooperated with National Engineering Center for Biochip at Shanghai during 2006-2008 are selected in this research. They were totally 126 men and 50 women, whose age range from 32 to 79 years (median: 61 years). Clinico-pathological characteristics in our study are presented in Table 1. Paraffin-embedded tumor specimens and paired adjacent nontumor specimens (≤1.5 cm away from the tumor) were carefully collected immediately after nephrectomy operation. All patients were followed up until September 2011 with a median observation time of 35 months. Patients had to provide a written consent to participate and after receiving written information regarding its course and purpose, then they could be enrolled into our observation. Approval for the study was received in advance from the Ethics Committee of our institution (Table 1).
Table 1.
Clinical-pathological characteristics of renal cell carcinoma patients
| Characteristics | N=176 | % |
|---|---|---|
| Gender | ||
| Male | 126 | 71.6 |
| Female | 50 | 28.4 |
| Age (yrs) | ||
| <65 | 93 | 52.8 |
| >65 | 83 | 47.2 |
| Tumor Size (cm) | ||
| <4 | 92 | 52.3 |
| ≥4 | 84 | 47.7 |
| Histological grade | ||
| I-II | 56 | 31.8 |
| III-IV | 120 | 68.2 |
| cTNM | ||
| I-II | 65 | 36.9 |
| III-IV | 111 | 63.1 |
| T stage | ||
| T1-T2 | 87 | 49.4 |
| T3-T4 | 89 | 50.6 |
| Lymph nodes metastasis | ||
| Absence | 122 | 69.3 |
| Presence | 54 | 30.7 |
| Distant Metastasis | ||
| Absence | 163 | 92.6 |
| Presence | 13 | 7.4 |
| Follow-ups | ||
| Dead | 61 | 34.7 |
| Survival or lost | 115 | 65.3 |
Construction and section of tissue microarray
Briefly, a tissue arraying instrument (Beecher Instruments, Inc) was used to create cylindershaped holes in a square recipient paraffin block, then to remove tissue cores from the donor block such as clinical biopsies or tumor samples by a hollow needle with an inner diameter of 1.5 mm, held in an X-Y axis precision guide. The cylindrical sample from a region in the donor block which is selected by an experienced pathologist was then inserted into a recipient paraffin block in a precisely spaced, array pattern. After the construction of arrayed block, a 5-μm section was cut with a microtome continuously with a high speed and picked a perfect piece to place on polylysine-coated slides. There are 2 tissue array blocks in this research containing totally 176 samples for each monoclonal antibody against Argonaute 1 and Argonaute 2 protein.
Preparation of antibodies against Argonaute proteins
Antibodies against Argonaute proteins in this research were prepared previously by immunizing rabbits with synthetic peptides derived from the sequences of Argonaute members. Then the antibody solution was fractionated from the rabbit antisera and affinity-purified on peptide affinity columns. After verifying by ELISA and Western blot, the antibody solution was used in immunohistochemistry analyses.
Immunohistochemistry on formalin-fixed sections
IHC staining for the Argonaute 1/Argonaute 2 on sections of the formalin-fixed samples on the tissue microarray was carried out by using the Envision ready-to-use methods. Slides were deparaffinized in xylene and rehydrated through graded concentrations of ethanol to distilled water and endogenous peroxidase activity was blocked by incubation with 30 mL/L H2O2 in methanol for 10 min at room temperature. Then sections were submitted to antigen retrieval in a pressure cooker containing 0.01 mmol/L natrium citricium buffer for 10 min. Slides were subsequently incubated in 100 mL/L normal goat serum for 20 min at room temperature. Sections were permeabilized in PBS-Triton and incubated overnight with primary antibody at 4°C. The antibodies were used in PBS-Trison with variable dilution. Rabbit anti-human polyclonal antibody to Argonaute 1/Argonaute 2 was used. Each section was then incubated with Envision rabbit peroxidase (GeneTech) for 30 min. Finally, the sections were treated with 0.02% 3,3’-diaminoberzidine and 0.005% H2O2 in 0.05 mmol/L Tris-HCl buffer and counterstained by hematoxylin.
