Abstract
Chronic histiocytic intervillositis of the placenta (CHI) is a rare and potentially recurrent disease. Characteristically it shows accumulation of CD68+ cells in the intervillous space but no destructive tissue infiltration. An immunopathological background is likely but it is unknown what attracts circulating monocytes to the placenta. Methods: We analysed the expression profile of 102 inflammation- and angiogenesis-associated genes with real-time reverse transcriptase-polymerase chain reaction (RT-PCR) in 16 placentas: CHI (n = 5) and, as controls, villitis of unknown aetiology (VUE, n = 4) and normal placenta (n = 7). Results: Compared to controls, CHI had significantly higher levels of matrix metallopeptidase 9 (MMP9) and transforming growth factor, beta receptor 1 (TGFBR1). MMP14 was lower in VUE than CHI (p < 0.05) and controls (not significant). Chemokine (C-X-C motif) ligand 9 (CXCL9), CXCL12, chemokine (C-C motif) ligand 5 (CCL5) and TIMP metallopeptidase inhibitor 1 (TIMP1) were significantly higher in VUE versus controls but not deregulated in CHI. The expression profile could not clearly discriminate CHI from VUE or controls but a tendency for grouping of massive CHI was found. Angiogenesis-associated factors were not deregulated in CHI. Conclusion: The discrepancy of massive histiocytic accumulation and the lack of striking up-regulation of cytokines might be the basis of the non-destructive behaviour of the histiocytes in CHI.
Keywords: Cytokine, chemokine, histiocyte, monocyte, placenta pathology, chronic histiocytic intervillositis, massive perivillous histiocytosis
Introduction
Chronic histiocytic intervillositis of the placenta (CHI, synonymously massive chronic intervillositis or massive perivillous histiocytosis) is a rare lesion (< 1% of pregnancies) and has first been described in 1987 by Labarrere and Mullen [1]. Similar to villitis of unknown aetiology (VUE), CHI is thought to have an immunopathological background in terms of “graft” rejection [2,3]. The infectious counterpart of primary CHI is histiocytic intervillositis secondary to placental malaria or human herpesvirus 5/cytomegalovirus (HHV5/CMV) [4,5]. VUE-like lesions can be found in association with bacterial or viral infection, in particular Treponema pallidum and CMV [3,6]. CHI can occur in all trimesters while VUE tend to manifest in later stages of gestation [2,3]. Both diseases have an elevated risk of recurrence in subsequent pregnancies and, in particular in CHI, high rates of perinatal mortality or growth restriction [2,3].
Diagnosis is established exclusively post-natally by histological evaluation of placenta specimens [2,3]. While monocytes circulate in the peripheral blood, activated histiocytes normally infiltrate into tissues and phagotise vital and avital materials. The very characteristic histopathological feature of CHI is the accumulation of non-circulating histiocytic cells in the intervillous space which lacks infiltration and inflammatory destruction of the placenta tissue [1,7]. A few T cells and eosinophils may be present and intervillous fibrin deposition and trophoblastic necrosis are variable features [8-10]. Usually > 70% of the intervillous space is occupied by monocytic cells [8-10]. Minor forms, so-called focal CHI, have been described; in these cases histocytes accumulate in few areas of the placenta [8-10].
CHI and VUE are asymptomatic and the only known biomarker which can indicate presence of CHI is elevation of maternal serum levels of alkaline phosphatase (ALP), but ALP alone is not sufficient to establish the diagnosis [11]. The gold standard is histology/immunohistology [7]. Any therapy options, such as corticoid treatment [12], are applicable only for the following pregnancies, because the first diagnosis is usually made postnatally.
The objective of this study was to investigate whether inflammation-associated factors, in particular chemokines, can be detected in CHI. The identification of a histiocytic accumulation-associated factor could effectively serve as a biomarker for the diagnosis and activity of CHI.
