Abstract
Ovarian cancer is one of the most common gynecological malignancies. Limited efficacy of cytotoxic chemotherapy is a key obstacle in the treatment of advanced ovarian cancer. This study aimed to investigate whether Mir-375 enhances Rawq01 (a ruthenium derived compound) induced cell death in ovarian cancer. Methods: Three human ovarian cancer cell lines were selected, and independently treated with Rawq01+mir-375 and Rawq01+control. MTT assay and flow cytometry were performed to detect the growth of ovarian cancer cells. Western blot was carried out to determine the expression of apoptotic associated proteins. In addition, ovarian cancer xenografts were established to explore whether mir-375 increased the in vivo chemosensitivity of ovarian cancer cells to RAWQ01. Results: Over-expression of mir-375 sensitized the ovarian cancer cells to RAWQ01. Mir-375 enhanced the in vitro sensitivity of ovarian cancer cells to RAWQ01 by inducing apoptosis. Mir-375 also increased the in vivo chemosensitivity of ovarian cancer cells to RAWQ01. Conclusion: Mir-375 can enhance Rawq01 induced cell death in human ovarian cancer both in vitro and in vivo.
Keywords: Ovarian cancer, mir-375, ruthenium derived compound, Rawq01
Introduction
Ovarian cancer is a leading cause of death in gynecological malignancies worldwide and the fifth most common cause of cancer death in women [1]. Due to lack of specific symptoms and absence of reliable screening strategies, ovarian cancer is usually diagnosed at an advanced stage. The current standard therapy for ovarian cancer is surgical intervention followed by adjuvant carboplatin and taxane-based chemotherapy [2]. Unfortunately, these strategies often achieve poor therapeutic efficacy. Chemoresistance is an important barrier in the treatment of recurrent ovarian cancer [3]. For these reasons, it is imperative to develop new and more effective therapeutic strategies.
MicroRNAs, a class of small non-coding RNAs, have been identified as a new kind of gene expression regulators through targeting the 3’-untranslated region (UTR) of mRNAs for translational repression, degradation or both [4-6]. It has been confirmed that miRNA abnormalities play an important role in gene regulation, apoptosis, maintenance of cell differentiation and tumorigenesis [5,7,8]. Several studies have shown that the differential expression of miRNAs is correlated with the survival of ovarian cancer patients [9,10]. Furthermore, mir-375 is shown to be frequently down-regulated in different cancers. The down-regulation of mir-375, which is mainly caused by the promoter methylation, has been regarded as one of the molecular mechanisms underlying the development and progression of cancers. Epigenetic silencing of mir-375 may induce an up-regulation of its targets, 3-phosphoinositide-dependent protein kinase-1 (PDK1) and insulin-like growth factor 1 receptor (IGF1R) [11,12], and can reduce the viability and induce the apoptosis of cancer cells [13].
It has also been confirmed that mir-375 is down-regulated in the ovarian cancer [14]. However, the functions and mechanisms of mir-375 in ovarian cancer are not fully elucidated, and the therapeutic application of mir-375 has not been reported.
Ruthenium complexes are now a proven effective alternative to Pt-based complexes in cancer chemotherapy, affording different mechanisms of action, a different spectrum of activity and the potential to overcome platinum-resistance, as well as lower toxicity [15].
In the present study, the anti-tumor activity of a novel ruthenium-derived compound Rawq01 was evaluated both in vitro and in vivo models of human ovarian cancer. Our findings revealed that mir-375 enhanced the inhibitory effect of Rawq01 on ovarian cancer cells. The combined treatment of mir-375 and Rawq01 may be a promising strategy for the chemotherapy of ovarian cancer.
Material and methods
Synthesis of Rawq01
All reagents were purchased from commercial suppliers without further purification. 1,10-phenanthroline-5,6-dione was prepared as described in previous study [16]. The ligand 2-(3-hydroxy-4-methoxyphenyl) imidazole [4,5-f][1,10] and phenanthrolene (m-OH-p-OMePIP) were prepared as described in literatures with some modifications and underwent further purification [17]. 1,10-phenanthroline-5,6-dione (1.6 mmol, 347 mg) and 3-hydroxy-4-methoxybenzaldehyde (1.6 mmol, 243.2 mg) in 20 ml of HAc solution containing 2.53 g of NH4Ac was refluxed at 110°C for 4 h. Then, 20 ml of water was added, and the pH value was adjusted to 7.0 at room temperature. Filtering and drying in vacuum were performed to obtain yellow precipitates which were purified in a silica gel column by using ethanol as eluent yielding 341 mg of final product (62.3%).