The evaluation of the immunohistochemical staining was performed independently by two authors without knowledge of the clinicpothological information. The immunoreactive scores besides Argonaute 1/Argonaute 2 were determined by the sum of extension and intensity as literature reported previously [16]. The intensity of the staining was scored using the following scale: 0, no staining of the tumor cells; +, mild staining; ++, moderate staining and +++, marked staining. The area of staining was evaluated and recorded as a percentage: 0, less than 5%; +, 5%-25%; ++, 26%-50%; 3+, 51%-75% and 4+, more than 75%. The combined scores were recorded and graded as follows: -, 0; +, 1-2; ++, 3-5; +++, 6-7.
Statistical analysis
Computerized statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS), version 12.0. Clinical and histopathologic information and the results from the immunohistochemical studies of the tissue microarray were entered into a database. The variances of Argonaute 1/Argonaute 2 expression among different tissues was analyzed using Mann-Whitney U-test and the clinicopathological data was analyzed with Spearman’s correlation test. In all statistical analyses, a two-tailed p value ≤0.05 was considered statistically significant.
Results
The expression of Argonaute 1 and Argonaute 2 were significantly higher in tumorous tissue than in adjacent tissue
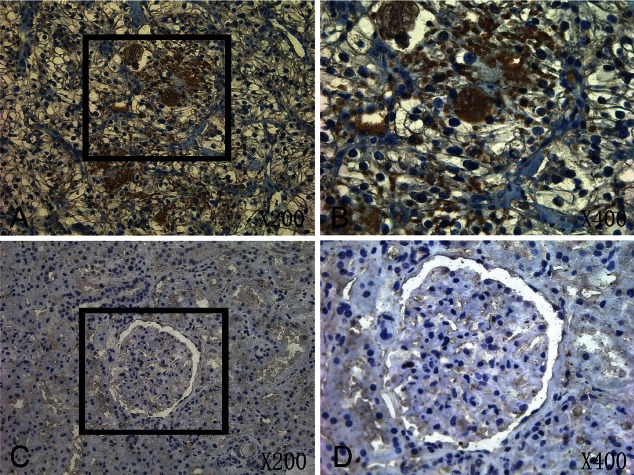
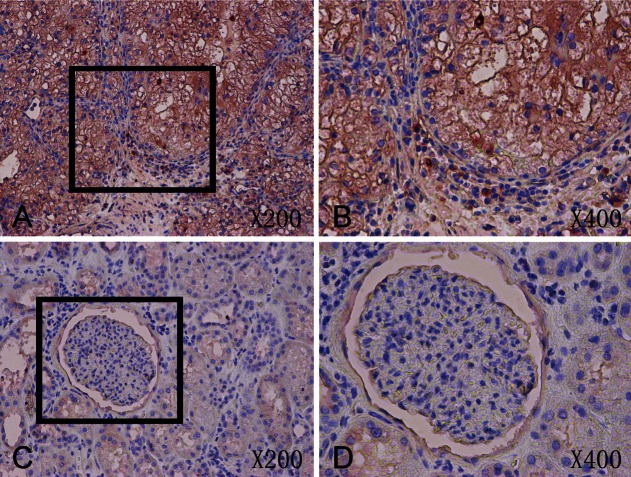
By using of a large tissue microarray (176 cores) we investigated the protein expression of Argonaute 1 and Argonaute 2 in renal cancer specimens and adjacent non-tumor tissue. The tumorous or nontumor staining was semiquantitatively scored by the intensity and the percentage of positive staining. Argonaute proteins expressions were detected mainly in cytoplasm of malignant cells (Figures 1 and 2). The positive expression of Argonaute 1 (p<0.05), Argonaute 2 (p<0.01) in tumor tissue was significantly higher than in adjacent non-tumor tissue. Images of representative immunostaining are presented in Figures 1 and 2. The results are shown in Table 2.
Figure 1.
A: Immunohistochemistry expression of Argonaute 1 in tumor tissue (×200). B: Immunohistochemistry expression of Argonaute 1 in tumor tissue (×400). C: Immunohistochemistry expression of Argonaute 1 in adjacent non-tumor tissue (×200). D: Immunohistochemistry expression of Argonaute 1 in adjacent non-tumor tissue (×400).
Figure 2.