Materials and methods
Study group
Placental samples from a total of 16 cases were included in this study: 5 CHI and, for control proposes, 4 VUE as well as 7 placentas without any histological sign of inflammation, in particular no chorioamnionitis, CHI or VUE. The latter 7 non-inflammatory control cases were age-matched for gestational age of CHI (mean 27, median 28 weeks, range 19-33) and VUE (mean 37, median 37 weeks, range 35-39) cases: 5/7 controls were matched for CHI (mean 27, median 28 weeks, range 20-33) and 2/7 controls (mean 37, median 37, range 36-38 weeks) were matched for VUE. Therefore, there was no difference regarding age of gestation between CHI/VUE and controls (each p > 0.05). Non-inflammatory control cases showed normal placenta maturation for the individual age of gestation and, similar to CHI, included preterm pregnancies.
All placental samples, including controls, had been evaluated as part of standard clinical care. Samples were formalin-fixed and paraffin-embedded (FFPE) and were retrieved from the tissue archive of the Institute of Pathology, Hannover Medical School.
The retrospective analysis of these clinical samples, after the diagnosis had been established, was approved by the local Ethics Committee, Hannover Medical School.
Immunohistochemistry and additional analysis for exclusion of virus infection
Placental tissue was evaluated with the following panel of antibodies: CD68, CD15, CD3, CD4, CD8, CD1a, CD56 (neural cell adhesion molecule 1/NCAM), CD25 (interleukin 2 receptor, alpha/ IL2RA), CD123 (interleukin 3 receptor, alpha (low affinity)/IL3RA), CD20 or CD79a and complement factor C4d. Virus infection was evaluated by immunohistochemistry for CMV in CHI and VUE. Furthermore, in CHI immunohistochemistry was performed for human herpes virus 1 and 2/herpes simplex virus 1 and 2 (HHV1-2/HSV1-2), human herpes virus 4/Epstein-Barr virus (HHV4/EBV) was tested with EBER in situ hybridisation and human papilloma viruses (HPV types 6, 11, 16, 18, 31, 33, 35, 39, 42, 44, 45, 51-54, 56, 58, 59, 61, 62, 66-68, 70, 72, 73, 81-84, 90, 91) were analysed with a PCR-based assay from FFPE tissue-extracted DNA as described [13].
RNA extraction and transcript expression assays
A total of 102 target gene transcripts were analysed. Inflammation-associated genes (n = 67/102) were evaluated in all cases while angiogenesis-associated genes (n = 35/102) were analysed in four CHI and three control cases.
Placental tissue samples (each 2-4 20 μm sections from FFPE blocks) were digested overnight at 55°C in 500 μL lysis solution and 500 μL proteinase K (50 μg). After organic extraction by phenol/chloroform, total RNA was precipitated from the aqueous phase with isopropanol and glycogen for at least 24 h at -20 °C. The RNA quality and concentration was determined with a Nanodrop 2000 (Thermo Scientific, Wilmington, DE, USA) and 20 ng RNA was used for real-time reverse transcriptase-polymerase chain reaction (RT-PCR)-based expression analysis. Two arrays were used which contained fibrosis-associated genes (a modified version of the previously used array [14,15]), angiogenesis-associated genes and, for each array, endogenous control gene polymerase (RNA) II (DNA directed) polypeptide A, 220kDa (POLR2A). PCR reagents and arrays were used as recommended by the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA). RT-PCR was performed with a 7900HT Fast Real-Time PCR system (Applied Biosystems) as described [14,15].
Data analyses
The sample- and detector-specific evaluation of amplification curves was accomplished with the RQ Manager 1.2 software (Applied Biosystems). CT values established in this manner were converted into ΔCT values and into 2-ΔCT values (normalised to endogenous control gene POLR2A). Statistical analysis was performed with Prism 5.0 (GraphPad Software, San Diego, CA, USA) by applying the non-parametric Kruskal-Wallis test followed by Dunns’ post test for comparison of multiple groups and Mann-Whitney test for two-group comparison. P values < 0.05 were considered as statistically significant. Cluster analysis was performed with the Qlucore Omics Explorer 2.2 (Qlucore AB, Lund, Sweden).
Results
Morphological characteristics of CHI cases
The age of gestation was higher in VUE than in CHI (mean 37 versus 27 weeks; p = 0.0159).
