Abstract
Dopamine D2 receptor is involved in reward-mediating mesocorticolimbic pathways. It plays an important role in major depressive disorder (MDD). Three gene polymorphisms Taq1A, C957T and -141C ins/del, were identified in the DRD2 gene among the Western population. These variants in the DRD2 gene might be associated with the susceptibility of MDD patients through affecting the bioeffects of endogenous dopamine neurotransmission. However, little is known about their occurrence in Chinese population and their association with the susceptibility of patients with major depressive disorder. In this study, a total of 338 unrelated adult Chinese Han population, including 224 healthy volunteers and 114 patients with major depressive disorder, were recruited. DRD2 polymorphisms (Taq1A and -141C ins/del) were detected using restriction fragment length polymorphism (RFLP) analysis and the C957T were detected by sequencing directly. As a result, three polymorphisms were identified in Chinese Han population and all were common SNP. However, we could detect no evidence of genetic association between 3 markers in DRD2 and major depressive disorder in the Chinese Han population. To conclude, this result suggests that Taq1A, C957T and -141C ins/del of DRD2 gene may not be associated with major depressive disorder, also may be the sample sizes too small to allow a meaningful test.
Keywords: Dopamine D2 receptor, polymorphism, major depressive disorder
Introduction
Major depressive disorder (MDD), is a common, chronic, and recurrent mental disease. MDD affects millions of individuals world-wide and contributes to heavy social and economic burden. In developed countries, MDD is one of the most important psychiatric problems of both genders [1,2]. It is associated with increased mortality, especially from suicide. A genetic component is very likely to contribute to the etiology of illness but the precise molecular mechanism by which MDD can be fully explained has yet to be determined. Twin studies estimate the heritability of MDD around 40% [3] or even higher [4], suggesting that genotyping is a promising field for research into the development of MDD. According to the dopamine theory of affective disorders, a deficiency in dopaminergic neurotransmission may play a role in the major symptoms of MDD. Specific polymorphisms in genes that affect dopamine transmission could increase susceptibility to MDD.
Dopamine (DA) is the most abundant catecholaminergic neurotransmitter in the brain, and plays a role in the regulation of emotions, motivation, reward, and reinforcement behavior, by the mesocorticolimbic pathway [5]. Evidence from animal studies [6] and several lines of evidence from human in vivo imaging studies [7,8] suggest that DA neurotransmission is critically involved in incentive-motivational mechanisms [9] but also in responses to salient aversive stimuli [10], in addition to its more traditional roles in cognitive and motor function. Recent literature has linked variation in genes related to DA function with reward [11-13] and emotional processing phenotypes [14-16]. Thus, the genes of dopaminergic system, including dopamine D2 receptor (DRD2) gene, are good candidates for genetics susceptibility studies on MDD.
The human DRD2 gene is located on chromosome 11q22-23 and is organized in eight exons spanning at least 270 kilobases [17]. Several studies proved that D2 antagonists disrupt the attribution of incentive-motivational value [18], and induced blunting of self-reported affective responses [19]. In the past few decades, many polymorphisms have been identified in this gene, such as Taq1A, rs6277 and -141Cins/del polymorphisms. A frequently investigated polymorphism in this gene is the Taq1A (rs1800497), a C (A2-allele) to T (A1-allele) substitution. A number of studies have investigated the association of this polymorphism with MDD, but findings are not always consistent [20-22]. Other investigated polymorphisms in the DRD2 gene are 141C Ins/Del (rs1799732) and C957T (rs6277). None of these SNPs show an association with MDD [23-27]. However, within a group of MDD patients, carriers of the T/T genotype of the C957T SNP had more depressive symptoms than carriers of the other genotypes [25]. There are several possible explanations for this discordance, such as small sample size, ethnic background, different types of mood disorders, and publication bias [28,29]. However, little is known about the association of the three polymorphisms of the DRD2 gene with the outcome of patients with major depressive disorder and the distribution of these polymorphisms among Chinese population.
