Abstract
Aims: The molecular mechanisms of the tumorigenesis and recurrence of cervical cancer are poorly understood. The objective of this study was to analyze the expression of phosphorylated c-Src (phospho-c-Src) and its clinical significance in human cervical cancer. Methods: The expression of phospho-c-Src was determined by immunohistochemistry in a total of 127 cervical specimens including 20 normal cervix tissues, 20 cases of carcinoma in situ of cervix (CIS), and 87 cases of cervical squamous cell carcinoma (CSCC). Results: The expression of phospho-Src in normal cervix, CIS, and CSCC increased gradually in ascending order (p=0.026). In addition, the expression of phospho-Src was correlated with overall (p=0.037) and recurrence (p=0.001) survival of cervical cancer. In multivariate Cox regression analysis, phospho-Src expression was an independent prognosis factor for recurrence-free survival (p=0.004). Conclusion: Our present study suggests that Src signaling may play essential role in cervical cancer progression. Phospho-Src expression may be considered as a prognostic marker to predict recurrence in CSCC.
Keywords: Phospho-Src, cervical squamous cell carcinoma, prognosis, recurrence
Introduction
Cervical squamous cell carcinoma (CSCC) is one of the most frequent malignant tumors in the female reproductive organs and accounts for 90% of cervical cancers [1]. Early stage CSCC could be cured on a rate of more than 80% with either radical resection or radiation [2], yet recurrence remains probably one of the most difficult challenges in the treatment of CSCC. The reported recurrence rates of stage I-II cervical cancer after radical hysterectomy or radiotherapy are between 10 and 18% [3]. Generally, the clinical outcome of recurrent cervical cancer patients is poor, with the 5-year survival rates ranging from 3.2 and 22.3% [4-6]. Although extensive researches have been done, there is a lack of therapeutic targets that can improve the treatment outcomes of recurrent cervical cancer.
Src belongs to a family of non-receptor intracellular tyrosine kinase proteins. It is consisted of seven parts including SH1-SH4 domain, unique domain, SH2-SH3 linker, and a c-terminal negative regulatory region [7]. Recent reports showed that Src kinase exert multifaceted functions by interacting with tyrosine kinase receptors, such as VEGF and EGFR receptor, or via a variety of intracellular signaling pathways involved in tumor cell growth, cell motility, angiogenesis, and invasion during tumor development [8]. Overexpression of Src has been associated with enhanced cancer cell growth, while depletion of Src by antisense oligonucleotides results in reduced cell division [9]. Indeed, tumor cells expressing higher levels of activated Src are associated with tumor invasiveness and metastasis both in vivo and in vitro models [10]. In contrast, inhibition of Src kinase significantly repressed the invasive and metastatic behavior of cancer cells [11]. Moreover, increased expression of Src kinase has been associated with the development and progression of a number of human malignancies, including pancreatic, colon, ovarian and breast carcinoma [7,12]. Stabile et al. demonstrated that the expression of phospho-c-Src is correlated with poor differentiation and lymph node metastases in patients with head and neck squamous cell carcinoma [13]. Campbell et al. suggested that nuclear localization of phosphorylated c-Src is associated with improved clinical outcome in patients with ER-positive breast cancer [14]. Wilson et al. shown that c-Src activation is correlatd with tumour grade, tumor cell proliferation, and HER2 expression in ductal carcinoma in situ [15]. Furthermore, elevated Src kinase activity contributes to attenuated response to endocrine therapy in breast cancer [16]. These studies together indicate the important role of Src kinase in tumor progression and potential therapeutic sensitivity of human malignancies.
Although increased phospho-Src expression has been found in cervical cell lines and clinical cervical cancer tissues, and downregulation of phospho-Src led to cell growth inhibition [17], elucidation of the biological functions of Src kinase expression on the tumor progression and prognosis of cervical cancer remains incomplete. In the present study, we investigated the expression of phospho-Src in histological sections of 87 cases of CSCC, 20 cases of carcinoma in situ of cervix, and 20 cases of normal cervix tissues using immunohistochemical technique. We report for the first time the correlation of phospho-Src expression and poor prognosis in patients with CSCC. Multivariate analysis suggested that phospho-Src expression was an independent prognostic marker for recurrence in patients with CSCC. Our data strongly suggest that Src family kinase may play essential role in cervical cancer progression, and that phospho-Src may be a valuable marker for predicting postoperative recurrence in CSCC patients.