Synthesis of [(C6H6)Ru(m-OH-p-OMPIP)Cl]Cl∙2H2O. (RAWQ01): A mixture of [(η6-C6H6)RuCl2]2 (0.15 mmol, 75 mg) and m-OH-p-OMPIP (0.3 mmol, 103.2 mg) in dichloromethane (40 mL) was refluxed under argon for 6 h until the solution color changed from brown to yellow [18]. A yellow precipitate was obtained by rotary evaporator and purified by recrystallization in methanol yielding 103 mg of products (54.5%). ESI-MS (in MeOH, m/z): 559.09, ([M-Cl]+). 1H NMR (DMSO-d6, δ/ppm) δ: 9.92 (d, J = 5.2 Hz, 2H), 9.40 (s, 2H), 9.18 (s, 2H), 8.17 (s, 2H), 7.79 (d, J = 7.6 Hz, 2H), 7.15 (d, J = 8.5 Hz, 2H), 6.30 (s, 6H).
Cell culture
Three human ovarian cancer cell lines OVCAR3, HO-8910 and SK-OV-3 were used in this study. They were cultured in RPMI medium 1640 (GIBCO® Invitrogen, No. 11875-093) supplemented with 5% fetal bovine serum (FBS, GIBCO® Invitrogen, No. 12484-010) and penicillin/streptomycin (GIBCO® Invitrogen, No. 10378-016; 10,000 U of penicillin and 10,000 μg of streptomycin per mL) in a humidified environment with 5% CO2 at 37°C.
Treatments
In the Rawq01 group, cells were treated with freshly prepared drug at a final concentration of IC50. In the Rawq01+mir-375 group, cells first underwent mir-375 transfection (mir-375 was purchased from the Shanghai GenePharma company [UUUGUUCGUUCGGCUCGCGUGA]). Twenty-four hours later, cells were then treated with Rawq01 at a final concentration of IC50.
MTT Assay
All of these cell lines (OVCAR3, HO-8910 and SK-OV-3) were seeded into 96-well plates (2×103 cells/well), followed by incubation overnight. Then, freshly prepared RAWQ01 at 100 μM, 50μM, 25 μM or 12.5μM were added to the corresponding cells. Forty-eight hours later, the cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. The absorbance was measured at 490 nm (A490) with a spectrophotometer. The concentration at which each drug produced 50% inhibition of growth (IC50) was estimated by the survival curve. Three independent experiments were performed in quadruplicate.
Apoptosis assay
Suspended cells were collected by centrifugation (1500 r/min for 5 min). These cells were washed twice with cold PBS and collected by centrifugation (1500 r/min for 5 min). These cells were re-suspended in 400 μl of 1×binding buffer to prepare single cell suspension at 1×106 cells/ml. Then, 5 μl of annexin V-FITC was added followed by mixing gently and incubation at 2-8°C for 15 min in dark. After adding of 10 μl of PI and incubation at 2-8°C in dark for 5 min, apoptosis was detected by flow cytometry within 1 h.
Western blot assay
Cells were harvested, washed once in PBS and lysed in the lysis buffer [25 mmol/L Tris-HCl (pH 7.4); 100 mmol/L NaCl; 2 mmol/L EDTA; 1% Triton X; 10 Ag/mL aprotinin, 10 Ag/mL leupeptin, 1 mmol/L Na3VO4, 1 mmol/L phenylmethyl sulfonyl fluoride]. SDS-PAGE and Western blot assay were done according to the standard procedures. β-actin on the same membrane served as an internal control. The protein bands were detected by secondary antibodies followed by labeling with ECL Detection System (GE Healthcare). The band density was determined by NIH-ImageJ.