The purpose of this study was to screen the distribution of Taq1A, C957T and -141C ins/del polymorphisms in the DRD2 gene among Chinese Han population, and investigate the functional significance of these polymorphism in terms of its linkage association with multiple organ dysfunction and sepsis in patients with MDD.
Material and methods
Study design and data collection
A total of 338 unrelated adult Chinese, including 224 healthy volunteers and 114 patients with MDD were recruited in this study. All of them were biologically unrelated individuals of Chinese Han origin living in Chongqing district, China. The 224 healthy volunteers were 128 men and 96 women with a median age of 35 years (range of 18-55 years). The 114 MDD patients of 75 men and 39 women were admitted to the Department of Burn Institute in the Southwest Hospital and the Chongqing Mental Health Center, Chongqing, China, between January 1, 2011 and October 1, 2012. All patients were assessed by using the Structured Clinical Interview for DSM-IVAxis I Disorders-Clinician Version [30] and interviewed by 2 independent psychiatrists. Additionally, bipolar spectrum was excluded according to the criteria pubulished previously [31] as well as by the application of the Mood Disorder Questionnaire [32]. Before sampling, it was verified by direct interview if they had current psychiatric problems or family history of psychiatric or neurological disorders. Controls were recruited from among volunteers randomly selected from local Chongqing communities no family history of psychiatric disorders. The protocol for this study was approved by the Ethical and Protocol Review Committee of the Third Military Medical University, and written informed consent for this genetic study was provided by each participant or their next of kin. Participant confidentiality was preserved according to the guidelines for studies of human subjects.
DNA isolation and genotyping
Genomic DNA was extracted from whole blood using the phenol/chloroform method. The MDD patients’ whole blood was collected immediately at admission to the hospital. The DRD2 gene Taq1A and -141C ins/del polymorphisms were detected by polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) method in plate thermal cycles (Mastercycler gradient, Eppendorf, Germany), and the C957T were detected by sequencing directly. Oligonucleotide primers for amplification were designed according to the published genomic sequences (GenBank NC_000011.9). The Primers, annealing temperature, restriction enzyme and fragment size were described in Table 1. A total of 25μL reaction mixture for PCR contained 2μl DNA, 2.5μl 10×ExTaq Buffer, 5μM of each primer, 25mM MgCl2, 2.5mM dNTPs and 1.25U of ExTaq (Takara Biotech, Japan). The PCR conditions were as follows: 5 min at 94°C followed by 30 cycles of 30s at 94°C, 40s at 68°C or 57°C, 40s at 72°C. PCR products were then digested with proper restriction endonucleases. Following digestion, 10μl portions of digested and undigested samples were electrophoresized on a 2% agarose gel with subsequent staining by ethidium bromide (0.5 μg/mL). The bands were visualized under UV light and photos were taken with a video camera. The results were confirmed by two independent observers. In addition, RFLP results were confirmed by DNA sequencing with 10 random samples.
Table 1.
Primers for DRD2 gene SNPs genotyping
| SNPs | Primers | Annealing temperature | Restriction enzyme | Allele | Fragment sizes (bp) |
|---|---|---|---|---|---|
| rs1799732 (-141delc) | P1: 5’-ACTGGCGAGCAGACGGTGAGGACCC-3’ | 68°C | BstN I | C | C/C: 144, 160 |
| P2: 5’-TGCGCGCGTGAGGCTGCCGGTTCGG-3’ | Del-c | C/-: 303, 144, 160 | |||
| -/-: 303bp | |||||
| rs6277 (C957T) | P1: 5’-TGCCTCAGTGACATCCTTGC-3’ | Sequencing | C | ||
| P2: 5’-TGGGACCTTTCACAGACCG-3’ | T | ||||
| rs1800497 (Taq1A) | P1: 5’-GCACGTGCCACCATACCC-3’ | 68°C | Taq I | C | CC: 401, 258 |
| P2: 5’-TGCAGAGCAGTCAGGCTG -3’ | T | CT: 659, 401, 258 | |||
| TT: 659 |
rs1799732 (-141delc) and rs1800497 (Taq1A) were detected using restriction fragment length polymorphism (RFLP) analysis; rs6277 (C957T) was detected by sequencing directly, no annealing temperature and fragment sizes.