Material and methods
Patients and tissue specimens
A total of 127 paraffin-embedded samples were obtained at Sun Yat-Sen University Cancer Center between Jan 2005 and Jan 2006. The samples were composed of 20 normal cervical tissues, 20 CIS, and 87 CSCC. For the use of these clinical materials for research purposes, prior patient consent and approval from the Institutional Research Ethics Committee were obtained. None of the patients had received chemotherapy or radiotherapy before surgery. The clinical characteristics of the 87 patients with CSCC are listed in Table 1. The disease stage of each patient was classified or reclassified according to the International Federation of Gynecology and Obstetrics criteria as follows: 30 were allocated to stage IB1, 30 to stage IB2, 13 to stage IIA1, 8 to stage IIA2, and 6 to stage IIB. The median age of the patients was 42.9 years (range 26-64 years).
Table 1.
Expression of phospho-Src in CSCC, CIS, and normal cervix
| Characteristics | No. | phospho-Src (+) N (%) | p |
|---|---|---|---|
| CSCC | 87 | 18 (20.7) | 0.026 |
| CIS | 20 | 1 (5.0) | |
| Normal cervix | 20 | 0 (0.0) |
Immunohistochemistry
Immunohistochemistry was done to study phospho-Src expression in 87 human CSCC, 20 CIS, and 20 normal cervical tissues. In brief, paraffin-embedded specimens were cut into 4 μm sections and mounted on poly-L-lysine-coated slides. The sections were baked at 65°C for 30 minutes, then deparaffinized in xylene and rehydrated. Antigen retrieval was performed by submerging the sections into a 10 μmol/L citrate buffer solution (pH 6.0) for 10 minutes in a microwave oven. The slides were then treated with 3% hydrogen peroxide in methanol to quench the endogenous peroxidase activity, followed by incubation with 1% fish skin gelatin to block the nonspecific staining. Tissue sections were incubated overnight with polyclonal rabbit antibody against phospho-Src (Tyr416) (Cell Signaling Technology, USA; 1:60). After washing, the sections were incubated with prediluted anti-rabbit secondary antibody (Zymed, San Francisco, CA), followed by further incubation with 3,3-diaminobenzidine tetrahydrochloride (DAB). Finally, the slides were counterstained with 10% Mayer’s hematoxylin and mounted in Crystal Mount.
The degree of immunostaining of formalin-fixed, paraffin-embedded sections was reviewed and scored by two independent observers. The proportion of the stained cells and the extent of the staining were used as criteria of evaluation. The intensity of membranous and cytoplasmic staining was graded semi-quantitatively into four levels: 0 (no staining); 1 (weak staining = light yellow); 2 (moderate staining = yellow brown) and 3 (strong staining = brown); and the percentage of stained cells was scored as: 0, negative; 1, 10% or less; 2, 11% to 50%; 3, 51% to 80%; or 4, 80% or more positive cells. A final score was defined by multiplying the percentage of positive cells by the intensity. The final score was defined as negative for score 0-4 and as positive for scores of 6-12.
Statistical analysis
All statistical analyses were carried out using the SPSS 16.0 statistical software. Chi-square test and Fisher’s exact was used to analyze the relationship between phospho-Src expression and clinicopathologic characteristics. Survival curves were plotted by the Kaplan-Meier method and compared by the log-rank test. Survival data was analyzed using univariate and multivariate Cox regression analyses. P < 0.05 in all cases was considered statistically significant.
