Abstract
Various tumors can arise in the urinary bladder (UB); most common is urothelial carcinoma (UC). UC of the UB have many variants. Other types of carcinomas such as adenocarcinoma (AC) and small cell carcinoma (SmCC) can occur in UB carcinomas. Expression of KIT and PDGFRA has not been reported. A 66-year-old man admitted to our hospital because of hematuria. Cystoscopy revealed papillary invasive tumor and a transurethral bladder tumorectomy (TUR-BT) was performed. The TUR-BT showed UC, AC, SmCC, large cell carcinoma (LCC), and pleomorphic carcinoma (PC). The UC component showed plasmacytoid, spindle, nested, clear cell, acantholytic variants. The AC element showed tubular adenocarcinoma and signet-ring cell carcinoma (Sig). Immunohistochemically, all of these subtypes were positive for cytokeratin (CK) AE1/3, CK CAM5.2, CK34BE12, CK5, CK6, CK7, CK8, CK18, CK19, CK20, EMA, CEA, p63, CA19-9, p53 (positive 45%), MUC1, NSE, NCAM, KIT, PDGFRA, and Ki-67 (87%). They were negative for vimentin, chromogranin, synaptophysin, S100 protein, CD34, CD14, α-smooth muscle actin, CD31, caldesmon, CD138, CD45, κ-chain, λ-chain, MUC2, MUC5AC and MUC6. Mucin histochemistry revealed mucins in AC element including Sig. A molecular genetic analysis using PCR-direct sequencing method identified no mutations of KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes. The carcinoma was highly aggressive and invaded into muscular layer. The nuclear grade was very high, and there were numerous lymphovascular permeations were seen. The surface showed carcinoma in situ involving von-Brunn’s nests. This case shows that carcinoma of UB can show diverse differentiations into numerous histological types and variants, and can express KIT and PDGFRA. The both genes showed no mutations in the present case.
Keywords: Urinary bladder, urothelial carcinoma, small cell carcinoma, adenocarcinoma, KIT, PDGFRA
Introduction
The urinary bladder (UB) carcinoma may shows various histologies such as urothelial carcinoma (UC), adenocarcinoma (AC), squamous cell carcinoma (SCC), small cell carcinoma (SmCC), large cell carcinoma (LCC), pleomorphic carcinoma (PC), urachal-type adenocarcinoma (UTA), signet-ring cell carcinoma (Sig), spindle cell carcinoma (SpCC), sarcomatoid carcinoma (SC) and so on [1-12]. The majority of UB cancers are UC. UC but most common is urothelial carcinoma (UC). The UC of UB shows diverse histological variants in addition to ordinary UC [1-12]. These variants (V) includes plasmacytoid V, nested V, sarcomatoid V, spindle cell V, lipoid cell V, micropapillary V, and clear cell V.
Asides from carcinoma, various types of sarcoma rarely occur in the UB. The most common is embryonal rhabdomyosarcoma. Small cell carcinoma and MALT lymphoma also rarely occurs in the UB [1-12].
KIT and PDGFRA, receptor tyrosine kinase oncoproteins, are known to be sometimes expressed in SmCC and SC [13-19]. However, the analysis of KIT and PDGFRA is still inadequate.
The author herein reports an extremely rare case of carcinoma of UB with expressions of KIT and PDGFRA and showing diverse differentiations into various variants of UC and other histological types.
Case report
A 66-year-old man admitted to our hospital because of hematuria. A blood land urine laboratory test showed no significant changes, Cystoscopy was performed and it revealed papillary invasive tumor. A transurethral bladder tumorectomy (TUR-BT) was performed.
The TUR-BT showed ordinary urothelial carcinoma (UC) (Figure 1A), adenocarcinoma (AC) (Figure 1B), small cell carcinoma (SmCC) (Figure 1C), large cell carcinoma (LCC) (Figure 1D), and pleomorphic carcinoma (PC) (Figure 1E). There are frequent merges among these histological types. The UC component showed plasmacytoid variant (V) (Figure 1F), spindle V (Figure 1G), nested V (Figure 1H), clear cell V (Figure 1I), acantholytic variants V (Figure 1J), and sarcomatous V (Figure 1K). The AC element showed tubular adenocarcinoma and signet-ring cell carcinoma (Sig) (Figure 1L). There were frequent gradual transitions among these UC Vs.