Statistical analysis
Allele frequencies for each SNP were determined by gene counting. Genotype distribution was tested for departure from Hardy-Weinberg equilibrium using X2 analyses. In case of a consistent trend reflecting an allele dose effect, a linear or logistic regression analysis was performed to quantify the association. In case of a dominant or recessive effect of the test allele, ANOVA and ANCOVA tests were performed. For dominant effects, test-allele carriers vs non-carriers were compared, whereas for recessive effects, subjects homozygous for the test allele were compared with heterozygous carriers and non-carriers. The association of genotypes with MDD rate was determined by X2 analyses. General linear model and logistic regression analyses were used to correct known confounding variables, such as age, gender ratio. Results were considered to be significant if P<0.05. All statistic analysis was carried out using SPSS Version 11.0 (Chicago, Illinois, USA).
Results
Allele frequencies and genotype distribution
In this study, we examined the allele frequency and genotype distribution of the DRD2 polymorphisms (Taq1A, C957T and -141C ins/del polymorphisms) in a relatively large group (n=338 Han Chinese). Our data showed that three polymorphisms were identified in Chinese Han population and were common SNP. The genotype distribution was in agreement with the Hardy-Weinberg equilibrium. (Figure 1, Table 2). No significant differences were found in allele frequencies and genotype distributions of age and gender.
Figure 1.
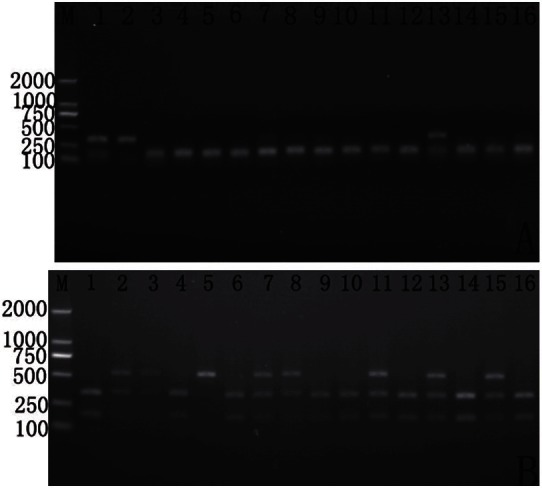
Genotyping of the DRD2 gene polymorphisms. A: Taq1A: Lanes 1, 3 and 9 indicate the CC genotype (401bp and 258bp). Lanes 4, 5, 6, 7 and 10 indicate the CT genotype (659bp, 401bp and 258bp). Lane 2 and 8 indicates the TT genotype (only a 659bp band). B: -141C ins/del: Lanes 3, 4, 5, 6, 7, 8, 9 and 10 indicate the CC genotype (144bp and 160bp). Lanes 1 indicate the C/- genotype (303bp, 144bp and 160bp). Lane 2 indicates the -/- genotype (only a 303bp band).
Table 2.
Genotypes and allele frequencies in the patient and control groups
| SNP | Genotype | MDD patients n=114 (%) | Healthy volunteers n=224 (%) | Hardy-Weinberg Equilibrium analysis | |
|---|---|---|---|---|---|
|
| |||||
| X2 | p | ||||
| rs1799732 (-141delc) | C/C | 98 (86.0) | 178 (79.5) | 3.1 | 0.09 |
| C/- | 12 (10.5) | 34 (15.2) | |||
| -/- | 4 (3.5) | 12 (5.3) | |||
| Allele: C | (91.2) | (87.1) | |||
| Allele: -/ | (8.8) | (12.9) | |||
| rs6277 (C957T) | CC | 102 (89.5) | 196 (88.3) | 1.6 | 0.20 |
| CT | 10 (8.8) | 24 (10.8) | |||
| TT | 2 (1.7) | 2 (0.9) | |||
| Allele: C | (93.9) | (93.7) | |||
| Allele: T | (6.1) | (6.3) | |||
| rs1800497 (Taq1A) | CC | 14 (12.2) | 28 (12.5) | 1.5 | 0.20 |
| CT | 50 (43.9) | 114 (50.9) | |||
| TT | 50 (43.9) | 82 (36.6) | |||
| Allele: C | (34.0) | (38.0) | |||
| Allele: T | (66.0) | (62.0) | |||
Abbreviations: MDD, Major Depressive Disorder; n, Number; (%), Percentage; Hardy-Weinberg Equilibrium analysis: P>0.05, suggested that all samples were in agreement with Hardy-Weinberg equilibrium.