Results
Expression of phospho-Src in CSCC, CIS, and normal cervix
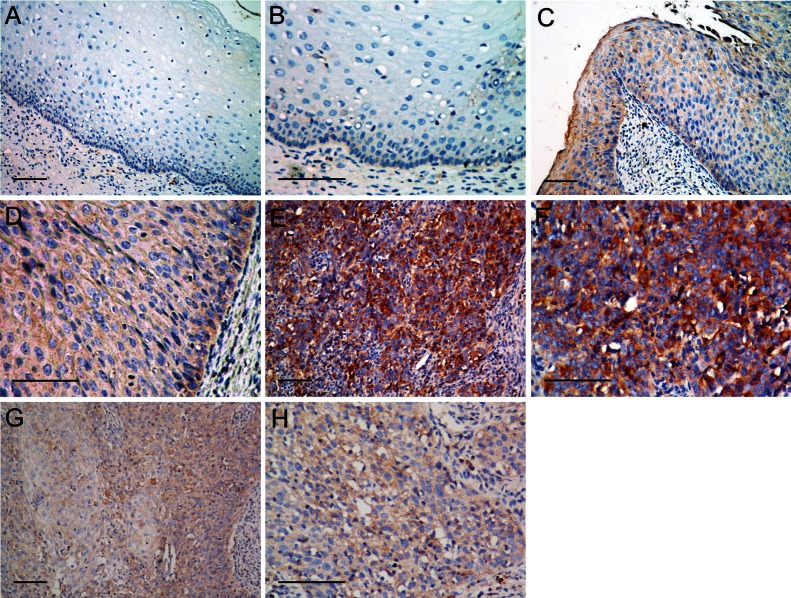
To investigate the potential roles of Src signaling in the tumorigenesis of CSCC, we examined the expression of phospho-Src in 87 paraffin-embedded CSCC, 20 CIS, and 40 normal cervical tissues by immunohistochemistry. The representative immunostaining of phospho-Src in normal cervix tissues (Figure 1A and 1B), CIS (Figure 1C and 1D), and CSCC (Figure 1E-H) were shown in Figure 1. Normal cervical tissue showed positive phospho-Src in 0 (0%) cases, CIS present 1 (5%), and CSCC positively stained 18 (20.7%) cases, respectively (Table 1). The expression of phospho-Src increases as CSCC progresses to more advanced stages (P=0.026). Taken together, these results suggested that phospho-Src was correlated with the tumorigenesis of CSCC.
Figure 1.
Representative immunostaining of phospho-Src in normal cervix tissues (A, B), carcinoma in situ of cervix (C, D), and cervical squamous cell cancer (E, F: positive; G, H: negative). Scale bar, 100 μm.
Expression of phospho-Src with the clinical features of CSCC
We next examined the correlation between the expression of phospho-Src and the clinicopathological characteristics of CSCC. As shown in Table 2, phospho-Src expression strongly correlated with tumor recurrence (p=0.008) of patients with CSCC, whereas it was not associated with age (p=0.793), FIGO stage (p=0.405), tumor differentiation (p=0.606), or lymph node metastasis (p=1.000). Based on the above results, we analyzed the correlation between phospho-Src expression and clinical prognosis. Patient survival analysis indicated a clear positive correlation between phospho-Src expression and both the overall survival (Figure 2A, p=0.037) and recurrence-free survival (Figure 2B, p=0.001) of CSCC patients. In a multivariate Cox regression analysis shown in Table 3, phospho-Src expression showed significant association with recurrence-free survival (P=0.004).
Table 2.
Expression of phospho-Src expression in CSCC patients according to clinicopathologic characteristics
| Characteristics | NO. | phospho-Src | p | |
|---|---|---|---|---|
|
| ||||
| Positive N (%) | Negative N (%) | |||
| Age (y) | 0.793 | |||
| ≤40 | 40 | 9 (22.5) | 31 (77.5) | |
| >40 | 47 | 9 (19.1) | 38 (80.9) | |
| FIGO Stage | 0.405 | |||
| IB1 | 30 | 8 (26.7) | 22 (73.3) | |
| >IB1 | 57 | 10 (17.5) | 47 (82.5) | |
| Differentiation | 0.606 | |||
| Grade 1/2 | 39 | 7 (17.9) | 32 (82.1) | |
| Grade 3 | 48 | 11 (22.9) | 37 (77.1) | |
| LN Metastasis | 1.000 | |||
| - | 79 | 17 (21.5) | 62 (78.5) | |
| + | 8 | 1 (12.5) | 7 (87.5) | |
| Recurrence | 0.008 | |||
| - | 79 | 13 (16.5) | 66 (83.5) | |
| + | 8 | 5 (62.5) | 3 (37.5) | |
Figure 2.