A: Immunohistochemistry expression of Argonaute 2 in tumor tissue (×200). B: Immunohistochemistry expression of Argonaute 2 in tumor tissue (×400). C: Immunohistochemistry expression of Argonaute 2 in adjacent non-tumor tissue (×200). D: Immunohistochemistry expression of Argonaute 2 in adjacent non-tumor tissue (×400).
Table 2.
Argonaute 1/Argonaute 2 expression in tumor tissue and adjacent non-tumor tissue
| Markers | tumor tissue | adjacent non-tumor tissue | p | z |
|---|---|---|---|---|
| Argonaute 1 | 4.67±2.013 | 4.21±1.753 | 0.042 | -2.15 |
| Argonaute 2 | 4.58±2.077 | 3.10±1.195 | 0.001 | -5.869 |
Relationship between the expression of Argonaute proteins and clinicopathological parameters
A significant correlation was observed between the higher expression of Argonaute proteins with the T stage, lymph node metastasis and cTNM. IHC was employed to investigate the association between Argonaute expression and clinicopathological features in the 176 renal cancer specimen. The expression level of Argonaute 1 in cytoplasm was significantly associated with T stage (r=0.189, p=0.014); The expression level of Argonaute 2 in cytoplasm was significantly associated with T stage (r=0.192, p=0.012), lymph node metastasis (r=0.257, p=0.015) and cTNM (r=0.379, p=0.032). There were no statistical differences among each protein expression and age, sex, tumor size and distant metastasis. Detailed data is shown in following Table 3.
Table 3.
Relationship between the expression of Argonaute 1/Argonaute 2 proteins and clinicopathological parameters
| Markers | Correlation coefficient (r) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Gender | Age | Tumor size | cTNM | T stage | LN metastasis | Distant metastasis | Histological grade | |
| Argonaute 1 | 0.111 | 0.027 | 0.032 | 0.126 | 0.189a | 0.028 | 0.040 | -0.036 |
| Argonaute 2 | 0.021 | 0.049 | 0.071 | 0.379a | 0.192a | 0.257a | 0.039 | -0.037 |
p<0.05; LN: lymph node.
Argonaute expression and survival analysis: univariate survival analysis
Follow-up information was available for 176 patients until September 2011, within the observation period, there were 61 renal cancer related deaths with a median follow-up time of 20 months (0~54 months). And the remaining 105 patients were still alive or lost to follow-up with a median follow-up time of 53 months ranging (42~61 months). Survival analysis by Kaplan-Meier survival curve and log-rank test demonstrated that patients with lower expression of Argonaute 1 and Argonaute 2 in tumor tissue had a better overall survival than patients with tumor with higher expression (p=0.040 and p=0.038, respectively), the 5-years survival rate of patients with higher expression was significantly lower than that of patients with lower expression (36.3% VS 67.1%; 37.3% VS 53.9%; respectively, Table 4). The survival curve was demonstrated in Figure 3.
Table 4.
The 5-year survival rate of the Argonaute 1/Argonaute 2 expression and other clinicopathologic features
| 5-year survival rate | ||||
|---|---|---|---|---|
|
| ||||
| Survival rate | Standard error | p value | ||
| Argonaute 1 | Negative | 0.671 | 0.088 | 0.040 |
| Positive | 0.363 | 0.040 | ||
| Argonaute 2 | Negative | 0.539 | 0.073 | 0.038 |
| Positive | 0.373 | 0.045 | ||
| Gender | Male | 0.435 | 0.046 | 0.655 |
| Female | 0.388 | 0.067 | ||
| Age | <65 | 0.522 | 0.056 | 0.008 |
| ≥65 | 0.321 | 0.052 | ||
| Tumor size | <4 cm | 0.579 | 0.060 | 0.000 |
| ≥4 cm | 0.302 | 0.046 | ||
| Histological grade | I-II | 0.575 | 0.075 | 0.023 |
| ≥III | 0.357 | 0.044 | ||
| cTNM | TNM1 | 0.764 | 0.119 | 0.011 |
| TNM2 | 0.461 | 0.076 | ||
Figure 3.