Clinical relevance of the Taq1A, rs6277 and -141Cins/del polymorphisms
With respect to the important role of DRD2 in outcome of MDD, we further examined the possible association of the Taq1A, C957T and -141C ins/del polymorphisms with the outcome of patients with major depressive disorder. 114 consecutive major depressive disorder patients were enrolled. The overall allele frequencies and genotype distribution of the Taq1A, C957T and -141Cins/del polymorphisms in MDD patients were similar to those in the healthy volunteers (Table 2). There were no significant differences in age, gender ratio between the different genotype groups of the Taq1A, C957T and -141C ins/del polymorphisms among the MDD patients (Table 3).
Table 3.
Association analysis of DRD2 gene among MDD patients and controls
| SNP | Genotype | patients (n=114) | Control (n=224) | Chi-square Test | ||
|---|---|---|---|---|---|---|
|
| ||||||
| X2 | p | OR (95% CI) | ||||
| rs1799732 (-141delc) | C/C | 98 | 178 | 2.2 | 0.3 | |
| C/- | 12 | 34 | ||||
| -/- | 4 | 12 | ||||
| C/C + C/- vs -/- | 0.6 | 0.5 | 1.6 (0.5-4.9) | |||
| C/C vs C/- + -/- | 2.1 | 0.1 | 1.6 (0.9-2.9) | |||
| rs6277 (C957T) | CC | 102 | 196 | 0.8 | 0.7 | |
| CT | 10 | 24 | ||||
| TT | 2 | 2 | ||||
| CC + CT vs TT | 0.5 | 0.5 | 0.5 (0.7-3.7) | |||
| CC vs CT + TT | 0.1 | 0.8 | 1.1 (0.6-2.3) | |||
| rs1800497 (Taq1A) | CC | 14 | 28 | 1.8 | 0.4 | |
| CT | 50 | 114 | ||||
| TT | 50 | 82 | ||||
| CC + CT vs TT | 1.7 | 0.2 | 0.7 (0.5-1.2) | |||
| CC vs CT + TT | 0.003 | 1.0 | 1.0 (0.5-2.0) | |||
Dominant effect (variant homozygotes + heterozygotes versus wild homozygotes) as analyzed by crosstab analysis; Recessive effect (variant homozygotes versus heterozygotes + wild homozygotes) as analyzed by crosstab analysis; only 2×2 crosstab analysis has OR and 95% CI.
Discussion
The present study, for the first time to our knowledge, investigated the occurrence of the Taq1A, C957T and -141C ins/del polymorphisms of the DRD2 gene in Chinese Han population and their possible clinical relevance in patients with major depressive disorder. Although the three SNPs have been identified in Caucasian and African-American populations, three polymorphisms exist in the cohort of 338 Han Chinese we studied, and all of them are common SNPs. The minor allele frequency of three polymorphisms in our studied cohort is shown to be similar to that in Western and other Asian populations.