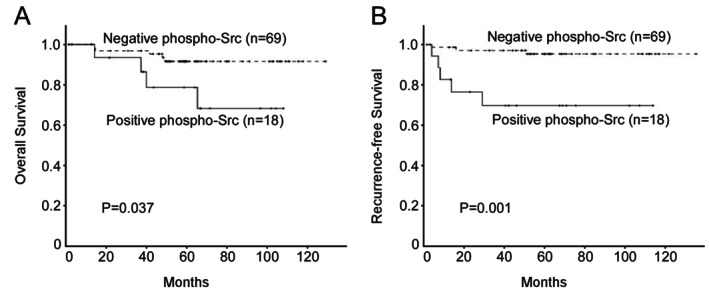
Kaplan-Meier analysis of overall survival (A) and recurrence-free survival (B) in relation to phospho-Src expression in 87 cervical squamous cell cancer (CSCC) patients.
Table 3.
Multivariate Cox regression analysis of overall survival (OS) and Recurrence-free survival (RFS) in patients with CSCC
| Prognostic variables | OS | RFS | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| Age (>40 vs ≤40) | 1.983 (0.469-8.399) | 0.352 | 4.344 (0.820-23.019) | 0.084 |
| FIGO Stage (>IB1 vs IB1) | 1.006 (0.239-4.239) | 0.993 | 2.213 (0.435-11.266) | 0.339 |
| Differentiation (Grade 3 vs 1/2) | 3.019 (0.622-14.659 | 0.170 | 1.526 (0.355-6.572) | 0.570 |
| LN Metastasis (+ vs -) | 1.348 (0.154-11.834) | 0.787 | 1.594 (0.187-13.582) | 0.670 |
| phospho-Src (+ vs -) | 3.714 (0.991-13.912) | 0.052 | 8.636 (2.013-37.046) | 0.004 |
Discussion
Currently, the exact molecular mechanisms of the progression and recurrence of cervical cancer are unclear, and there is lack of biomarkers to predict cervical cancer recurrence. In this study, we observed that the expression of phospho-Src in normal cervix, CIS, and CSCC increased gradually in ascending order. We also demonstrated that expression of phospho-Src was correlated with overall survival and recurrence of cervical cancer. Patients with high phospho-Src expression had shorter overall and recurrence-free survival time, and patients with low phospho-Src expression had longer survival time. Multivariate analysis indicated that phospho-Src expression was an independent prognosis factor for recurrence-free survival. Our present study reveals that phospho-Src may play essential role in cervical cancer progression, and that it may represent a valuable marker to predict recurrence in CSCC.
It has been demonstrated previously that Src family kinases are implicated in squamous epithelial differentiation and in cancer development and progression [18]. Cancer-associated c-Src can suppress cell attachment and motility by affecting the process of actin-filament or integrin-actin cytoskeleton assembly [19]. In addition, activated Src/focal adhesion kinase complex promotes tumor cell survival and invasion via activation of the PI3K/Akt pathway [20]. Moreover, overexpression of c-Src can enhance epidermal growth factor-induced DNA synthesis in cancer cells, which in turn leads to tumor cell proliferation and migration [21]. Furthermore, Src kinases can induce tumor angiogenesis either by regulating the gene expression of angiogenic growth related factors/cytokines or by cooperating with angiogenic growth factor receptors [22,23]. These studies indicated that Src family kinases may contribute to the development and progression of cancer.
Src protein has been reported to be highly expressed in several cancers including head and neck cancers, malignant skin cancers, thyroid papillary carcinomas, and pancreatic carcinomas [24-27]. In several precancerous lesions, elevated Src kinase activity is also detected [28,29]. To investigate whether Src kinases are correlated to the progression of cervical cancer, we performed immunohistochemical studies to characterize the expression of phospho-Src in normal cervical tissues, carcinoma in situ of cervix, and CSCC specimens. We found that the expression of phospho-Src increases as CSCC progresses to more advanced stages, which is in agreement with previous studies. The stepwise and remarkable increase in the phospho-Src expression from normal cervical tissues through CIS to cervical cancer implicated that Src signaling may play a critical role in the progression of cervical carcinoma.