Figure 1.
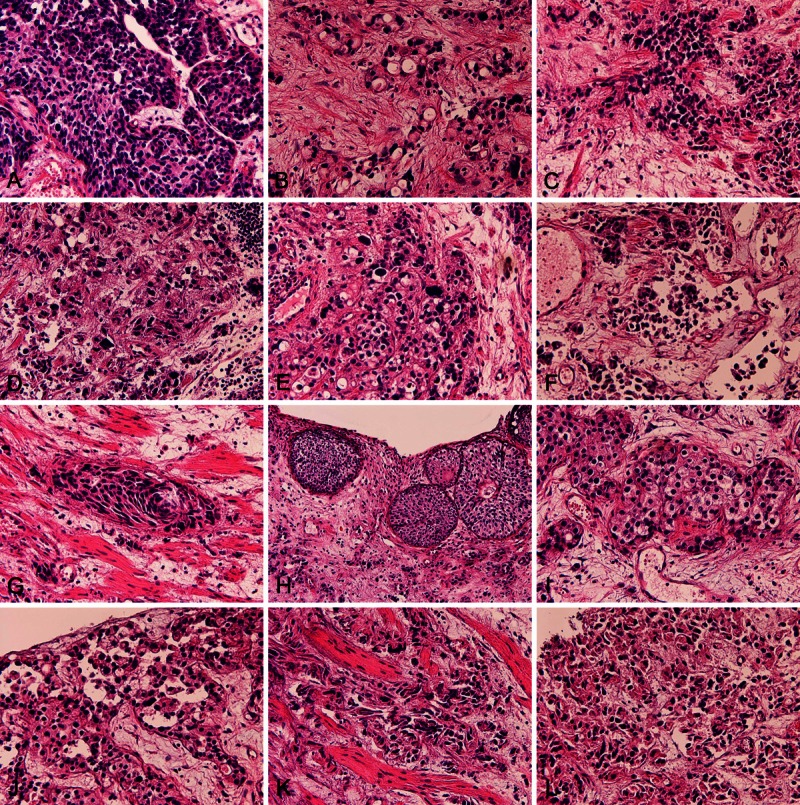
Histological findings. The biopsy of the present tumor showed ordinary urothelial carcinoma (A), adenocarcinoma (B), small cell carcinoma (C), large cell carcinoma (D), and pleomorphic carcinoma (E). There are frequent merges among these histological types. The urothelial component showed plasmacytoid variant (V) (F), spindle cell V (G), nested V (H), clear cell V (I), anantholitic variants V (J), and sarcomatous V (K). The adenocarcinoma element showed tubular adenocarcinoma and signet-ring cell carcinoma (L). There were frequent gradual transitions among these UC variants. HE: A-L: x150.
Histochemistry was performed with the use of mucicarmine, colloidal iron, periodic acid Schiff (PAS), diastase-pre-digestion PAS (d-PAS), Alcian blue at pH2.5 and pH1.0, and these combined techniques. It was found that the AC and Sig were positive for neutral, carboxylate, and sulfated mucins. Other histological types and UC variants are free of mucins, but contained glycogen. The clear cell V and plasmacytoid V of UC had glycogen, but not mucins.
An Immunohistochemical study was performed by Dako’ Envidion method. As previously reported [20,21]. Immunohistochemically, all of these subtypes were positive for cytokeratin (CK) AE1/3, CK CAM5.2, CK34BE12, CK5 (Figure 2A), CK6, CK7 (Figure 2B), CK8, CK18, CK19, CK20, EMA (Figure 2C), CEA (Figure 2D), p63 (Figure 2E), CA19-9, p53 (positive 45%) (Figure 2F), MUC1, NSE, NCAM, KIT (Figure 2G), PDGFRA (Figure 2H) and Ki-67 (87%) (Figure 2I). They were negative for vimentin, chromogranin, synaptophysin, S100 protein, CD34, CD14, α-smooth muscle actin, CD31, caldesmon, CD45, CD138, κ-chain, λ-chain, MUC2, MUC5AC and MUC6. Mucin histochemistry revealed mucins in AC element including Sig.