In our study, we did not find any association between DRD2 gene Taq1A, C957T and -141C ins/del polymorphisms and MDD. The DRD2 TaqI A1 polymorphism is a single nucleotide transition which creates a restriction fragment polymorphism [17]. Studies found that this polymorphism modulated the density of DRD2 receptors, and the A1 allele was associated with a 30-40% reduction inDRD2 density, compared to individuals homozygous for the A2 allele [33,34] . This reduction is particularly prominent in ventral parts of caudate and putamen. Additionally, Noble et al. [35] reported that reduced glucose metabolism was observed in carriers of the A1 allele in the striatum and remote areas. Given the importance of these areas for regulation of emotions, the reduction of glucose metabolism and the reduced density of DRD2 in carriers of the A1 allele may make individuals more susceptible to MDD. However, no association of DRD2 gene TaqI A1 polymorphism with MDD was found in the current study. Recent data indicated that this polymorphism may be a marker of both DRD2 and the ankyrin repeat and kinase domain containing 1 (ANKK1) genetic variants [36]. These two genes overlap and are transcribed from the opposite directions. Proximity of the two genes may be non-incidental and reflect functional relationship. Thus, this polymorphism could associate with dopaminergic phenotypes by being in linkage disequilibrium with other polymorphisms in DRD2 or by reflecting functional variations of the ANKK1 that are implicated in dopaminergic transmission. DRD2 gene -141Cins/del polymorphism was located in the 58 flanking region of the DRD2 gene at position -141. Studies showed that expression was distinctly reduced in constructs containing allele 1 (-141Cdel allele) when compared to constructs containing allele 2 (-141Cins allele), and the lower-expressing allele 1 was significantly reduced in schizophrenic patients when compared to controls [37]. The -141Cins/del polymorphism may provide a powerful test of the role ofDRD2 in psychiatric disorders. However, our results do not support a major role for the DRD2 gene -141Cins/del polymorphism in the etiology of mood disorders. The C957T polymorphism (rs6277) was located in the exon 7 of the human DRD2 gene which causes a synonymous coding C-T transition at position 957. The SNP changes the receptor’s affinity and regulates DRD2 availability in vivo, but its effect differs depending on the brain region under investigation [38,39]. Previous studies [40,41] have proved that C957T polymorphism could significantly impact on the working memory task performance in the way that the T-allele was associated with a better performance and the higher density of extrastriatal D2 receptors (C957T CC) were associated with better backward serial recall, an episodic memory task with high encoding and retrieval demands. A recent PET study provides further evidence for a role of striatal dopamine in the attentional blink phenomenon (AB) [42]. However, in this study, we found no association between this polymorphism and MDD.
The above results suggest that the Taq1A, C957T and -141C ins/del polymorphism of the DRD2 gene might be of less clinical relevance although it has been shown to be associated with mood disorder [3,4]. In addition, there exist other possible reasons for the negative results. One important reason might be due to polygenetic and multifactorial involvement in the pathogenesis of MDD. In fact, any one gene should not determine the clinical phenotype after MDD although the gene is a pivotal one. Therefore, one gene polymorphism should have much less power on the MDD outcome. The susceptibility to MDD might be the result of combination of numerous genetic polymorphisms. Another possible reason might be due to small sample size we recruited in this study (n=114). The power is only 34.5% for three polymorphisms at a significance of 0.05 to detect recessive effect that confers a risk of 0.803. The inadequate power may result in false negative associations.
In conclusion, this study for the first time investigates the occurrence of the Taq1A, C957T and -141C ins/del polymorphisms of the DRD2 gene in Chinese Han population and finds that all three polymorphisms might be exist in Chinese Han population. The Taq1A, C957T and -141C ins/del polymorphisms of the DRD2 gene might be less relevant for risk estimation of MDD.
Acknowledgments
We acknowledge Dr. Han Luo, Qian-ying Tand, Jun-wei Guo and Shan-shan Gu for their clinical help, Prof. Yi Ren, Rutgers, The State University of New Jersey, the United States of America for reviewing the article.
This work was supported by the National Natural Science Foundation of China (81101422) and Third Military Medical University Southwest Hospital Clinical innovation fund (SWH2012LC14).
Conflict of interest
The authors have declared that no conflict of interest exists.
