Abstract
We have previously identified pancreatic and duodenal homeobox 1 (Pdx1) autoantibodies (PAA) in sera from both non-obese diabetic (NOD) mice and human type 1 diabetic (T1D) patients. A suitable non-radioactive, sensitive and specific assay is needed for large-scale testing to determine the clinical utility of PAA. Here we reported a liquid-phase luciferase immunoprecipitation system (LIPS) assay by generating a renilla luciferase (Rluc)-Pdx1 fusion protein as a sensitive non-radioactive antigen from mammalian cells combined with immunoprecipitation to detect PAA in human sera. Sera from healthy donors and the University of Florida Pathology Laboratories, Endocrine Autoantibody Laboratory were used to validate the LIPS assay for PAA. Antigenic specificity to Pdx1 was confirmed by using a Rluc-only control compared to Rluc-Pdx1 fusion antigen and by competition assays using purified recombinant Pdx1 protein. We then used the LIPS assay to assess the prevalence of triple autoantibodies (GADA, IA-2A, and IA-2βA), and PAA in non-T1D control sera, recent onset (RO)-T1D sera (mean duration of T1D = 9.5 weeks), and long standing (LS)-T1D sera. Compared to clinical radioimmunoprecipitation assays (RIPA), the LIPS assay showed comparable sensitivity and specificity for detection of GADA and IA-2A. PAA were detectable in human serum samples and higher in triple-positive T1D autoantibodies (21% PAA positive in triple positive sera and 4% PAA positive in triple negative sera). Interestingly, PAA were found to be highest in the non-T1D population, suggesting that PAA might have a clinical utility in screening high-risk population susceptible for developing T1D. In conclusion, we have developed a liquid-phase, non-radioactive, sensitive and specific LIPS assay to detect PAA in human sera, providing a useful tool for evaluating the clinical relevance of PAA.
Keywords: Pancreatic and duodenal homeobox 1 (Pdx1), Pdx1 autoantibodies (PAA), type I diabetes, luciferase immunoprecipitation systems (LIPS) assay
Introduction
Type 1 diabetes (T1D) is an autoimmune cell-mediated destruction of pancreatic beta cells within the islets of Langerhans [2,3]. Mounting evidence indicates that in T1D, beta cell proteins are perceived by patients’ immune cells as autoantigens (AAgs) and thereby trigger an autoimmune inflammatory reaction around and within the islets called insulitis. AAgs can invoke autoantibodies and specific reactive T cells that are ultimately responsible for beta cell death. Therefore, detection of autoantibodies to beta cell AAgs facilitates the prediction and diagnosis of T1D and investigation of antigen-specific T cells provides insights into the immunopathogenesis of T1D, which in turn uncovers new targets for immunomodulatory therapeutic approaches for the treatment of T1D. Multiple beta cell-related or beta cell-specific AAgs including insulin [27], glutamic acid decarboxylase (GAD) [4], insulinoma associated protein 2 (IA-2) [6,19], IA-2β [22], and zinc transporter 8 protein (ZnT8) [31] and their corresponding autoantibodies have been identified and characterized in T1D and populations at high risk for the disease. These autoantibodies are useful for T1D diagnosis and the AAgs may be useful for specific immunotherapy [33]. Autoantibodies can serve as an early indicator of susceptibility to T1D because they are present in 70-80% of newly diagnosed patients as opposed to their limited presence in the general population [3]. Insulin autoantibodies (IAA) [27], islet cell autoantibodies (ICA) [7], GAD autoantibodies (GADA) [4], and IA-2 autoantibodies (IA-2A) [6] are useful markers for the diagnosis and prediction of T1D onset, and the latter may have a role for determining the timing of intervention [33].