Xenograft model of ovarian cancer
Animal experiments were conducted in accordance with the Guidelines for the Care and Use of Animals for research purposes. Female athymic nude mice (3-4 weeks old) were purchased from the Shanghai Laboratory Animal Co., Ltd (SLAC, Shanghai, China). The animals were housed and fed in accordance with the guidelines established by the National Science Council of China. SKOV3 cells (1×106 cells) were injected subcutaneously into the right axillary fossa. The mice were randomly divided into three groups (n = 5 per group): control group, Rawq01+control group, Rawq01+mir-375 group. In the control group, mice were treated with normal saline; in the Rawq01+control group, mice were treated with Rawq01 and normal saline; in the Rawq01+mir-375 group, mice were treated with Rawq01 and mir-375. Treatments were done intravenously once every other day for 21 days. The tumor size was measured every 4 days, and the tumor volume was calculated using the formula: V = L×W2/2; where V is the volume (mm3), L is the long diameter (mm) and W is the short diameter (mm). These mice were killed and the tumors were weighed at 21 days after inoculation.
Statistical analysis
Data were presented as the mean ± standard deviation (SD). Student’s t-test was performed for comparisons using SPSS version 14.0. A value of P < 0.05 was considered statistically significant.
Results
Over-expression of mir-375 sensitized ovarian cancer cells to RAWQ01
To investigate the growth inhibitory effect of RAWQ01 on the ovarian cancer cells, MTT assay was performed. After treatment with RAWQ01 at various concentrations (100 μM, 50 μM, 25 μM and 12.5 μM) for 48 h, the growth of ovarian cancer cells was significantly inhibited as compared to the control group.
Studies have reported that mir-375 is frequently down-regulated in many cancers including esophageal cancer, hepatocellular carcinoma, breast cancer, etc [19-22]. Restoration of mir-375 expression in these cancer cells might inhibit the proliferation of cancer cells by targeting PDK1 and IGF1R. Therefore, we hypothesized that mir-375 enhances the antitumor activity of ruthenium in the ovarian cancer cells. The baseline expression of mir-375 in ovarian cancer cell lines was detected by real time PCR. Results showed that the expression of mir-375 was at a very low level in the OVCAR3 cells, HO-8910 cells and SK-OV-3 cells. Then, transfection of mir-375 was introduced to these cancer cells by transfection. The expression of mir-375 in these cells was further confirmed by real-time PCR at 48 h after transfection. Furthermore, our results revealed that the restoration of mir-375 expression in ovarian cancer cell lines down-regulated the expression of both PDK1 and IGF1R (Figure 1A). Then, the cells undergoing transfection were exposed to Rawq01. As shown in Figure 1B, MTT assay revealed cells transfected with mir-375 exhibited significantly decreased IC50 as compared to those undergoing transfection of control miRNA, suggesting that mir-375 increases the sensitivity of ovarian cancer cells to Rawq01.
Figure 1.
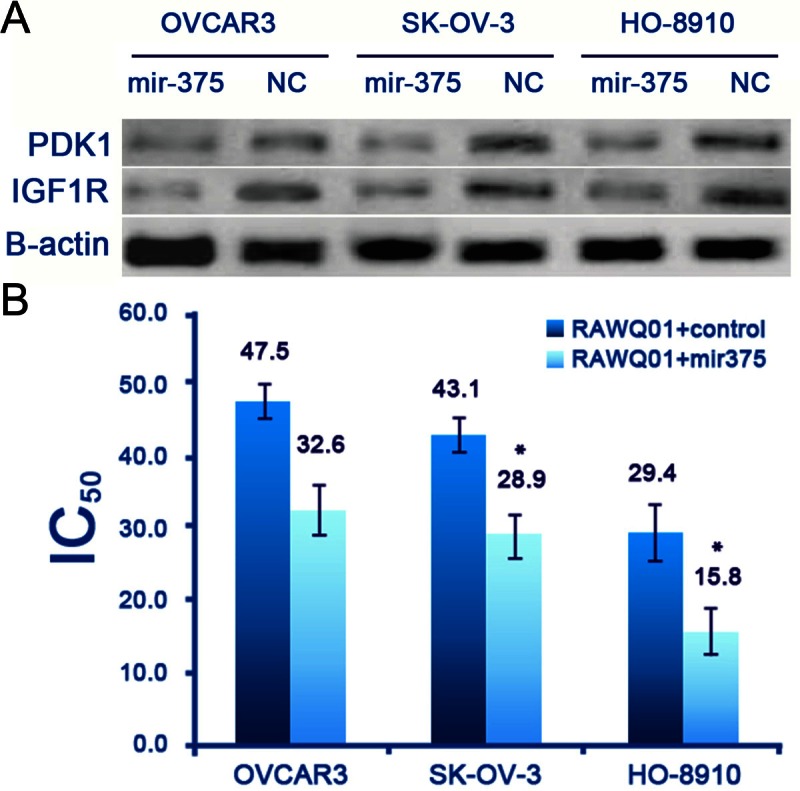
Mir-375 enhanced the growth inhibitory effect of RAWQ01 on ovarian cancer cells. A: Over-expression of mir-375 inhibited PDK1 and IGF1R expression in ovarian cancer cells. B: MTT assay revealed cells transfected with mir-375 exhibited significantly decreased IC50 as compared to those transfected with control miRNA. (*P < 0.05).