Among the CHI, 3/5 have been characterised in our previous work (cases #1, #3 and #4 in Traeder et al. [8]); tissue samples of one of the previously described cases was not available for analysis (external case). In the last two years we have diagnosed two additional cases (#5, #6) which were included in this analysis (Table 1, Figure 1). Both showed typical accumulation of intervillous CD68+ cells with minor fibrin deposition, few T cells (equally CD4+ and CD8+, few CD25+ cells) and few eosinophils. In these cases, a subfraction of histiocytes weakly expressed CD4 and were negative for CD1a and CD123. Minor C4d and fibrin deposition did not appear to be different among CHI, VUE and controls.
Table 1.
Clinical and morphological characteristics of pregnancies with CHI of the placenta
| Cases | #5 | #6 |
|---|---|---|
| Maternal data (age of mother) | Gravida I, Para 0 (25 years) | Gravida III, Para I (35 years) |
| Relevant history | 6 years earlier: abortion (14th week of gestation) | Adiposities, allergy, recurrent miscarriages |
| Course of pregnancy | Foetal growth restriction, premature rupture of membranes in the 18+6 week of gestation, anhydramnios | Foetal growth restriction, oligohydramnios, alkaline phosphatase level 625 U/l (31+6 week of gestation) |
| Mode of delivery (gestation age, week+day) | Induction of abortion after severe complications (19+1) | Caesarean section (32+1) |
| Gender of child (birth weight, percentile) | Male (137 g, < 3) Post mortem examination: no malformation | Male (835 g, < 3) No malformation |
| Apgar score | Not applicable | 8/9/10 |
| Placenta parameters (percentile) | 10×6×2.5 cm, 47 cm2 (< 10), 87 g (< 10) | 11×11×2 cm, 95 cm2 (< 10), 229 g (< 10) |
| Diagnosis of placenta (% of intervillous space occupied by histiocytes) | CHI (~90%) +acute chorioamnionitis | CHI (30-40%) |
Note that CHI cases #1-#4 have been described earlier by us (Traeder et al., 2010).
Figure 1.
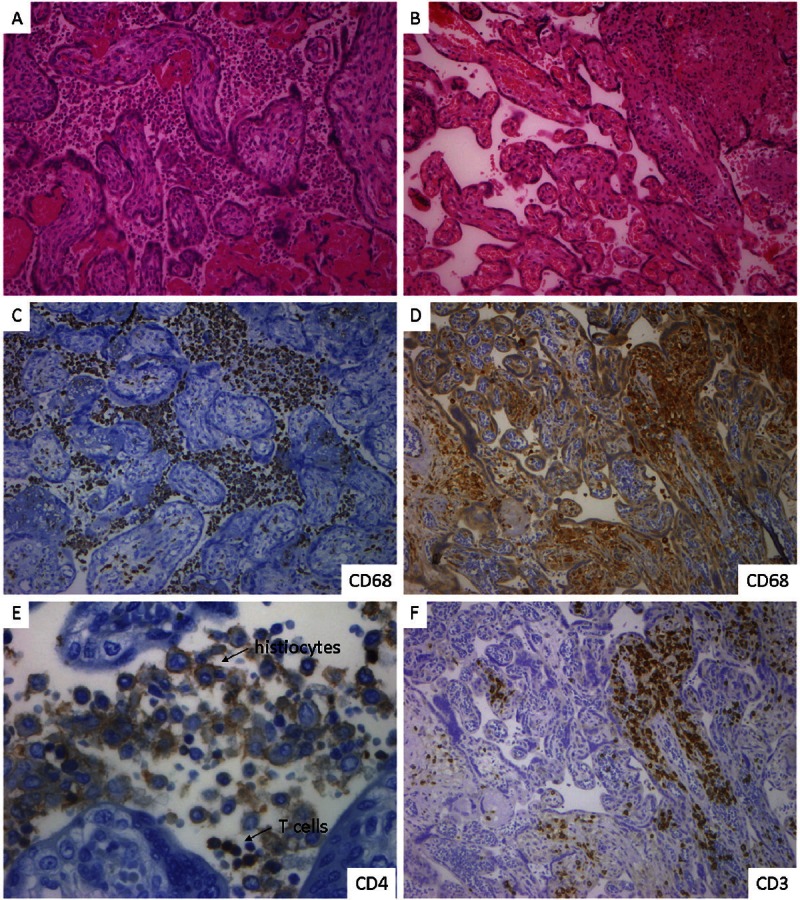
Histology and immunohistology of CHI and VUE. CHI-placenta of case #5 shows massive histiocytic infiltration of the intervillous space with no infiltration of the villi (A, haematoxylin-eosin/HE, x100). The majority of cells are CD68+ histiocytes (C, x100). A subfraction of histocytes show a weak membranous CD4 expression. Only very few T cells are intermingled, equally CD4+ and CD8+ cells (E, CD4 is depicted, x400). VUE shows patchy infiltration of villi by small to medium sized leukocytes but no accumulation of intervillous histiocytes (B, HE, x100). The intravillous cells are CD68+ histiocytes (D, x100) and T cells (F, x100). Images were produced with a DP71 Camera (Olympus, Hamburg, Germany) on an Axiophot microscope with Plan-Neofluar 10×/0.30 and 40×/0.75 objectives (Zeiss, Jena, Germany), image processing with Soft Imaging System software (Olympus, Hamburg, Germany).