Pancreatic and duodenal homeobox 1 (Pdx1) is a master-control transcription factor for pancreatic development and maintenance of beta cell function that also plays important roles in pancreatic beta cell survival and regeneration [15]. Pdx1 protein contains a protein transduction domain (PTD), which is a specific argine- and lysine-enriched amino acid sequence that allows the protein to be transported across cellular membranes by lipid raft-mediated macropinocytosis [24-26]. Utilizing this property, we have demonstrated that in vivo treatment of chemically induced diabetic mice with recombinant Pdx1 protein (rPdx1) [21] reversed diabetes by reprogramming liver cells into insulin-producing cells and promoting pancreatic islet beta cell regeneration [18]. Unexpectedly, we discovered that Pdx1 is also a beta-cell specific AAg recognized by Pdx1 autoantibodies (PAA) found in sera of non-obese diabetic (NOD) mice and patients with T1D [20]. We also have reported T cell proliferative responses to Pdx1 in NOD mice. Interestingly, PAA levels in NOD mice often peaked before the onset of diabetes and then decreased to very low or undetectable levels by the time of T1D onset [20]. Furthermore, our unpublished observation suggest that treatment of pre-diabetic female NOD mice with a biologically inactive rPdx1 (with deletion of the PTD domain) prevented the onset of T1D due to up-regulation of Th2 inhibitory cytokines and down-regulation of Th1 proinflammatory cytokines (manuscript in preparation).
Although we detected the presence of PAA in human serum by western blotting, we were unable to detect human PAA from the same samples by ELISA, possibly due to an altered conformation of the PAA epitope. In view of the key role of Pdx1 in beta cell development, function, survival, and regeneration [15], we were interested in the clinical relevance of PAA in human T1D and high-risk populations and developed a sensitive, specific, non-radioactive, high throughput assay for detection of PAA [20].
Most clinical assays for the detection of T1D-related autoantibodies utilize a liquid-phase radioimmunoprecipitation assay (RIPA). However, an alternative, non-radioactive, assay known as the luminescence immunoprecipitation system (LIPS) assay [8,9], has been developed recently to detect GADA [10,11], IA-2A [11,12], and IA-2βA [11] with equal or greater sensitivity and specificity compared to RIPA assays. Here, we report a LIPS assay for detecting PAA in human sera using a renilla luciferase (Rluc)-Pdx1 fusion protein produced in mammalian cells providing a liquid-phase, non-radioactive, and high-throughput means of detecting PAA in human sera. We then evaluated the prevalence of GADA, IA-2A, IA-2βA, and PAA in both recent onset and long standing diabetic populations.
Materials and methods
Plasmid construction
The human Rluc-Pdx1 fusion plasmid was constructed by cloning the Rluc gene (from the pRen2 plasmid) upstream of the Pdx1 gene in pCMV-XL5 (Open Biosystems) using HindIII/BamHI restriction sites for expression in mammalian cells. A stop codon was introduced at the 3’ end of the Rluc gene by site-directed mutagenesis to construct the Rluc-only expression control. Rluc fusion construct plasmids (Rluc-GAD65 [10], Rluc-IA-2 [12], and Rluc-IA-2β [11]) were generous gifts from Dr. Peter Burbelo (NIH, Bethesda, MD). All plasmids were purified using the Plasmid Maxi Kit (Qiagen).
Fusion protein lysate
Mammalian fusion protein lysates were prepared by transfecting human embryonic kidney (293) cells with each plasmid using Lipofectamine 2000 Reagent (Invitrogen) according to manufacturer’s protocol in 10cm2 culture dishes. Forty-eight hours following transfection, lysates were harvested using 1ml Passive Lysis Buffer (Promega) per dish and supernatant (lysate) was collected following centrifugation. 293 cells were cultured at 37°C in DMEM containing 10% fetal bovine serum and 1% Penicillin/Streptomycin. 1µl of each reagent can yield ≥ 2 × 108 RLUs in Berthold Lumat LB9507.