Mir-375 enhanced in vitro sensitivity of ovarian cancer cells to RAWQ01 by inducing apoptosis
The drug resistance of various cancer cells has been linked to a reduced susceptibility to drug-induced apoptosis, which was shown to be a consequence, at least in some cases, of over-expression of anti-apoptotic proteins, such as BCL2, IAPs, and BCL-XL [23-25]. Since mir-375 enhanced the growth inhibitory effect of RAWQ01, we hypothesized that mir-375 might play a role in the modulation of apoptosis. To validate this hypothesis, flow cytometry was performed to detect the apoptosis of ovarian cancer cells. In all cell lines undergoing mir-375 transfection, markedly increased apoptosis was observed after RAWQ01 treatment, when compared with those transfected with control miRNA (Figure 2A). In addition, the expression of Bcl-2 and Bcl-2-associated X protein (Bax) was also detected by Western blot assay. When compared with the cells without mir-37 transfection, the Bax expression was strikingly up-regulated but the Bcl-2 expression significantly down-regulated in cells transfected with mir-375 after RAWQ01 treatment (Figure 2B).
Figure 2.
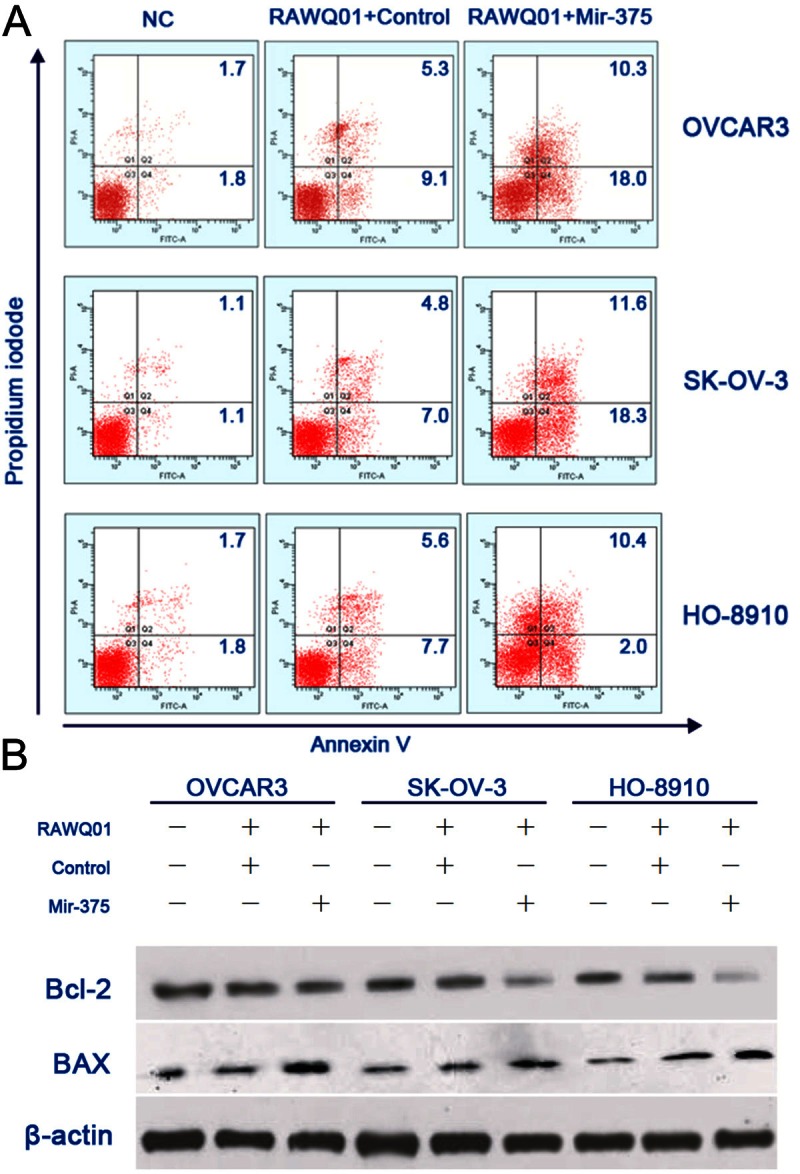
Mir-375 sensitized ovarian cancer cells to RAWQ01- induced apoptosis. A: Flow cytometry showed a marked increase in apoptosis in mir-375 transfected cells after RAWQ01 treatment. B: Expression of Bcl-2 and Bax was measured by Western blot assay. When compared with cells without mir-375 transfection, the Bax expression was strikingly up-regulated but the Bcl-2 expression significantly down-regulated in cells transfected with mir-375 mimic after RAWQ01 treatment.