In case #5, the chronic disease was complicated by additional acute chorioamnionitis after preterm rupture of membranes. In this case, abortion had been initiated and ALP was not determined. In case #6, shortly before birth, the ALP level was 625 U/l.
None of the viruses under investigation (CMV, HSV, EBV, HPV) were detectable. Hepatitis viruses B and C (HBV, HCV) and human immunodeficiency virus (HIV) infection had been excluded during pregnancy in CHI cases.
Expression of inflammatory factors in CHI and VUE
All samples yielded sufficient quality and amount of RNA (determined with a Nanodrop 2000) for further transcript expression analysis (determined by expression of endogenous control genes; mean CT value for POLR2A 33.4).
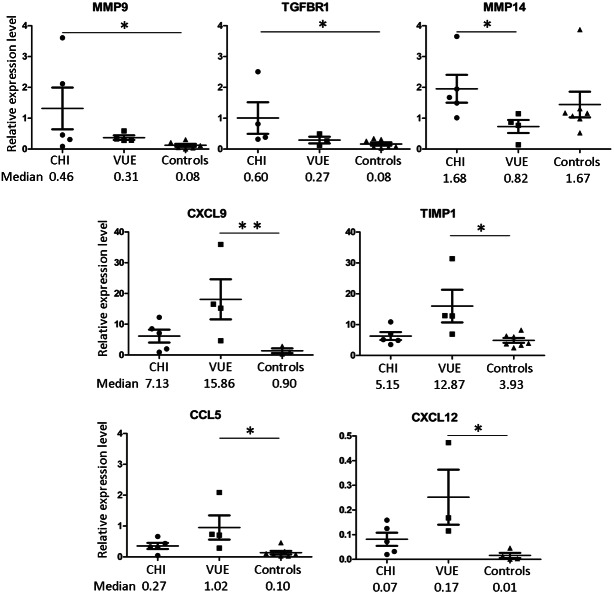
In CHI and VUE the 10 inflammation-associated factors with the highest mean relative expression levels were as follows: thrombospondin 1 (THBS1, involved in cell-to-cell and cell-to-matrix interactions), chemokine (C-X-C motif) ligand 9 (CXCL9, involved in T cell trafficking), TIMP metallopeptidase inhibitor 1 (TIMP1, anti-fibrogenic factor), transforming growth factor, beta 1 (TGFB1, among other functions the protein regulates cell adhesion and migration), bone morphogenetic protein receptor, type IB (BMPR1B, receptor of matrix modulating BMPs; note that this receptor was expressed in only a small subfraction of cases), collagen, type III, alpha 1 (COL3A1, component of the placental extracellular matrix), chemokine (C-C motif) ligand 3 (CCL3, involved in inflammatory response), serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 (SERPINE1, inhibitor of fibrinolysis), TIMP2 (involved in extracellular matrix remodelling) (Table 2, Supplementary Table 1). Signi-ficant differences (p < 0.05) were found for matrix metallopeptidase 9 (MMP9; 11-fold mean up-regulation in CHI versus controls) and transforming growth factor, beta receptor 1 (TGFBR1; 6-fold mean up-regulation in CHI versus controls) as well as MMP14 (3-fold mean up-regulation in CHI versus VUE) (Figure 2). CXCL9 (mean 13-fold), CXCL12 (mean 12.5-fold), CCL5 (mean 7-fold) and TIMP1 (mean 3-fold) were significantly higher in VUE versus controls but not deregulated in CHI (Figure 2). The monocyte attractor CCL2 and other factors were not significantly deregulated in CHI and VUE.