Recent studies have also correlated Src kinase overexpression with tumor progression and clinical prognosis. Ben-Izhak et al. demonstrated an inverse correlation between phospho-Src kinase expression and 5-year overall survival rate in patients with tongue cancer [30]. Zhang et al. suggested that patients with positive c-Src expression exhibited significantly worse progression-free and disease-specific survival in breast cancer [31]. Chatzizacharias et al. revealed that Src expression correlated significantly with the tumor stage and overall survival in patients with pancreatic ductal adenocarcinoma [32]. In this study, we analyzed the correlation between phospho-Src expression and prognosis in CSCC patients, and we found that high expression of phospho-Src is associated with poor overall survival, which is in agreement with most previous studies. In contrast to our findings, Campbell et al. reported that phosphospho-Src kinase in the nucleus is associated with favorable outcome in ER-positive breast cancer patients [14]. As nuclear expression of phospho-Src was rarely observed either in our study or in other cancers, their finding may be associated with ER-positive breast cancer cohort [14].
It is particularly noteworthy that phospho-Src overexpression has been found, in our study, to be associated with shorter recurrence-free survival. Our finding is supported by Wilson [15], who suggested that high levels of activated Src may indicate a greater risk of tumor recurrence. Cheng et al. also described that Src expression is significantly correlated with the progression and recurrence in patients with oral squamous cell carcinoma [17]. Similarly to these studies, de Heer et al. discovered that high levels of FAK and Src were associated with shorter time to recurrence of colorectal cancer [33]. Redmond et al. described that Src-1 was a strong independent predictor of shorter disease-free survival in breast cancer [34]. Together these findings underline an important role of phospho-Src in predicting tumor recurrence in patients with CSCC.
In conclusion, this study showed a significantly higher phospho-Src expression in CSCC than in CIS and normal cervical specimens. We also found a significant correlation of phospho-Src expression and overall survival. Moreover, CSCC patients with phospho-Src expression are more likely to have recurrent disease than those without phospho-Src expression. These results indicate that phospho-Src can be use as a biomarker for prediction of the progression and recurrence of patients with CSCC.
Funding source
This work was supported by grant from the Guangdong Medical Science Foudation (2012B031800385).
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Hertel H, Kohler C, Michels W, Possover M, Tozzi R, Schneider A. Laparoscopic-assisted radical vaginal hysterectomy (LARVH): prospective evaluation of 200 patients with cervical cancer. Gynecol Oncol. 2003;90:505–511. doi: 10.1016/s0090-8258(03)00378-0. [DOI] [PubMed] [Google Scholar]
- 3.Elit L, Fyles AW, Devries MC, Oliver TK, Fung-Kee-Fung M. Follow-up for women after treatment for cervical cancer: a systematic review. Gynecol Oncol. 2009;114:528–535. doi: 10.1016/j.ygyno.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Burke TW, Hoskins WJ, Heller PB, Shen MC, Weiser EB, Park RC. Clinical patterns of tumor recurrence after radical hysterectomy in stage IB cervical carcinoma. Obstet Gynecol. 1987;69:382–385. [PubMed] [Google Scholar]
- 5.Poolkerd S, Leelahakorn S, Manusirivithaya S, Tangjitgamol S, Thavaramara T, Sukwattana P, Pataradule K. Survival rate of recurrent cervical cancer patients. J Med Assoc Thai. 2006;89:275–282. [PubMed] [Google Scholar]