Mir-375 increased in vivo chemosensitivity of ovarian cancer to RAWQ01
To explore the enhanced inhibitory effect of combined treatment on ovarian cancer cells, mir-375 precursor was cloned and transfected into SK-OV-3 cells. The expression of mature mir-375 was confirmed by quantitative PCR. Tumor xenografts developed after inoculation of mir-375 transfected SK-OV-3 cells were used to evaluate the antitumor effect of combined treatment in vivo, while control vector transfected cells served as controls. As shown in Figure 3, the tumor weight of mice with mir-375 xenograft was significantly reduced after RAWQ01 treatment when compared with mice inoculated with control cells, indicating that mir-375 enhanced the in vivo antitumor effect of RAWQ01.
Figure 3.
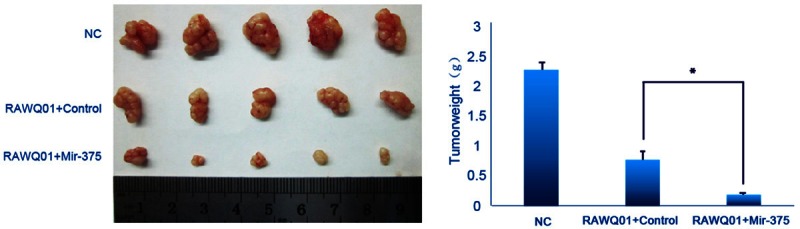
In vivo effects of mir-375 on chemosensitivity of ovarian cancer cells to RAWQ01. Tumors from each group were weighed immediately after removal. The average tumor weight was expressed as mean ± SD. The tumor weight of mice with mir-375 xenograft was significantly reduced after RAWQ01 treatment when compared with mice innoculated with control cells (*P < 0.05).
Discussion
Ovarian cancer is the fifth common gynecological malignancy and one of the leading causes of death in women. Due to the lack of effective strategies for early diagnosis, ovarian cancer is often detected at an advanced stage. Cisplatin-based chemotherapy has been a standard therapy for women with advanced epithelial ovarian cancer for more than two decades. The overall clinical response rate of cisplatin-based chemotherapy is about 67% and its failure is often associated with severe neurotoxicity, nephrotoxicity and gastrointestinal adverse effects as well as myelosuppression. Other drugs including paclitaxel, docetaxel, vinorelbine, irinotecan and gemcitabine are currently being used in combination with cisplatin to achieve better survival. However, the limited efficacy of cytotoxic chemotherapy remains a key obstacle in the treatment of advanced ovarian cancer [3,26,27]. Therefore, a reasonable strategy would be one that not only decreases the dose of chemotherapeutics but enhances the sensitivity of cancer cells to chemotherapeutics.
A great attention has been paid to the ruthenium compounds as an alternative to platinum-based tumor inhibitors. Due to the presence of the aromatic π-ligand which makes them easily penetrate the cell membrane and thus enhance the cellular uptake, organometallic arene ruthenium - based complexes have been considered as one of the most promising substitutes for cisplatinum as an anti-tumor drug in clinic practice with low toxicity to normal cells and high selectivity to cancer cells [28-31].