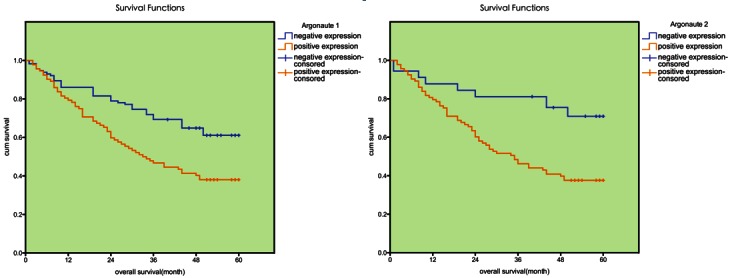
The survival analysis of Argonaute 1 and Argonaute 2. Patients with higher Argonaute 1 and Argonaute 2 expression in tumor tissue were closely correlated with poorer overall survival than patients with tumor with lower expression (p=0.040, p=0.038, respectively).
Multivariate cox regression analysis
To avoid the influence caused by univariate analysis, the expression of Argonaute 1 and Argonaute 2 as well as other parameters was examined in multivariate Cox analysis. The Argonaute 2 was found to be a significant independent prognostic factor for poor overall survival in our study (B=0.97; p=0.03; Exp (B)=2.671; Table 4), which indicated that the Argonaute protein could act as a potential biomarker for prognosis evaluation of renal cancer. Of other parameters, tumor size, histological grade, age and cTNM were also found to be independent prognostic factors for patient survival (Table 5).
Table 5.
Multivariate Cox’s proportional hazards regression analysis of prognostic factors for renal cancer
| Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 95.0% CI for Exp (B) | ||||||||
|
|
||||||||
| B | SE | Wald | df | Sig. | Exp (B) | Lower | Upper | |
| Argonaute 1 | -0.191 | 0.302 | 0.377 | 1 | 0.538 | 0.826 | 0.447 | 1.520 |
| Argonaute 2 | 0.970 | 0.449 | 4.712 | 1 | 0.030 | 2.671 | 1.101 | 6.449 |
| Tumor size | 0.067 | 0.028 | 5.433 | 1 | 0.018 | 1.070 | 1.010 | 1.133 |
| Histological grade | 0.500 | 0.202 | 5.956 | 1 | 0.015 | 1.649 | 1.104 | 2.458 |
| Age | 0.024 | 0.012 | 4.479 | 1 | 0.035 | 1.026 | 1.003 | 1.048 |
| cTNM | 0.538 | 0.187 | 8.816 | 1 | 0.003 | 1.750 | 1.211 | 2.527 |
Discussion
Small non-coding RNA has drawn more and more attention due to its rapidly rising role in the gene transcriptional and posttranscriptional regulation. There is about approximately 30% gene expression in human body regulated by the mechanism of gene silencing such as RNAi [17], and all of these biological process needs the participation of the Argonaute proteins which has being an area of intense investigation. However, the presumed role of Argonaute proteins in the etiology of cancer remains unclear. The present study demonstrated that Argonaute proteins were up-regulated in the renal cell cancer, and explored available evidence of close correlation of Argonaute expression and the total patients’ survival during a five-year follow-up survey.
Argonaute proteins are the essential components of the miRNA-induced silencing complex (miRISC) that directly recruit miRNAs and function as the interface between miRNAs and their mRNA targets. Mammals have four Argonauts (Ago 1-4) that are involved in the miRNA pathway and among them, Ago 2 is unique, with the slicer activity that mediates the cleavage of perfectly matched targets for miRNAs and siRNAs [18,19]. To date, exhaustive bioinformatic and experimental analyses have failed to identify a large number of perfectly matched miRNA:mRNA regulatory sequences. It remains unclear why Ago 2 is universally important for miRNA functions in diverse organs and tissues of mammals. In addition the functional significance of Ago 1, Ago 3, and Ago 4 for miRNA activity is poorly understood, probably due to the lack of developmental phenotypes in knockout (KO) mouse models. The function of individual Argonauts and the quantitative nature of their contribution to the miRNA pathway during mammalian development were under investigation in the recent year [20].
Genome-wide shotgun proteomics and absolute quantification of Argonauts demonstrates that Ago 2 is the most abundant Argonaute and so associates with the largest pool of miRNAs in both mouse epidermal cells and human melanoma cells. With more in-deph research, data demonstrated that miRNA activity can be quantitatively manipulated by controlling individual Argonauts [21].