Figure 2.
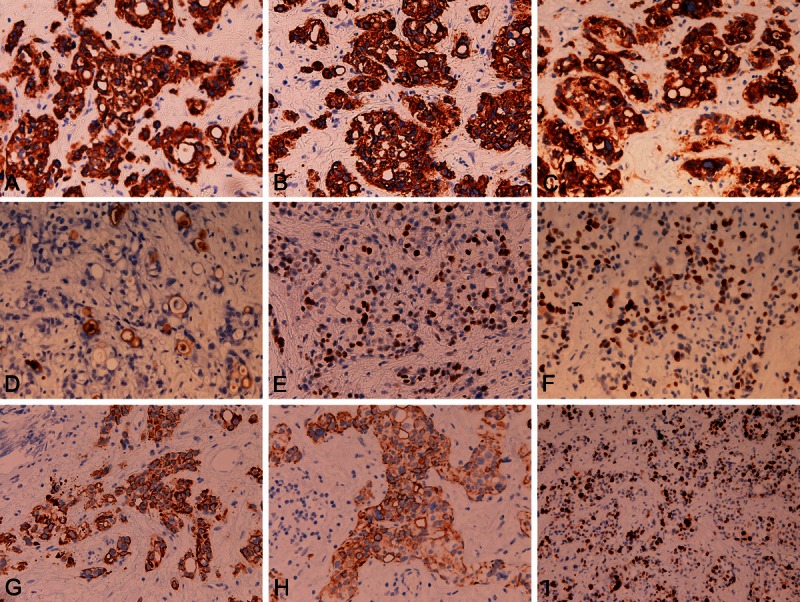
Immunohistochemical findings. All of these subtypes of the present study were positive for cytokeratin (CK) AE1/3, CK CAM5.2, CK34BE12, CK5 (A), CK6, CK7 (B), CK8, CK18, CK19, CK20, EMA (C), CEA (D), p63 (E), CA19-9, p53 (positive 45%) (F), MUC1, NSE, NCAM, KIT (G), PDGFRA (H) and Ki-67 (87%) (I). They were negative for vimentin, chromogranin, synaptophysin, S100 protein, CD34, CD14, α-smooth muscle actin, CD31, caldesmon, CD45, CD138, κ-chain, λ-chain, MUC2, MUC5AC and MUC6. A-I: x200.
A molecular genetic analysis of KIT gene (exons 9, 11, 13, and 17) and PDGFRA gene (exons 12 and 18) was performed by the PCR direct sequencing method, as previously reported [13-19]. This was performed because the tumor cells were positive for KIT and PDGFRA. The author always investigates the mutational status of these two genes when the author encounters tumors positive for KIT and PDGFRA. This is because, if activating mutations were found, imatinib mesylate, a gene targeting drug, may be effective. The exons of both genes were selected because they are frequent mutation sites. The primers were used as previously reported, and were shown in Table 1. In brief, the genomic DNA was extracted from the paraffin sections containing the SmCC cells with proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94°C for one minute, 52°C for one minute, 72°C for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The annealing temperature was 53°C. PCR products were extracted, and subjected to a computed automatic DNA sequencer (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems, ABI, CA). Two cases of gastric GIST and two cases of uterine leiomyoma were used as positive controls and negative controls, respectively.
Table 1.
Primer sequence
| Forward | Reverse |
|---|---|
| KIT exon 9 | |
| 5’-TCC TAG AGT AAG CCA GGG CTT-3’ | 5’-TGG TAG ACA GAG CCT AAA CAT CC-3’ |
| KIT exon11 | |
| 5’-GAT CTA TTT TTC CCT TTC TC-3’ | 5’AGC CCC TGT TTC ATA CTG AC-3’ |
| KIT exon 13 | |
| 5’-GCT TGA CAT CAG TTT GCC AG -3’ | 5’-AAA GGC AGC TTG GAC ACG GCT TTA-3’ |
| KIT exon 17 | |
| 5’-CTC CTC CAA CCT AAT AGT GT-3’ | 5’-GTC AAG CAG AGA ATG GGT AC-3’ |
| PDGFRA exon12 | |
| 5’-TTG GAT ATT CAC CAG TTA CCT GTC-3’ | 5’-CAA GGG AAA AGC TCT TGG-3’ |
| PDGFRA exon 18 | |
| 5’-ACC ATG GAT CAG CCA GTC TT-3’ | 5’-TGA AGG AGG ATG AGC CTG ACC-3’ |
The molecular analysis revealed no mutations of genes of KIT (exons 9, 11, 13, and 17) and PDGFRA (exons 12 and 18) genes in this bladder tumor, Imatinib may be ineffective.