References
- 1.O’Donovan MC, Craddock NJ, Owen MJ. Genetics of psychosis; insights from views across the genome. Hum Genet. 2009;126:3–12. doi: 10.1007/s00439-009-0703-0. [DOI] [PubMed] [Google Scholar]
- 2.Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Numata S, Iga J, Nakataki M, Tayoshi S, Tanahashi T, Itakura M, Ueno S, Ohmori T. Positive association of the pericentrin (PCNT) gene with major depressive disorder in the Japanese population. J Psychiatry Neurosci. 2009;34:195–198. [PMC free article] [PubMed] [Google Scholar]
- 4.Fan M, Liu B, Jiang T, Jiang X, Zhao H, Zhang J. Meta-analysis of the association between the monoamine oxidase-A gene and mood disorders. Psychiatr Genet. 2010;20:1–7. doi: 10.1097/YPG.0b013e3283351112. [DOI] [PubMed] [Google Scholar]
- 5.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 6.Hamidovic A, Dlugos A, Skol A, Palmer AA, de Wit H. Evaluation of genetic variability in the dopamine receptor D2 in relation to behavioral inhibition and impulsivity/sensation seeking: an exploratory study with d-amphetamine in healthy participants. Exp Clin Psychopharmacol. 2009;17:374–383. doi: 10.1037/a0017840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badgaiyan RD. Dopamine is released in the striatum during human emotional processing. Neuroreport. 2010;21:1172–1176. doi: 10.1097/WNR.0b013e3283410955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobiella A, Vollstadt-Klein S, Buhler M, Graf C, Buchholz HG, Bernow N, Yakushev IY, Landvogt C, Schreckenberger M, Grunder G, Bartenstein P, Fehr C, Smolka MN. Human dopamine receptor D2/D3 availability predicts amygdala reactivity to unpleasant stimuli. Hum Brain Mapp. 2010;31:716–726. doi: 10.1002/hbm.20900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 10.Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–656. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 11.Aarts E, Roelofs A, Franke B, Rijpkema M, Fernandez G, Helmich RC, Cools R. Striatal dopamine mediates the interface between motivational and cognitive control in humans: evidence from genetic imaging. Neuropsychopharmacology. 2010;35:1943–1951. doi: 10.1038/npp.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camara E, Kramer UM, Cunillera T, Marco-Pallares J, Cucurell D, Nager W, Mestres-Misse A, Bauer P, Schule R, Schols L, Tempelmann C, Rodriguez-Fornells A, Munte TF. The effects of COMT (Val108/158Met) and DRD4 (SNP -521) dopamine genotypes on brain activations related to valence and magnitude of rewards. Cereb Cortex. 2010;20:1985–1996. doi: 10.1093/cercor/bhp263. [DOI] [PubMed] [Google Scholar]
- 13.Hahn T, Heinzel S, Dresler T, Plichta MM, Renner TJ, Markulin F, Jakob PM, Lesch KP, Fallgatter AJ. Association between reward-related activation in the ventral striatum and trait reward sensitivity is moderated by dopamine transporter genotype. Hum Brain Mapp. 2011;32:1557–1565. doi: 10.1002/hbm.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, de Quervain DJ, Papassotiropoulos A. Aversive stimuli lead to differential amygdala activation and connectivity patterns depending on catechol-O-methyltransferase Val158Met genotype. Neuroimage. 2010;52:1712–1719. doi: 10.1016/j.neuroimage.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Swart M, Bruggeman R, Laroi F, Alizadeh BZ, Kema I, Kortekaas R, Wiersma D, Aleman A. COMT Val158Met polymorphism, verbalizing of emotion and activation of affective brain systems. Neuroimage. 2011;55:338–344. doi: 10.1016/j.neuroimage.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Williams LM, Gatt JM, Grieve SM, Dobson-Stone C, Paul RH, Gordon E, Schofield PR. COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. Neuroimage. 2010;53:918–925. doi: 10.1016/j.neuroimage.2010.01.084. [DOI] [PubMed] [Google Scholar]