Recent newly up surging findings demonstrated that the Argonauts proteins have a more close relation with tumorigenesis and progression of different kinds of human cancer [22,23]. The interaction of Argonaute proteins with small RNAs or other parts of RISC involved in carcinogenesis has not been completely elucidated. Gene specific translational control induced by some miRNA species has been reported to have an effect on cancer development [24]. As a component of RISC, Argonaute 2 binds to miRNAs or piRNAs (Piwi-interacting RNA), and aberrant regulation of these small RNAs by Argonaute 2 might induce the malignant phenotype of these cells. SND1, also reported to be a component of RISC, is over-expressed in human colonic carcinoma tissues, even in early-stage lesions [25]. Identification of target mRNA species and interacting partners of Argonaute 2 might lead us to the further insights into the complex roles of Argonaute 2 in renal cancer carcinogenesis.
To directly address the potential roles for Argonaute proteins in the occurrence and development of kidney cancer, an elaborate experiment was conducted and a rigorous analysis was performed of human Argonaute proteins on a cohort of 176 renal cancer specimens. Our results revealed that the positive rate of Argonaute 1 and Argonaute 2 expression in gastric cancer tissue was remarkably higher than that in non-tumor tissue (p<0.05), which further proving the conclusion gained by Wang [26] et al. In Wang’s study, by contrasting the expression of Argonaute 2 in colonic cancer tissues and normal tissue, they had shown that the abnormal expression of Argonaute 2 might be correlated with colon oncogenic event.
Besides, a significant correlation was observed between the higher expression of Argonaute proteins with the T stage, lymph node metastasis and cTNM in this research. Argonaute subfamily might be involved in the development and progression of renal cancer as we supposed before. In the present study, we found the expression level of Argonaute 1 in cytoplasm was significantly associated with T stage (r=0.189, p=0.014); the expression level of Argonaute 2 in cytoplasm was significantly associated with T stage (r=0.192, p=0.012), lymph node metastasis (r=0.257, p=0.015) and cTNM (r=0.379, p=0.032). It is suggested that Argonaute 1 and Argonaute 2 are associated with tumor progression to advanced stage and may promote tumor invasion. We further imagined that the Argonaute subfamily proteins may interfere with the activation of cellular signal transduction pathway, cell division cycle and tumor angiogenesis to influence biological behavior of tumor, and this had just been unraveled in the latest relevant researches. Chen et al. demonstrated the regulatory roles of Argonaute 1 in MDA-MB-231 injected SCID mice through promoting angiogenesis. In this study, the author combined bioinformatics in conjunction with lab experimental validation and revealed that let-7 and miR-103/107 are robustly induced in vascular endothelial cells and consequently target Argonaute 1. Subsequently, a translational desuppression pathway up-regulates VEGF and enhances angiogenesis in vitro and in vivo. Specimens from human breast cancer provided evidence that this pathway may be implicated in tumor angiogenesis and progression. These findings suggest a novel role of hypoxia-responsive microRNAs (HRMs) in hypoxia-induced gene expression and the consequent angiogenesis via targeting Argonaute 1 [27].
Ultimately, A total of 176 patients histologically proven renal cancer with follow-up information were conducted a systematically analysis to confirm the relationship of the Argonaute proteins and outcome of patient initially. Our finding demonstrated that patients with lower expression of Argonaute 1 and Argonaute 2 in tumor tissue had a better overall survival than patients with higher expression (p=0.040, p=0.038, respectively), providing an evidence that elevated expression of Argonaute 1 or Argonaute 2 in renal cancer might facilitate an increased malignant and worse prognostic phenotype. It is noteworthy that by multivariate Cox analysis combining expression of Argonaute proteins with other parameters, Argonaute 2 was found as an independent prognostic factor (p=0.03) for patient survival. The aberrant expression of Argonaute protein linked to a poor prognosis of patients has never been investigated in renal cancer before.
Conclusions
In summary, the role of Argonaute proteins with small RNAs or other part of RISC involved in carcinogenesis has been partly demonstrated in the study. We postulate that at least some members of the human Argonaute family may be involved in the development and progression of renal cancer and can serves as an independent biomarker for prognosis. We also place special emphasis on the over-expression of Argonaute may identifies patients at high risk and be a novel therapeutic molecular target for renal cancer. However, molecular mechanisms are poorly understood and for the limitations of our study samples, which has so far only scratched the surface of the unknown arena, further investigations are urgently required.