The carcinoma was highly aggressive and invaded into muscular layer. The nuclear grade was very high, and there were numerous lymphovascular permeations were seen. The surface showed carcinoma in situ (CIS) involving von-Brunn’s nests. The patient referred to another hospital specializing in urological cystectomy.
Discussion
It is well recognized that carcinoma of bladder shows various histology [1-12]. The most common is urothelial carcinoma (UC), followed in order by adenocarcinoma (AC), squamous cell carcinoma (SCC), and sarcomatoid carcinoma (SC). Small cell carcinoma (SmCC), and urachal-type AC were very rarely seen in the UB. In the present case, the carcinoma of UB consisted of many cells types including ordinary UC, AC, SmCC, signet-ring cell carcinoma (Sig), large cell carcinoma (LCC), and pleomorphic carcinoma (PC). Squamous cell carcinoma (SCC) was not recognized. The LCC and PC in UB have not been reported in the literatures. The present tumor is quite interesting because one carcinoma of the UB showed many histological types. Such a case has not been reported in the literature.
Among these various histological types of the present tumor of UB, the most prevalent was AC, followed by UC, SmCC, Sig, LCC and PC [1-12]. In general, urinary bladder carcinoma is UC. The immunohistochemical data demonstrated p63 antigen which is expressed of basal layer cells of UC and SCC as well as basal and myoepithelial cells and their malignant counterparts. P63 is usually negative in AC. In addition, the CK profiles of the present UB carcinoma showed wide ranges of CK profiles containing high molecular weight CK (CK34BE12, CK5, and CK6), which are often seen in UC and SCC. AC, in contrast, shows low molecular weight cytokeratins such as CK8 and CK18. In the present study, almost all histological types of the tumor showed the same immunophenotype. From there reasons, it seem very probable that the main tumor of the present UB carcinoma is ordinary UC. Also, it seems very probable that other histological types of the present tumor are derived from UC. That is, it seems that the ordinary UC gives rise to or transdifferentiates into AC, Sig, SmCC, LCC, PC and other types in the present study. The fact that there were gradual merges among the histological types supports this suggestion. Thus, it seems that UC may transdifferentiate into various other histological types.
In the present study, the UC showed many variants (V); the present UC showed areas of nested V, clear cell V, plasmacytoid V, acantholytic V, spindle cell V, and sarcomatous V. The each V was certain histologically. However, there were merges among these Vs. Thus, these Vs reflect the diversity of UC differentiations. The biological behaviors of these Vs are similar to each others except for spindle V and sarcomatous V, which shows poor outcome. Therefore, each V of these Vs seems not to be a clinicopathological entity. With regards to spindle cell V and sarcomatous V, they may be used in major histological types in significant number of papers, not variants of UC. The author thinks that the spindle and sarcomatous carcinomas should be categorized as spindle cell carcinoma and sarcomatous carcinoma, not UC variants.