- 6.Qiu JT, Abdullah NA, Chou HH, Lin CT, Jung SM, Wang CC, Chen MY, Huang KG, Chang TC, Lai CH. Outcomes and prognosis of patients with recurrent cervical cancer after radical hysterectomy. Gynecol Oncol. 2012;127:472–477. doi: 10.1016/j.ygyno.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–595. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci. 2012;33:122–128. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karmakar S, Foster EA, Smith CL. Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-alpha transcriptional activity. Endocrinology. 2009;150:1588–1596. doi: 10.1210/en.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez L, Agullo-Ortuno MT, Garcia-Martinez JM, Calcabrini A, Gamallo C, Palacios J, Aranda A, Martin-Perez J. Role of c-Src in human MCF7 breast cancer cell tumorigenesis. J Biol Chem. 2006;281:20851–20864. doi: 10.1074/jbc.M601570200. [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Dering J, Ginther C, Wilson CA, Glaspy P, Tchekmedyian N, Slamon DJ. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal-type/“triple-negative” breast cancer cell lines growing in vitro. Breast Cancer Res Treat. 2007;105:319–326. doi: 10.1007/s10549-006-9463-x. [DOI] [PubMed] [Google Scholar]
- 12.Huang YW, Chen C, Xu MM, Li JD, Xiao J, Zhu XF. Expression of c-Src and phospho-Src in epithelial ovarian carcinoma. Mol Cell Biochem. 2013;376:73–79. doi: 10.1007/s11010-012-1550-1. [DOI] [PubMed] [Google Scholar]
- 13.Stabile LP, He G, Lui VW, Henry C, Gubish CT, Joyce S, Quesnelle KM, Siegfried JM, Grandis JR. c-Src Activation Mediates Erlotinib Resistance in Head and Neck Cancer by Stimulating c-Met. Clin Cancer Res. 2013;19:380–92. doi: 10.1158/1078-0432.CCR-12-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell EJ, McDuff E, Tatarov O, Tovey S, Brunton V, Cooke TG, Edwards J. Phosphorylated c-Src in the nucleus is associated with improved patient outcome in ER-positive breast cancer. Br J Cancer. 2008;99:1769–1774. doi: 10.1038/sj.bjc.6604768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson GR, Cramer A, Welman A, Knox F, Swindell R, Kawakatsu H, Clarke RB, Dive C, Bundred NJ. Activated c-SRC in ductal carcinoma in situ correlates with high tumour grade, high proliferation and HER2 positivity. Br J Cancer. 2006;95:1410–1414. doi: 10.1038/sj.bjc.6603444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan L, Gee J, Pumford S, Farrow L, Finlay P, Robertson J, Ellis I, Kawakatsu H, Nicholson R, Hiscox S. Elevated Src kinase activity attenuates Tamoxifen response in vitro and is associated with poor prognosis clinically. Cancer Biol Ther. 2009;8:1550–1558. doi: 10.4161/cbt.8.16.8954. [DOI] [PubMed] [Google Scholar]
- 17.Cheng SJ, Kok SH, Lee JJ, Yen-Ping Kuo M, Cheng SL, Huang YL, Chen HM, Chang HH, Chiang CP. Significant association of SRC protein expression with the progression, recurrence, and prognosis of oral squamous cell carcinoma in Taiwan. Head Neck. 2012;34:1340–1345. doi: 10.1002/hed.21923. [DOI] [PubMed] [Google Scholar]
- 18.Jin F, Reynolds AB, Hines MD, Jensen PJ, Johnson KR, Wheelock MJ. Src induces morphological changes in A431 cells that resemble epidermal differentiation through an SH3- and Ras-independent pathway. J Cell Sci. 1999;112:2913–2924. doi: 10.1242/jcs.112.17.2913. [DOI] [PubMed] [Google Scholar]
- 19.Brunton VG, MacPherson IR, Frame MC. Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochim Biophys Acta. 2004;1692:121–144. doi: 10.1016/j.bbamcr.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12:3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 21.Dimri M, Naramura M, Duan L, Chen J, Ortega-Cava C, Chen G, Goswami R, Fernandes N, Gao Q, Dimri GP, Band V, Band H. Modeling breast cancer-associated c-Src and EGFR overexpression in human MECs: c-Src and EGFR cooperatively promote aberrant three-dimensional acinar structure and invasive behavior. Cancer Res. 2007;67:4164–4172. doi: 10.1158/0008-5472.CAN-06-2580. [DOI] [PubMed] [Google Scholar]