Rawq01 is a novel ruthenium-derived compound. Studies have shown that Rawq01 is effective in treatment of esophageal cancer [32]. Our findings clearly showed that Rawq01 was also effective to inhibit the growth of ovarian cancer cells and induce cell death.
MicroRNAs are a family of endogenously synthesized small non-coding RNAs (approx. 22 nucleotides in length) that regulate gene expression by 1) influencing the protein translational machinery and/or 2) inducing the degeneration of target mRNAs [13]. These miRNAs appear to play important roles in vital cellular processes such as development, differentiation, cell cycle, apoptosis, metabolism, and proliferation.
Increasing evidence suggests that miRNA may serve as a potential adjunct in the chemotherapy of cancer. Recently, it has been reported that mir-375 down-regulation is a common phenomenon in pancreatic cancer, hepatocellular cancer, gastric cancer, head and neck cancer and esophageal cancer [3,14,15,25,26]. Mir-375 can inhibit the proliferation, invasion and cell motility of cancer cells [14,15,29-31], implicating a anti-tumor activity of mir-375. In line with previous findings [14,15], our findings also revealed that mir-375 was down-regulated in ovarian cancer cells, which suggests that mir-375 may play a role as a potent inhibitor of ovarian cancer. Introduction of mir-375 to ovarian cancer cell lines (OVCAR3, HO-8910 and SK-OV-3) down-regulated the expression of both PDK1 and IGF1R.
The present study clearly indicated that mir-375 enhanced the Rawq01-induced growth inhibition and apoptosis of human ovarian cancer cells in vivo and in vitro.
In conclusion, our findings demonstrate that combined treatment with mir-375 and Rawq01 may sensitize ovarian cancer cells to chemotherapy. Therefore, combined therapy with mir-375 and Rawq01 may represent a new strategy for the clinical treatment of human ovarian cancer.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Muggia F. Platinum compounds 30 years after the introduction of cisplatin: implications for the treatment of ovarian cancer. Gynecol Oncol. 2009;112:275–281. doi: 10.1016/j.ygyno.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 3.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 4.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 5.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 6.Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- 7.Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 8.Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- 9.Shahab SW, Matyunina LV, Mezencev R, Walker LD, Bowen NJ, Benigno BB, McDonald JF. Evidence for the complexity of microRNA-mediated regulation in ovarian cancer: a systems approach. PLoS One. 2011;6:e22508. doi: 10.1371/journal.pone.0022508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hui A, How C, Ito E, Liu FF. Micro-RNAs as diagnostic or prognostic markers in human epithelial malignancies. BMC Cancer. 2011;11:500. doi: 10.1186/1471-2407-11-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Lin R, Li J. Epigenetic silencing of microRNA-375 regulates PDK1 expression in esophageal cancer. Dig Dis Sci. 2011;56:2849–2856. doi: 10.1007/s10620-011-1711-1. [DOI] [PubMed] [Google Scholar]
- 12.Kong KL, Kwong DL, Chan TH, Law SY, Chen L, Li Y, Qin YR, Guan XY. MicroRNA-375 inhibits tumour growth and metastasis in oesophageal squamous cell carcinoma through repressing insulin-like growth factor 1 receptor. Gut. 2012;61:33–42. doi: 10.1136/gutjnl-2011-300178. [DOI] [PubMed] [Google Scholar]
- 13.Anindo MI, Yaqinuddin A. Insights into the potential use of microRNAs as biomarker in cancer. Int J Surg. 2012;10:443–449. doi: 10.1016/j.ijsu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Jiang Q, Xia N, Yang H, Hu C. Decreased expression of microRNA-375 in nonsmall cell lung cancer and its clinical significance. J Int Med Res. 2012;40:1662–1669. doi: 10.1177/030006051204000505. [DOI] [PubMed] [Google Scholar]