Sera
54 serum samples from the University of Florida Pathology Laboratories, Endocrine Autoantibody Laboratory were used to validate the LIPS assay, 29 of which tested triple-positive for ICA (by indirect immunofluorescence), GADA, and IA-2A (by RIPA). The remaining 25 sera from this group tested triple negative for ICA, GADA, and IA-2A (by RIPA). The ICA assay has been validated previously and was the basis for the Diabetes Prevention Trial-1 study [1] and is used in the current T1D TrialNet studies [23]. The GADA and IA-2A RIPAs were manufactured by Kronus, Inc. (Star, Indiana) and validated previously. Sera from healthy human donors (n = 10) with no known history of autoimmune diseases were used to establish the cut-off between positive and negative for detecting GADA, IA-2A, and PAA when using the LIPS assays for these 54 sera. We next obtained 100 non-T1D sera (Ctrl), 100 recent-onset (RO)-T1D sera (mean T1D duration = 9.5 weeks), and 50 long-standing (LS)-T1D sera (patients were on a renal transplant list) to measure the prevalence of GADA, IA-2A, IA-2βA, and PAA in these populations by LIPS assay. The 100 Ctrl sera had a mean age of 19.6 years (range = 2.7 - 52.3 years) with a male to female ratio of 1.0 and the 100 RO-T1D sera had a mean age of 14.8 (range = 5.9 - 41 years) with a male to female ratio of 0.8. In addition, four sera from the LS-T1D group (that were previously identified as PAA positive) were used for determination of PAA antigenic specificity. All sera were measured in a blinded fashion. Human sample collection was approved by Institutional Review Board of University of Florida.
LIPS assay
LIPS assays were performed similarly to the previously published protocols [8,9] with minor modifications. Rluc-Pdx1 fusion protein cell lysate (≥ 2 × 107 RLUs in Berthold Lumat LB9507) was incubated with 10μl human serum for PAA (or 1μl to 5µl serum for GADA, IA-2A, and IA-2βA as described) in 96-well round-bottom plates at a total volume of 100μl in PBS overnight with agitation. Samples were then transferred to 96-well filter plates containing 10μl Immobilized Protein A/G Plus Beads (Pierce) of 50% concentration (beads/volume) and incubated at 4°C for 2hrs with agitation. All samples were washed 8 times with Buffer A as previously described [8,9]. 20μl PBS was added to each well before reading in a LUMIstar Omega plate reader (BMG Labtech). Competition assays used purified recombinant Pdx1 protein (rPdx1) [21] or bovine serum albumin (BSA) at indicated concentrations and were incubated with Rluc-Pdx1 fusion cell lysate overnight. Due to limited sera volumes, all assays were performed in singlet. We obtained extra sera for negative controls and some strong PAA positives that were used for competition assays to determine Pdx1 antigenic specificity.
Results
Establishment of LIPS assay
Because PAA are a recent discovery, there is no standard assay for detecting these autoantibodies in the clinical setting. To validate the LIPS assay, we first compared our detection of GADA (Figure 1A) and IA-2A (Figure 1B) in patient samples by LIPS assay to the results determined by established clinical RIPA. Sera from patients (n = 54) and control subjects (n = 10) were screened for GADA or IA-2A by LIPS assay and a positive cutoff was arbitrarily set at 3 standard deviations (SD) above the mean of the 10 control subjects. For GADA, LIPS identified 28 out of 29 (97%) RIPA-positive sera and 6 additional LIPS positive sera that were RIPA-negative (24%). For IA-2A, LIPS identified all 29 (100%) RIPA-positive sera and one additional LIPS positive serum that was RIPA-negative (4%). However, the degree of positivity for individual samples varied greatly between RIPA and LIPS assay. For example, a particular sample may yield a high positive value by RIPA but a low to moderate value by LIPS assay, or vice versa, suggesting that the two assays may recognize different epitopes or have different affinity. Compared to the results of clinical RIPA, the LIPS assay gives 97% sensitivity and 76% specificity for GADA detection and 100% sensitivity and 96% specificity for IA-2A detection. However, since the accuracy of the reference assay (RIPA) is unknown, the low specificity could alternatively indicate that the LIPS assay is actually more sensitive than RIPA. Taken together, these results suggest that the LIPS assay has sensitivity comparable to RIPA for detecting GADA and IA-2A.
Figure 1.