Acknowledgements
This work was partially supported by grants from the National Natural Science Foundation of China (No. 81000311 and No. 81270831).
Conflict of interest
The authors declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
Abbreviations and acronyms
- RCC
Renal cell carcinoma
- ccRCC
clear cell renal cell carcinoma
- ELISA
enzyme-linked immuno sorbent assay
- TMA
tissue microarray
- OS
overall survival
- RISC
RNA induced silencing complex
- IHC
Immunohistochemistry
- siRNAs
small interfering RNAs
- PBS
Phosphate buffer solution
- HRMs
hypoxia-responsive microRNAs
- VEGF
vascular endothelial growth factor
- SCID
severe combined immune deficiency
- MDA-MB-231
a type of frequently-used breast cancer cell line
References
- 1.Miyamoto H, Miller JS, Fajardo DA, Lee TK, Netto GJ, Epstein JI. Non-invasive papillary urothelial neoplasms: The 2004 WHO/ISUP classification system. Pathol Int. 2010;60:1–8. doi: 10.1111/j.1440-1827.2009.02477.x. [DOI] [PubMed] [Google Scholar]
- 2.Montironi R, Santinelli A, Pomante R, Mazzucchelli R, Colanzi P, Filho AL, Scarpelli M. Morphometric index of adult renal cell carcinoma. Comparison with the Fuhrman grading system. Virchows Arch. 2000;437:82–9. doi: 10.1007/s004280000216. [DOI] [PubMed] [Google Scholar]
- 3.Matsuura K, Nakada C, Mashio M, Narimatsu T, Yoshimoto T, Tanigawa M, Tsukamoto Y, Hijiya N, Takeuchi I, Nomura T, Sato F, Mimata H, Seto M, Moriyama M. Downregulation of SAV1 plays a role in pathogenesis of high-grade clear cell renal cell carcinoma. BMC Cancer. 2011;11:523. doi: 10.1186/1471-2407-11-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaycioglu O, Eskicorapci S, Karabulut E, Soyupak B, Gogus C, Divrik T, Turkeri L, Yazici S, Ozen H Society of Urooncology Study Group for Kidney Cancer Prognosis. A preoperative prognostic model predicting recurrence-free survival for patients with kidney cancer. Jpn J Clin Oncol. 2013;43:63–8. doi: 10.1093/jjco/hys192. [DOI] [PubMed] [Google Scholar]
- 5.Braun JE, Huntzinger E, Izaurralde E. The role of GW182 proteins in miRNA-mediated gene silencing. Adv Exp Med Biol. 2013;768:147–63. doi: 10.1007/978-1-4614-5107-5_9. [DOI] [PubMed] [Google Scholar]
- 6.Kim WC, Lee CH. The role of mammalian ribonucleases (RNases) in cancer. Biochim Biophys Acta. 2009;1796:99–113. doi: 10.1016/j.bbcan.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Joshua-Tor L. The Argonauts. Cold Spring Harb Symp Quant Biol. 2006;71:67–72. doi: 10.1101/sqb.2006.71.048. [DOI] [PubMed] [Google Scholar]
- 8.Zha X, Xia Q, Yuan YA. Structural insights into small RNA sorting and mRNA target binding by Arabidopsis Argonaute Mid domains. FEBS Lett. 2012;586:3200–7. doi: 10.1016/j.febslet.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Wei KF, Wu LJ, Chen J, Chen YF, Xie DX. Structural evolution and functional diversification analyses of Argonaute protein. J Cell Biochem. 2012;113:2576–85. doi: 10.1002/jcb.24133. [DOI] [PubMed] [Google Scholar]
- 10.Juvvuna PK, Khandelia P, Lee LM, Makeyev EV. Argonaute identity defines the length of mature mammalian microRNAs. Nucleic Acids Res. 2012;40:6808–20. doi: 10.1093/nar/gks293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson T, Lin H. The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Annu Rev Cell Dev Biol. 2009;25:355–76. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou X, Guo H, Chen K, Cheng H, Zhou R. Identification, chromosomal mapping and conserved synteny of porcine Argonaute family of genes. Genetica. 2010;138:805–12. doi: 10.1007/s10709-010-9462-z. [DOI] [PubMed] [Google Scholar]