- 15.Bergamo A, Masi A, Peacock AF, Habtemariam A, Sadler PJ, Sava G. In vivo tumour and metastasis reduction and in vitro effects on invasion assays of the ruthenium RM175 and osmium AFAP51 organometallics in the mammary cancer model. J Inorg Biochem. 2010;104:79–86. doi: 10.1016/j.jinorgbio.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Zelonka RA, Baird MC. Benzene Complexes of Ruthenium(II) Can J Chem. 1972;50:3063–3072. [Google Scholar]
- 17.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 18.Fan SH, Zhang AG, Ju CC, Gao LH, Wang KZ. A triphenylamine-grafted imidazo[4,5-f] [1,10] phenanthroline ruthenium(II) complex: acid-base and photoelectric properties. Inorg Chem. 2010;49:3752–3763. doi: 10.1021/ic902100v. [DOI] [PubMed] [Google Scholar]
- 19.Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, Yao H, Liu X, Ke Y, Si J, Zhou T. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res. 2010;20:784–793. doi: 10.1038/cr.2010.79. [DOI] [PubMed] [Google Scholar]
- 20.Liu AM, Poon RT, Luk JM. MicroRNA-375 targets Hippo-signaling effector YAP in liver cancer and inhibits tumor properties. Biochem Biophys Res Commun. 2010;394:623–627. doi: 10.1016/j.bbrc.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Mathe EA, Nguyen GH, Bowman ED, Zhao Y, Budhu A, Schetter AJ, Braun R, Reimers M, Kumamoto K, Hughes D, Altorki NK, Casson AG, Liu CG, Wang XW, Yanaihara N, Hagiwara N, Dannenberg AJ, Miyashita M, Croce CM, Harris CC. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res. 2009;15:6192–6200. doi: 10.1158/1078-0432.CCR-09-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris T, Jimenez L, Kawachi N, Fan JB, Chen J, Belbin T, Ramnauth A, Loudig O, Keller CE, Smith R, Prystowsky MB, Schlecht NF, Segall JE, Childs G. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am J Pathol. 2012;180:917–928. doi: 10.1016/j.ajpath.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia L, Zhang D, Du R, Pan Y, Zhao L, Sun S, Hong L, Liu J, Fan D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int J Cancer. 2008;123:372–379. doi: 10.1002/ijc.23501. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Yang D, Lippman ME. Targeting Bcl-2 and Bcl-XL with nonpeptidic small-molecule antagonists. Semin Oncol. 2003;30:133–142. doi: 10.1053/j.seminoncol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Reed JC. Drug insight: cancer therapy strategies based on restoration of endogenous cell death mechanisms. Nat Clin Pract Oncol. 2006;3:388–398. doi: 10.1038/ncponc0538. [DOI] [PubMed] [Google Scholar]
- 26.Fisher DE. Apoptosis in cancer therapy: crossing the threshold. Cell. 1994;78:539–542. doi: 10.1016/0092-8674(94)90518-5. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt CA, Lowe SW. Apoptosis is critical for drug response in vivo. Drug Resist Updat. 2001;4:132–134. doi: 10.1054/drup.2001.0188. [DOI] [PubMed] [Google Scholar]
- 28.Suss-Fink G. Arene ruthenium complexes as anticancer agents. Dalton Trans. 2010;39:1673–1688. doi: 10.1039/b916860p. [DOI] [PubMed] [Google Scholar]
- 29.Smith GS, Therrien B. Targeted and multifunctional arene ruthenium chemotherapeutics. Dalton Trans. 2011;40:10793–10800. doi: 10.1039/c1dt11007a. [DOI] [PubMed] [Google Scholar]
- 30.Hu W, Luo Q, Ma X, Wu K, Liu J, Chen Y, Xiong S, Wang J, Sadler PJ, Wang F. Arene control over thiolate to sulfinate oxidation in albumin by organometallic ruthenium anticancer complexes. Chemistry. 2009;15:6586–6594. doi: 10.1002/chem.200900699. [DOI] [PubMed] [Google Scholar]
- 31.Liu HK, Sadler PJ. Metal complexes as DNA intercalators. Acc Chem Res. 2011;44:349–359. doi: 10.1021/ar100140e. [DOI] [PubMed] [Google Scholar]
- 32.Chatterjee S, Kundu S, Bhattacharyya A, Hartinger CG, Dyson PJ. The ruthenium(II)-arene compound RAPTA-C induces apoptosis in EAC cells through mitochondrial and p53-JNK pathways. J Biol Inorg Chem. 2008;13:1149–1155. doi: 10.1007/s00775-008-0400-9. [DOI] [PubMed] [Google Scholar]