- 17.Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, Makam H, Reed L, Magenis RE, Civelli O. The human dopamine D2 receptor gene is located on chromosome 11 at q22-q23 and identifies a TaqI RFLP. Am J Hum Genet. 1989;45:778–785. [PMC free article] [PubMed] [Google Scholar]
- 18.Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacol Biochem Behav. 2010;96:40–47. doi: 10.1016/j.pbb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizrahi R, Rusjan P, Agid O, Graff A, Mamo DC, Zipursky RB, Kapur S. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry. 2007;164:630–637. doi: 10.1176/ajp.2007.164.4.630. [DOI] [PubMed] [Google Scholar]
- 20.Vaske J, Makarios M, Boisvert D, Beaver KM, Wright JP. The interaction of DRD2 and violent victimization on depression: an analysis by gender and race. J Affect Disord. 2009;112:120–125. doi: 10.1016/j.jad.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Berman SM, Ozkaragoz T, Noble EP, Antolin T, Sheen C, Siddarth P, Conner BT, Ritchie T. Differential associations of sex and D2 dopamine receptor (DRD2) genotype with negative affect and other substance abuse risk markers in children of alcoholics. Alcohol. 2003;30:201–210. doi: 10.1016/j.alcohol.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Elovainio M, Jokela M, Kivimaki M, Pulkki-Raback L, Lehtimaki T, Airla N, Keltikangas-Jarvinen L. Genetic variants in the DRD2 gene moderate the relationship between stressful life events and depressive symptoms in adults: cardiovascular risk in young Finns study. Psychosom Med. 2007;69:391–395. doi: 10.1097/psy.0b013e31806bf365. [DOI] [PubMed] [Google Scholar]
- 23.Cusin C, Serretti A, Lattuada E, Lilli R, Lorenzi C, Smeraldi E. Association study of MAO-A, COMT, 5-HT2A, DRD2, and DRD4 polymorphisms with illness time course in mood disorders. Am J Med Genet. 2002;114:380–390. doi: 10.1002/ajmg.10358. [DOI] [PubMed] [Google Scholar]
- 24.Furlong RA, Coleman TA, Ho L, Rubinsztein JS, Walsh C, Paykel ES, Rubinsztein DC. No association of a functional polymorphism in the dopamine D2 receptor promoter region with bipolar or unipolar affective disorders. Am J Med Genet. 1998;81:385–387. [PubMed] [Google Scholar]
- 25.Huuhka K, Anttila S, Huuhka M, Hietala J, Huhtala H, Mononen N, Lehtimaki T, Leinonen E. Dopamine 2 receptor C957T and catechol-o-methyltransferase Val158Met polymorphisms are associated with treatment response in electroconvulsive therapy. Neurosci Lett. 2008;448:79–83. doi: 10.1016/j.neulet.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Koks S, Nikopensius T, Koido K, Maron E, Altmae S, Heinaste E, Vabrit K, Tammekivi V, Hallast P, Kurg A, Shlik J, Vasar V, Metspalu A, Vasar E. Analysis of SNP profiles in patients with major depressive disorder. Int J Neuropsychopharmacol. 2006;9:167–174. doi: 10.1017/S1461145705005468. [DOI] [PubMed] [Google Scholar]
- 27.Manki H, Kanba S, Muramatsu T, Higuchi S, Suzuki E, Matsushita S, Ono Y, Chiba H, Shintani F, Nakamura M, Yagi G, Asai M. Dopamine D2, D3 and D4 receptor and transporter gene polymorphisms and mood disorders. J Affect Disord. 1996;40:7–13. doi: 10.1016/0165-0327(96)00035-3. [DOI] [PubMed] [Google Scholar]