- 13.Sasaki T, Shiohama A, Minoshima S, Shimizu N. Identification of eight members of the Argonaute family in the human genome small star, filled. Genomics. 2003;82:323–30. doi: 10.1016/s0888-7543(03)00129-0. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Yu C, Gao H, Li Y. Argonaute proteins: potential biomarkers for human colon cancer. BMC Cancer. 2010;10:38. doi: 10.1186/1471-2407-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo NJ, Hur SY, Kim MS, Lee JY, Lee SH. Immunohistochemical analysis of RNA-induced silencing complex-related proteins AGO2 and TNRC6A in prostate and esophageal cancers. APMIS. 2010;118:271–6. doi: 10.1111/j.1600-0463.2010.02588.x. [DOI] [PubMed] [Google Scholar]
- 16.Lynch HT, Smyrk TC. Identifying hereditary nonpolyposis colorectal cancer. N Engl J Med. 1998;338:1537–8. doi: 10.1056/NEJM199805213382109. [DOI] [PubMed] [Google Scholar]
- 17.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Chahar HS, Abraham S, Wu H, Pierson TC, Wang XA, Manjunath N. Ago-2-mediated slicer activity is essential for anti-flaviviral efficacy of RNAi. PLoS One. 2011;6:e27551. doi: 10.1371/journal.pone.0027551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shekar PC, Naim A, Sarathi DP, Kumar S. Argonaute-2-null embryonic stem cells are retarded in self-renewal and differentiation. J Biosci. 2011;36:649–57. doi: 10.1007/s12038-011-9094-1. [DOI] [PubMed] [Google Scholar]
- 20.Wang D, Zhang Z, O’Loughlin E, Lee T, Houel S, O’Carroll D, Tarakhovsky A, Ahn NG, Yi R. Quantitative functions of Argonaute proteins in mammalian development. Genes Dev. 2012;26:693–704. doi: 10.1101/gad.182758.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Y, Deng H, Bi F, Liu J, Bemis LT, Norris D, Wang XJ, Zhang Q. MicroRNA-137 targets carboxyl-terminal binding protein 1 in melanoma cell lines. Int J Biol Sci. 2011;7:133–7. doi: 10.7150/ijbs.7.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parker JS, Barford D. Argonaute: A scaffold for the function of short regulatory RNAs. Trends Biochem Sci. 2006;31:622–30. doi: 10.1016/j.tibs.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Siddiqi S, Matushansky I. Piwis and piwi-interacting RNAs in the epigenetics of cancer. J Cell Biochem. 2012;113:373–80. doi: 10.1002/jcb.23363. [DOI] [PubMed] [Google Scholar]
- 24.Vaksman O, Hetland TE, Trope’ CG, Reich R, Davidson B. Argonaute, Dicer, and Drosha are up-regulated along tumor progression in serous ovarian carcinoma. Hum Pathol. 2012;43:2062–9. doi: 10.1016/j.humpath.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya N, Ochiai M, Nakashima K, Ubagai T, Sugimura T, Nakagama H. SND1, a component of RNA-induced silencing complex, is up-regulated in human colon cancers and implicated in early stage colon carcinogenesis. Cancer Res. 2007;67:9568–76. doi: 10.1158/0008-5472.CAN-06-2707. [DOI] [PubMed] [Google Scholar]
- 26.Wang YX, Zhang XY, Zhang BF, Yang CQ, Gao HJ. Study on the clinical significance of Argonaute2 expression in colonic carcinoma by tissue microarray. Int J Clin Exp Pathol. 2013;6:476–84. [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Z, Lai TC, Jan YH, Lin FM, Wang WC, Xiao H, Wang YT, Sun W, Cui X, Li YS, Fang T, Zhao H, Padmanabhan C, Sun R, Wang DL, Jin H, Chau GY, Huang HD, Hsiao M, Shyy JY. Hypoxia-responsive miRNAs target Argonaute 1 to promote angiogenesis. J Clin Invest. 2013;123:1057–67. doi: 10.1172/JCI65344. [DOI] [PMC free article] [PubMed] [Google Scholar]