Table 2.
Summary of the 10 highest mean expressed inflammation-associated genes in CHI
| CHI (n = 5), median 28 weeks of gestation weeks (range 19-33) | VUE (n = 4), median 37 weeks of gestation weeks (range 35-39) | Control (n = 7), median 30 weeks of gestation (range 20-38) | |
|---|---|---|---|
| THBS1 | 6.70 (4.73, 2.18-15.16) | 8.08 (8.75, 3.81-11.01) | 6.15 (4.60, 1.78-16.30) |
| CXCL9 | 6.15 (7.13, 0.90-12.24) | 18.09 (15.86, 4.67-35.96) | 1.37 (0.90, 0.35-2.88) |
| TIMP1 | 6.30 (5.15, 3.54-10.88) | 16.02 (12.87, 6.93-31.39) | 4.90 (3.93, 2.53-8.25) |
| TGFB1 | 6.08 (5.11, 2.01-10.01) | 6.57 (5.15, 3.21-12.78) | 4.33 (4.31, 1.64-8.68) |
| BMPR1B | 5.47 (ND in 3/5 cases) | 5.52 (ND in 3/4 cases) | 0.27 (ND in 6/7 cases) |
| COL3A1 | 4.43 (4.00, 2.41-8.58) | 3.46 (3.36, 1.05-6.09) | 9.26 (5.71, 3.77-25.94) |
| CCL3 | 3.62 (0.27, 0.08-17.02) | 3.93 (1.02, 0.19-13.50) | 0.17 (0.10, 0.03-0.54) |
| SERPINE1 | 3.32 (2.57, 1.12-8.00) | 3.53 (3.47, 1.51-5.65) | 4.38 (4.37, 1.43-7.45) |
| HGF | 2.87 (2.38, 1.76-5.45) | 1.58 (1.54, 1.08-2.15) | 2.84 (2.40, 1.31-7.12) |
| TIMP2 | 2.29 (1.40, 0.27-4.40) | 2.03 (1.92, 1.06-3.21) | 2.05 (1.89, 0.87-3.65) |
Gene names are described in the text. The mean (median, range) relative expression levels (2-ΔCT values normalised to endogenous control gene POLR2A) are listed. An overview of all analysed genes is provided in Supplementary Tables 1 and 2. Abbreviation: not detectable (ND).
Figure 2.
Deregulated expression of inflammation-associated genes in CHI and VUE. Mean and standard deviation are depicted and median values are summarised. Abbreviations: p < 0.05 (*), p < 0.01 (**).
Cluster analysis revealed a tendency for grouping of CHI with VUE. In particular, massive CHI cases #1, #3, #5 and #6 shared a similar expression profile while focal CHI case #4 had a profile more similar to two VUE than to massive CHI, e.g. focal CHI showed the lowest MMP9 expression level among all CHI. However, the transcript profile of genes under investigation could not clearly discriminate between CHI and VUE or controls (Supplementary Figure 1).
Angiogenesis-associated factors are not deregulated in CHI
In CHI and controls, the five angiogenesis-associated factors with the highest mean relative expression levels were as follows: fms-related tyrosine kinase 1 (FLT1, vascular endothelial growth factor/vascular permeability factor receptor), platelet/endothelial cell adhesion molecule 1 (PECAM1/CD31), podoplanin (PDPN), polymerase I and transcript release factor (PTRF) and hypoxia inducible factor 1, alpha subunit (HIF1A). These genes and all other genes under investigation showed no significant deregulation in CHI versus controls (Supplementary Table 2).