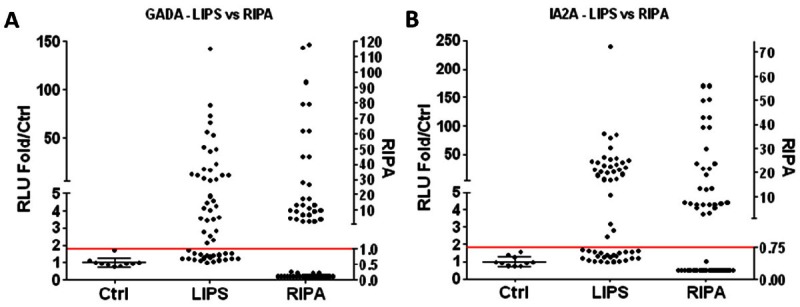
Detection of GADA and IA-2A by LIPS. Sera (n = 54) that were tested for ICA, GADA, and IA-2A clinically by RIPA or healthy normal donor control sera (n = 10) were used in panels A, B, and the transverse line is 3SD above control mean. RLU (relative light units) are expressed as fold relative to control mean (left y-axis). Standard LIPS assays (1μl serum) were performed with (A) Rluc-GAD65 fusion protein lysate or (B) Rluc-IA-2 fusion protein lysate and are compared to clinical RIPA data (right y-axis).
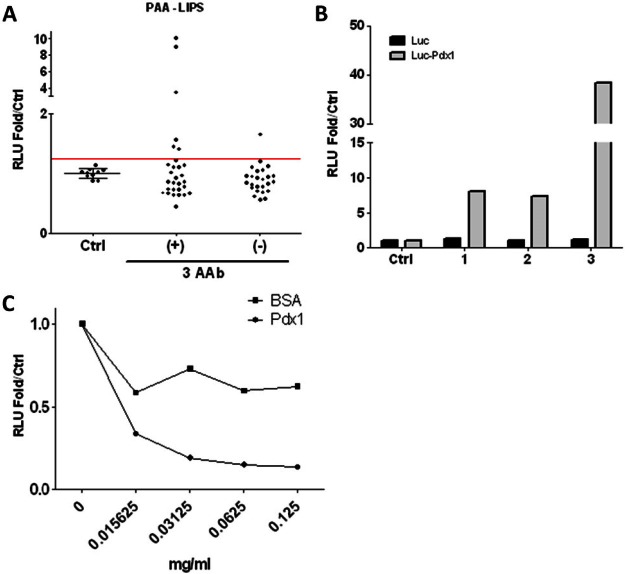
Next, we tested the same serum samples (54 patients and 10 healthy controls) for PAA by LIPS assay (Figure 2A). We arbitrarily set the positive cutoff at 3 SD above the mean of 10 healthy control sera and defined any sample above this cutoff as positive for PAA. Using this cutoff, 7 out of 54 sera were positive for PAA (13%). Among the PAA positive samples, 6 out of 29 triple-positive serum samples were PAA positive (21%) and 1 out of 25 triple-negative serum samples was PAA positive (4%).
Figure 2.
Detection and antigenic specificity of PAA in human sera. A. Sera (n = 54) that were tested for ICA, GADA, and IA-2A clinically by RIPA or healthy normal donor control sera (n = 10) were used to detect PAA using a standard LIPS assay (10μl serum) with Rluc-Pdx1 fusion protein lysate. RLU are expressed as fold relative to control mean. AAb = autoantibody. B. Sera (10μl) that were negative- (Ctrl), medium- (A & B), or high-signal positive (C) for PAA (from T1D patients awaiting renal transplants) were used in a standard LIPS assay using either Rluc-Pdx1 antigen or Rluc-only antigen for detection. C. A standard LIPS assay was performed to detect PAA from our highest signal positive serum (10μl) incubated with indicated concentrations of rPdx1 or BSA.