In the present case, a broad range of immunohistochemistry was performed. Immunohistochemically, all of these subtypes were positive for CK AE1/3, CK CAM5.2, CK34BE12, CK5, CK6, CK7, CK8, CK18, CK19, CK20. The present tumor has wide ranges of CK profiles from low molecular weight CK to high molecular weight CK. The CK7+/CK20+ pattern is compatible with primary UC of the UB. The positive CK indicate that the current tumor is an epithelial tumor. EMA was positive, indicating the epithelial nature of the tumor. CEA and CA-19-9 was positive, indicating AC nature of the tumor. p63 was positive, suggesting the UC, SCC, basal cell nature of the present tumor. p53 was strongly positive, suggesting p53 gene mutations and malignant potential of the present tumor. MUC1 (transmembranous non-secretory mucin) and mucins were positive, suggesting glandular differentiation. NSE and NCAM were positive, suggesting neuroendocrine differentiation of the present case. Ki-67 labeling was very high (87%), indicating high cell proliferation of the present study and suggesting poor prognosis. Vimentin was negative, suggesting that the tumor is not mesenchymal tumor. S100 protein was negative, suggesting non-neural, non-lipomatous nature. CD31 and CD34 were negative, suggesting non-vascular tumor of the present tumor. Caldesmon and α-smooth muscle actin were negative, suggesting non-smooth muscle natures. CK14 was negative, suggesting non-basal and non-myoepithelial natures of the present tumor. CD45 was negative, suggesting that the present tumor is not leukocytic malignancy. CD138, κ-chain and λ-chain were negative, suggesting that the present tumor is not plasma cell dyscrasia and the plasmacytoid variant of UC (CK-positive) is not composed of plasma cells. MUC2, MUC5AC and MUC6 were negative, suggesting that the present tumor does not contain mucin phenotypes of goblet cells mucins (MUC2), gastric foveolar cells mucins (MUC5AC) and pyloric glands mucins of stomach (MUC6). Mucin histochemistry revealed mucins in AC element including Sig. Other types or UC variants showed glycogen but no mucins. The clear cell V and plasmacytoid V of UC did not show mucins. Taken together, the mucins of the AC and Sig elements of the present study appear not to contain MUC2, MUC5AC and MUC6 secretory mucin though MUC1 transmembranous non-secretory mucins are present.
In the present study, KIT and PDGFRA were positive. KIT and PDGFRA positivity have been reported in SmCC and sarcomatous carcinomas by the author [2-21]. In both tumors, strong expression of KIT and PDGFRA were seen, but no mutations of KIT and PDGFRA were noted. In the present study, since SmCC element was seen in the tumor, the author performed the immunostaining and genetics of KIT and PDGFRA. Very surprisingly, KIT and PDGFRA were expressed all other carcinomatous elements in addition to the SmCC element. Such an UC case with strong KIT and PDGFRA expression has not been reported.
SmCC of the lung and the exptrapulmonary organs very frequently express KIT and PDGFRA [13-21]. In the present study also, the author investigated the gene status of KIT and PDGFRA. It was found that no mutations were seen in the hot spots. KIT and PDGFRA, both mapped to 4q12, encode transmembranous receptor tyrosine kinase oncoproteins called KIT (CD117) and PDGFRA, respectively. Both molecules are transmembranous oncoproteins involved in tumorigenesis, particularly in gastrointestinal stromal tumor. It is famous that SmCC of the lung and extrapulmonary organs express KIT and PDGFRA, but no mutations at the hot spots of GIST; namely KIT gene (exons 9, 11, 13, and 17) and PDGFRA gene (exons 12 and 18). In future, mutations of other exons and all introns should be examined in SCLC and SmCC. In GIST, imatinib mesylate, a gene targeting drug, is relatively very effective. However, imatinib has little effect in SCLC. In GIST, many kinds of gain-of-function mutations are seen in KIT and PDGFRA genes. Imatinib appears effective in cases with mutations of KIT and PDGFRA. Because the KIT and PDGFRA expressions are very characteristics of SmCC of the lung and extrapulmonary locations, analyses of all exons of introns of the both genes are mandatory. If mutations would be found, creating new molecular targeting drugs are possible.
Finally, the present UB carcinoma showed high grade nuclear grade. The invasion is severe and the invasion is at least muscular later (stage pT2). Numerous lymphovascular permeations were seen. From these observations, it seems that the prognosis in not good. In addition, this histological and immunohistochemical studies were those only in small TUR-BT. It is strongly suggested that many other differentiations of the UC is really seen in this neoplasm.
In conclusion, the authors reported an extremely rare case of high grade urothelial carcinoma with diverse and numerous differentiation into other histological type and urothelial variants. The tumor is also characterized by strong expression of KIT and PDGFRA, which showed no mutations in hot spots in the present case.
Declaration
The author has no conflict of interest.