- 22.Petreaca ML, Yao M, Liu Y, Defea K, Martins-Green M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol Biol Cell. 2007;18:5014–5023. doi: 10.1091/mbc.E07-01-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesslie DP, Summy JM, Parikh NU, Fan F, Trevino JG, Sawyer TK, Metcalf CA, Shakespeare WC, Hicklin DJ, Ellis LM, Gallick GE. Vascular endothelial growth factor receptor-1 mediates migration of human colorectal carcinoma cells by activation of Src family kinases. Br J Cancer. 2006;94:1710–1717. doi: 10.1038/sj.bjc.6603143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Oijen MG, Rijksen G, ten Broek FW, Slootweg PJ. Overexpression of c-Src in areas of hyperproliferation in head and neck cancer, premalignant lesions and benign mucosal disorders. J Oral Pathol Med. 1998;27:147–152. doi: 10.1111/j.1600-0714.1998.tb01931.x. [DOI] [PubMed] [Google Scholar]
- 25.Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Luhrs H, Friess H, Buchler MW, Adler G. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun. 1998;243:503–508. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- 26.Lee JH, Pyon JK, Kim DW, Lee SH, Nam HS, Kim CH, Kang SG, Lee YJ, Park MY, Jeong DJ, Cho MK. Elevated c-Src and c-Yes expression in malignant skin cancers. J Exp Clin Cancer Res. 2010;29:116. doi: 10.1186/1756-9966-29-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michailidi C, Giaginis C, Stolakis V, Alexandrou P, Klijanienko J, Delladetsima I, Chatzizacharias N, Tsourouflis G, Theocharis S. Evaluation of FAK and Src expression in human benign and malignant thyroid lesions. Pathol Oncol Res. 2010;16:497–507. doi: 10.1007/s12253-010-9269-3. [DOI] [PubMed] [Google Scholar]
- 28.Cartwright CA, Coad CA, Egbert BM. Elevated c-Src tyrosine kinase activity in premalignant epithelia of ulcerative colitis. J Clin Invest. 1994;93:509–515. doi: 10.1172/JCI117000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pena SV, Melhem MF, Meisler AI, Cartwright CA. Elevated c-yes tyrosine kinase activity in premalignant lesions of the colon. Gastroenterology. 1995;108:117–124. doi: 10.1016/0016-5085(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Izhak O, Cohen-Kaplan V, Nagler RM. The prognostic role of phospho-Src family kinase analysis in tongue cancer. J Cancer Res Clin Oncol. 2010;136:27–34. doi: 10.1007/s00432-009-0633-1. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Teng Y, Zhang Y, Liu J, Xu L, Qu J, Hou K, Yang X, Liu Y, Qu X. c-Src expression is predictive of poor prognosis in breast cancer patients with bone metastasis, but not in patients with visceral metastasis. APMIS. 2012;120:549–557. doi: 10.1111/j.1600-0463.2011.02864.x. [DOI] [PubMed] [Google Scholar]
- 32.Chatzizacharias NA, Giaginis C, Zizi-Serbetzoglou D, Kouraklis GP, Karatzas G, Theocharis SE. Evaluation of the clinical significance of focal adhesion kinase and SRC expression in human pancreatic ductal adenocarcinoma. Pancreas. 2010;39:930–936. doi: 10.1097/MPA.0b013e3181d7abcc. [DOI] [PubMed] [Google Scholar]
- 33.de Heer P, Koudijs MM, van de Velde CJ, Aalbers RI, Tollenaar RA, Putter H, Morreau J, van de Water B, Kuppen PJ. Combined expression of the non-receptor protein tyrosine kinases FAK and Src in primary colorectal cancer is associated with tumor recurrence and metastasis formation. Eur J Surg Oncol. 2008;34:1253–1261. doi: 10.1016/j.ejso.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Redmond AM, Bane FT, Stafford AT, McIlroy M, Dillon MF, Crotty TB, Hill AD, Young LS. Coassociation of estrogen receptor and p160 proteins predicts resistance to endocrine treatment; SRC-1 is an independent predictor of breast cancer recurrence. Clin Cancer Res. 2009;15:2098–2106. doi: 10.1158/1078-0432.CCR-08-1649. [DOI] [PubMed] [Google Scholar]