Discussion
The basis of severe CHI-associated complication (foetal growth restriction, intrauterine death) is placental insufficiency due to decreased perfusion of the intervillous space as well as the increased distance between maternal erythrocytes and foetal surface of the villi. The pathophysiology of aberrant intervillous histiocytic cell accumulation in CHI is still not known [2]. An overt underlying maternal autoimmune disease or assisted reproduction fertilisation do not appear to be major risk factors [8,11]. It is thought that micro-lesions of the trophoblast and activation of complement factors may initiate the inflammatory processes [1,2]. However, trophoblast degeneration and fibrin deposition are present at variable extents in CHI and, to a certain degree, are almost normal in late placental development [1,2,8-12]. In our previous analysis, we found histiocytic expression of membranous and cytoplasmic complement cascade-associated factor CD55 in CHI but also in acute granulocytic placentitis [8]. Therefore, a complement-associated immunological process might be involved in CHI but CD55 expression or complement factor deposition is not specific for CHI. Acute atherosis-like lesions and IgM deposition have been linked to the pathobiology of CHI [1] but we could not find any hint that altered angiogenesis might be a major defect in CHI.
Currently, the only biomarker which can indicate presence of CHI is the increase of the ALP level above 600 U/l [11] and this was also present in one case of this current analysis. However, ALP elevation is not specific (absent in > 40% of cases) [11] and, therefore, a marker for disease and disease activity is still lacking. In this current analysis, we hypothesised that local cytokine secretion and production of other inflammation-associated factors play a major role in attracting circulation monocytes to the placenta. CHI is dominated by intervillously “resting” histiocytes and only few T cells are present, while in VUE the intravillous infiltration is predominated by T cells and equivalent amounts of histiocytes [1-3,6-12]. In VUE, the T cells are of maternal origin and induce activation of foetal histiocytes/Hofbauer cells, as could be demonstrated by XY fluorescence in situ hybridisation [3,6,16]. In CHI, as could be expected, histiocytes are of maternal origin and a significant involvement of foetal histiocytes/Hofbauer cells could not be found [8]. It is remarkable that, despite striking histopathological differences and the different origin of involved leukocytes, on the one hand CHI shows only minor differences with normal placenta and on the other hand only minor differences with VUE. While CCL2, a chemoattractant and activator of monocytes/histiocytes and T cells [17], and other inflammation-associated cytokines were not increased, we found an increase of TGFBR1 expression in CHI. This might indicate that up-regulation of inflammation-associated receptors could contribute to histiocytic accumulation by sensitising the cells for local cytokines such as TGFB1 and TGFB2. In addition to TGFBR1, MMP9 was increased in CHI. Leukocyte-derived MMP9 is activated via MMP13 by MMP2 and MMP9 and MMP2 share similar functions [18]. Both factors can not only cleave extracellular matrix components but also cytokines such as tumour necrosis factor and TGFB1 [18]. However, MMP2 production was not deregulated and MMP13 was not expressed, neither in CHI nor in VUE or controls. In addition to its cleavage function, MMP9 is involved in haematopoietic stem cell mobilisation [18], while another factor which also mobilises cells, IL8, was not markedly up-regulated in CHI. Furthermore, MMP9 is not specifically involved in CHI but can also be detected in acute chorioamnionitis [19]. Taken together, TGFBR1 and MMP9 can contribute to CHI but are unlikely to be the major drivers of histiocytic accumulation.
VUE showed up-regulation of a different set of cytokines than CHI, including CXCL9 and CCL5. The only study which has analysed cytokine expression in VUE also revealed increased expression of CXCL9 and CCL5 as well as up-regulation of CXCL10, CXCL11, CXCL13, CCL4, CXCR3 and CCR5 [20]. In our analysis, we also found elevated levels of CXCL10 and CCL4 in VUE and CHI but, in comparison to controls, the differences were not significant (CXCL11, CXCL13 and CXCR3 were not part of the analysis platform used in our study). Paralleling the inflammatory processes in the stroma of the villi, TIPM2 were increased in VUE while MMP14 was decreased, which is likely to be associated with focal matrix remodelling in the affected villi. Of note, although focal CHI showed an expression profile similar to VUE, these two genes were not specifically increased/decreased in focal CHI since histiocytes did not invade the stroma.