- 28.Hayden EP, Klein DN, Dougherty LR, Olino TM, Laptook RS, Dyson MW, Bufferd SJ, Durbin CE, Sheikh HI, Singh SM. The dopamine D2 receptor gene and depressive and anxious symptoms in childhood: associations and evidence for gene-environment correlation and gene-environment interaction. Psychiatr Genet. 2010;20:304–310. doi: 10.1097/YPG.0b013e32833adccb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sipila T, Kananen L, Greco D, Donner J, Silander K, Terwilliger JD, Auvinen P, Peltonen L, Lonnqvist J, Pirkola S, Partonen T, Hovatta I. An association analysis of circadian genes in anxiety disorders. Biol Psychiatry. 2010;67:1163–1170. doi: 10.1016/j.biopsych.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Maffei C, Fossati A, Agostoni I, Barraco A, Bagnato M, Deborah D, Namia C, Novella L, Petrachi M. Interrater reliability and internal consistency of the structured clinical interview for DSM-IV axis II personality disorders (SCID-II), version 2.0. J Pers Disord. 1997;11:279–284. doi: 10.1521/pedi.1997.11.3.279. [DOI] [PubMed] [Google Scholar]
- 31.Ghaemi SN, Ko JY, Goodwin FK. The bipolar spectrum and the antidepressant view of the world. J Psychiatr Pract. 2001;7:287–297. doi: 10.1097/00131746-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hirschfeld RM, Williams JB, Spitzer RL, Calabrese JR, Flynn L, Keck PE Jr, Lewis L, McElroy SL, Post RM, Rapport DJ, Russell JM, Sachs GS, Zajecka J. Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry. 2000;157:1873–1875. doi: 10.1176/appi.ajp.157.11.1873. [DOI] [PubMed] [Google Scholar]
- 33.Jonsson EG, Nothen MM, Grunhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol Psychiatry. 1999;4:290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 34.Ritchie T, Noble EP. Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem Res. 2003;28:73–82. doi: 10.1023/a:1021648128758. [DOI] [PubMed] [Google Scholar]
- 35.Noble EP, Gottschalk LA, Fallon JH, Ritchie TL, Wu JC. D2 dopamine receptor polymorphism and brain regional glucose metabolism. Am J Med Genet. 1997;74:162–166. [PubMed] [Google Scholar]
- 36.Ponce G, Perez-Gonzalez R, Aragues M, Palomo T, Rodriguez-Jimenez R, Jimenez-Arriero MA, Hoenicka J. The ANKK1 kinase gene and psychiatric disorders. Neurotox Res. 2009;16:50–59. doi: 10.1007/s12640-009-9046-9. [DOI] [PubMed] [Google Scholar]
- 37.Arinami T, Gao M, Hamaguchi H, Toru M. A functional polymorphism in the promoter region of the dopamine D2 receptor gene is associated with schizophrenia. Hum Mol Genet. 1997;6:577–582. doi: 10.1093/hmg/6.4.577. [DOI] [PubMed] [Google Scholar]
- 38.Hirvonen MM, Lumme V, Hirvonen J, Pesonen U, Nagren K, Vahlberg T, Scheinin H, Hietala J. C957T polymorphism of the human dopamine D2 receptor gene predicts extrastriatal dopamine receptor availability in vivo. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:630–636. doi: 10.1016/j.pnpbp.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 39.Hirvonen MM, Laakso A, Nagren K, Rinne JO, Pohjalainen T, Hietala J. C957T polymorphism of dopamine D2 receptor gene affects striatal DRD2 in vivo availability by changing the receptor affinity. Synapse. 2009;63:907–912. doi: 10.1002/syn.20672. [DOI] [PubMed] [Google Scholar]
- 40.Colzato LS, Slagter HA, de Rover M, Hommel B. Dopamine and the management of attentional resources: genetic markers of striatal D2 dopamine predict individual differences in the attentional blink. J Cogn Neurosci. 2011;23:3576–3585. doi: 10.1162/jocn_a_00049. [DOI] [PubMed] [Google Scholar]
- 41.Li SC, Papenberg G, Nagel IE, Preuschhof C, Schroder J, Nietfeld W, Bertram L, Heekeren HR, Lindenberger U, Backman L. Aging magnifies the effects of dopamine transporter and D2 receptor genes on backward serial memory. Neurobiol Aging. 2013;34:358, e1–10. doi: 10.1016/j.neurobiolaging.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Slagter HA, Tomer R, Christian BT, Fox AS, Colzato LS, King CR, Murali D, Davidson RJ. PET evidence for a role for striatal dopamine in the attentional blink: functional implications. J Cogn Neurosci. 2012;24:1932–1940. doi: 10.1162/jocn_a_00255. [DOI] [PMC free article] [PubMed] [Google Scholar]