References
- 1.Lopes-Beltran A, Knowles MA, Sauter G, et al. World Health Organization of tumours. Infiltrating urothelial carcinoma. In: Eble JN, Sauter G, Epstain JI, Sesterhenn IA, editors. Pathology and genetics of tumours of the urinary system and male genital organs. Lyon: IARC Press; 2004. pp. 93–109. [Google Scholar]
- 2.Murphy WM, Grignon DJ, Perlman EJ. Tumors of the kidney, bladder, and related urinary structures. Series 4. Washington DC: Armed Forces Institute of Pathology; 2004. Urothelial carcinoma, nested type. AFIP Atlas of Tumor Pathology. pp. 284–285. [Google Scholar]
- 3.Terada T. Urinary bladder carcinoma with triplicate differentiations into giant cell sarcomatoid carcinoma, squamous cell carcinoma, and papillary urothelial transitional cell carcinoma: a case report. Cases J. 2009;2:9111. doi: 10.1186/1757-1626-2-9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terada T. Synchronous squamous cell carcinoma of the kidney, squamous cell carcinoma of the ureter, and sarcomatoid carcinoma of the urinary bladder: a case report. Pathol Res Pract. 2010;206:379–383. doi: 10.1016/j.prp.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Sarcomatoid carcinoma of the urinary bladder: a case report with immunohistochemical and molecular genetic analysis. Med Oncol. 2010;27:547–553. doi: 10.1007/s12032-009-9247-3. [DOI] [PubMed] [Google Scholar]
- 6.Terada T. Autopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutations. Pathol Int. 2009;59:247–250. doi: 10.1111/j.1440-1827.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 7.Terada T. Spindle cell carcinoma progressed from transitional cell carcinoma of the urinary bladder. Int J Clin Exp Pathol. 2012;5:83–88. [PMC free article] [PubMed] [Google Scholar]
- 8.Terada T. Primary pure signet-ring cell adenocarcinoma of the urinary bladder: a report of three cases with an immunohistochemical study. Med Oncol. 2012;29:2866–2869. doi: 10.1007/s12032-011-0122-7. [DOI] [PubMed] [Google Scholar]
- 9.Terada T. An Autopsy Case of Clear Cell Adenocarcinoma of the Urinary Bladder. Appl Immunohistochem Mol Morphol. 2011 Oct 20; doi: 10.1097/PAI.0b013e31821b193b. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Terada T. Primary CD5-positive mucosa-associated lymphoid tissue lymphoma of the urinary bladder. Ann Diagn Pathol. 2011;15:382–384. doi: 10.1016/j.anndiagpath.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Terada T. Small cell carcinoma of the urinary bladder. Int J Clin Exp Pathol. 2012;5:596–600. [PMC free article] [PubMed] [Google Scholar]
- 12.Terada T. Nested variant of urothelial carcinoma of the urinary bladder. Rare Tumors. 2011 Oct 21;3:e42. doi: 10.4081/rt.2011.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terada T. Gastrointestinal stromal tumor of the digestive organs: a histopathologic study of 31 cases in a single Japanese institute. Int J Clin Exp Pathol. 2010;3:162–168. [PMC free article] [PubMed] [Google Scholar]
- 14.Terada T. Primary small cell carcinoma of the ureter: a case report with immunohistochemical and molecular genetic analysis of KIT and PDGFRA genes. Pathology. 2010;42:101–102. doi: 10.3109/00313020903443018. [DOI] [PubMed] [Google Scholar]
- 15.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 16.Terada T. Amelanotic malignant melanoma of the esophagus: report of two cases with immunohistocheimcal and molecular genetic study of KIT and PDGFRA . World J Gastroenterol. 2009;15:2679–2683. doi: 10.3748/wjg.15.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 18.Terada T. Gastrointestinal stromal tumor of the uterus: A case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 19.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terada T, Ashida K, Endo K, Horie S, Maeta H, Matsunaga Y, Takashima K, Ohta T, Kitamura Y. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325–331. doi: 10.1046/j.1365-2559.1998.00496.x. [DOI] [PubMed] [Google Scholar]
- 21.Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175–80. doi: 10.1016/s0046-8177(98)90229-5. [DOI] [PubMed] [Google Scholar]