The discrepancy of massive histiocytic accumulation and the lack of striking up-regulation of cytokines (e.g. CCL2) and other factors might be the basis of the non-destructive behaviour of the histiocytes and prevent tissue infiltration and increased attraction of other leukocytes.
Administration of low-dose acetyl salicylic acid (ASA) helps to prevent severe preeclampsia and foetal growth retardation [21] and ASA in combination with corticoids or corticoids alone can also prevent CHI and VUE [12]. However, the therapeutic result regarding CHI and VUE are based on case reports [12,21]. In our study, we found very low expression levels of the ASA-target gene prostaglandin-endoperoxide synthase 1 (PTGS1/COX1). It cannot be excluded that PTGS1 proteins, for example in platelets, could be targeted by ASA and prevent fibrin accumulation. In summary, our knowledge on prevention is still sparse.
It will be interesting to continue the research on CHI by evaluating leukocyte adhesion molecules and receptors while, due to the rarity of this placenta lesion and therefore limited samples, serum profiling of proteins will be difficult. However, even up-regulation of such molecules might be secondary to a still unknown (autoimmune) trigger which is responsible for this unusual histiocytic disease.
Conclusion
CHI is a rare disease of still unknown aetiology which is associated with increased MMP9 and TGFBR1 expression in a subfraction of cases, but CHI is not mediated by CCL2 or other inflammation-associated factors. Therefore a chemokine suitable for the diagnosis of CHI or clinical management in pregnancy is not yet available.
Acknowledgements
The authors would like to thank Dr. Henning Feist (formerly Institute of Pathology, Hannover Medical School).
Authors’ contribution: Conception of the analysis (LF, KH), histopathology (KH, HK), clinical data (LF, CvK, KH), molecular analysis (KH), interpretation of data, manuscript preparation (LF, CvK, HK, KH).
Supporting Information
References
- 1.Labarrere C, Mullen E. Fibrinoid and trophoblastic necrosis with massive chronic intervillositis: an extreme variant of villitis of unknown etiology. Am J Reprod Immunol Microbiol. 1987;15:85–91. doi: 10.1111/j.1600-0897.1987.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 2.Contro E, deSouza R, Bhide A. Chronic intervillositis of the placenta: a systematic review. Placenta. 2010;31:1106–10. doi: 10.1016/j.placenta.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Redline RW. Villitis of unknown etiology: noninfectious chronic villitis in the placenta. Hum Pathol. 2007;38:1439–46. doi: 10.1016/j.humpath.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive chronic intervillositis of the placenta associated with malaria infection. Am J Surg Pathol. 1998;22:1006–11. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Taweevisit M, Sukpan K, Siriaunkgul S, Thorner PS. Chronic Histiocytic Intervillositis with Cytomegalovirus Placentitis in a Case of Hydrops Fetalis. Fetal Pediatr Pathol. 2012 Dec;31:394–400. doi: 10.3109/15513815.2012.659405. [DOI] [PubMed] [Google Scholar]
- 6.Kapur P, Rakheja D, Gomez AM, Sheffield J, Sanchez P, Rogers BB. Characterization of inflammation in syphilitic villitis and in villitis of unknown etiology. Pediatr Dev Pathol. 2004;7:453–8. doi: 10.1007/s10024-004-2124-3. [DOI] [PubMed] [Google Scholar]
- 7.Heller DS. CD68 immunostaining in the evaluation of chronic histiocytic intervillositis. Arch Pathol Lab Med. 2012;136:657–9. doi: 10.5858/arpa.2011-0328-OA. [DOI] [PubMed] [Google Scholar]
- 8.Traeder J, Jonigk D, Feist H, Bröcker V, Länger F, Kreipe H, Hussein K. Pathological characteristics of a series of rare chronic histiocytic intervillositis of the placenta. Placenta. 2010;31:1116–9. doi: 10.1016/j.placenta.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 9.Rota C, Carles D, Schaeffer V, Guyon F, Saura R, Horovitz J. Perinatal prognosis of pregnancies complicated by placental chronic intervillitis. J Gynecol Obstet Biol Reprod (Paris) 2006;35:711–9. doi: 10.1016/s0368-2315(06)76468-7. [DOI] [PubMed] [Google Scholar]
- 10.Doss BJ, Greene MF, Hill J, Heffner LJ, Bieber FR, Genest DR. Massive chronic intervillositis associated with recurrent abortions. Hum Pathol. 1995;26:1245–1251. doi: 10.1016/0046-8177(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 11.Marchaudon V, Devisme L, Petit S, Ansart-Franquet H, Vaast P, Subtil D. Chronic histiocytic intervillositis of unknown etiology: clinical features in a consecutive series of 69 cases. Placenta. 2011;32:140–5. doi: 10.1016/j.placenta.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 12.Boog G, Le Vaillant C, Alnoukari F, Jossic F, Barrier J, Muller JY. Combining corticosteroid and aspirin for the prevention of recurrent villitis or intervillositis of unknown etiology. J Gynecol Obstet Biol Reprod (Paris) 2006;35:396–404. doi: 10.1016/s0368-2315(06)76411-0. [DOI] [PubMed] [Google Scholar]
- 13.Pilatz A, Altinkilic B, Rusz A, Izykowski N, Traenkenschuh W, Rische J, Lehmann U, Herbst C, Maegel L, Becker J, Weidner W, Jonigk D. Role of human papillomaviruses in persistent and glucocorticoid-resistant juvenile phimosis. J Eur Acad Dermatol Venereol. 2013;27:716–21. doi: 10.1111/j.1468-3083.2012.04542.x. [DOI] [PubMed] [Google Scholar]
- 14.Bock O, Muth M, Theophile K, Winter M, Hussein K, Büsche G, Kröger N, Kreipe H. Identification of new target molecules PTK2, TGFBR2 and CD9 overexpressed during advanced bone marrow remodelling in primary myelofibrosis. Br J Haematol. 2009;146:510–20. doi: 10.1111/j.1365-2141.2009.07808.x. [DOI] [PubMed] [Google Scholar]
- 15.Jonigk D, Merk M, Hussein K, Maegel L, Theophile K, Muth M, Lehmann U, Bockmeyer CL, Mengel M, Gottlieb J, Welte T, Haverich A, Golpon H, Kreipe H, Laenger F. Obliterative airway remodeling: molecular evidence for shared pathways in transplanted and native lungs. Am J Pathol. 2011;178:599–608. doi: 10.1016/j.ajpath.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Z, Abrahams VM, Mor G, Guller S. Placental Hofbauer cells and complications of pregnancy. Ann N Y Acad Sci. 2011;1221:103–8. doi: 10.1111/j.1749-6632.2010.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–26. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirvaikar N, Marquez-Curtis LA, Janowska-Wieczorek A. Hematopoietic Stem Cell Mobilization and Homing after Transplantation: The Role of MMP-2, MMP-9, and MT1-MMP. Biochem Res Int. 2012;2012:685267. doi: 10.1155/2012/685267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oh KJ, Park KH, Kim SN, Jeong EH, Lee SY, Yoon HY. Predictive value of intra-amniotic and serum markers for inflammatory lesions of preterm placenta. Placenta. 2011;32:732–6. doi: 10.1016/j.placenta.2011.07.080. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Romero R, Kim CJ, Tarca AL, Chhauy S, LaJeunesse C, Lee DC, Draghici S, Gotsch F, Kusanovic JP, Hassan SS, Kim JS. Villitis of unknown etiology is associated with a distinct pattern of chemokine up-regulation in the feto-maternal and placental compartments: implications for conjoint maternal allograft rejection and maternal anti-fetal graft-versus-host disease. J Immunol. 2009;182:3919–27. doi: 10.4049/jimmunol.0803834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberge S, Giguère Y, Villa P, Nicolaides K, Vainio M, Forest JC, von Dadelzen P, Vaiman D, Tapp S, Bujold E. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol. 2012;29:551–6. doi: 10.1055/s-0032-1310527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.