Validation of LIPS for PAA
To validate the LIPS assay for PAA, we selected serum samples from three LS-T1D patients awaiting renal transplantation that previously tested negative, medium, or high positive for PAA by LIPS assay. To exclude the possibility of antibody binding to Rluc, immunoreactivity with both Rluc-Pdx1 antigen and with Rluc antigen alone was tested, demonstrating that the PAA-positive sera were only detected using Rluc-Pdx1 containing lysate (Figure 2B). We next confirmed Pdx1 antigenic specificity using a competition assay with purified rPdx1 (Figure 2C). First, we used rabbit anti-mouse Pdx1 immune serum to confirm that anti-Pdx1 antibodies can be selectively removed by an increasing rPDX1 protein concentration, indicating that LIPS assay for Pdx1 antibodies is specific to Pdx1 protein (data not shown). We then selected the highest-PAA positive sample and carried out the LIPS assay in the absence or presence of various concentrations of rPdx1 or BSA as a control. The PAA signal was reduced by incubation with increasing concentrations of rPdx1 but not with BSA, confirming that the PAA signal is specific for Pdx1 protein. BSA non-specifically reduced the signal but was unable to block detection of PAA even at high concentrations (0.125mg/ml).
Prevalence of PAA in T1D populations
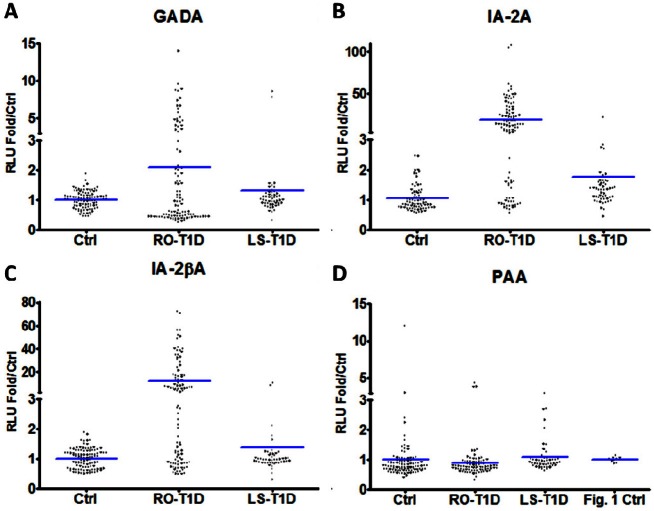
To determine the prevalence of PAA in the general and T1D populations, we first obtained serum samples from RO-T1D patients (n = 100) along with age-matched controls (n = 100) from the UF serum bank, and serum from LS-T1D patients (n = 50) waiting for renal transplant from the pathology-immunology laboratory. We used the LIPS assay to assess the prevalence of GADA (Figure 3A), IA-2A (Figure 3B), IA-2βA (Figure 3C), and PAA (Figure 3D) in these groups. Using 3 SD above mean to identify positive sera, the RO-T1D group had 32/100 (32%) positive for GADA, 69/100 (69%) positive for IA-2A, and 59/100 (59%) positive for IA-2βA. Using the same method for cutoff, the LS-T1D group had 2/50 (4%) positive for GADA, 4/50 (8%) positive for IA-2A, and 3/50 (6%) positive for IA-2βA. These results indicate that not every RO-T1D patient is positive for all three autoantibodies and a loss of GADA, IA-2A, and IA-2βA occurs after clinical onset of T1D. Using the cut off of 3 SD above mean of non-T1D controls for identification of PAA positive sera would indicate that there are no PAA positive sera in any RO-T1D or LS-T1D individuals. However, since the clinical history of this population is unknown and since they could have other diseases that lead to development of PAA, we also used the cut-off set by the previous normal 10 healthy control sera to reexamine our data. It is interesting to note that the non-T1D group has the highest prevalence of PAA positive sera among these three groups of serum samples using this method of analysis.
Figure 3.
Detection of autoantibodies in T1D patient sera. Non-T1D control (ctrl) sera (n = 100), RO-T1D sera (n = 100, mean duration of T1D = 9.5 weeks), and LS-T1D sera (n = 50) were used for all panels and the transverse lines represent means. Standard LIPS assays (using 5μl serum) were performed with (A) Rluc-GAD65 fusion protein lysate, (B) Rluc-IA-2 fusion protein lysate, or (C) Rluc-IA-2β fusion protein lysate. D. Standard LIPS assays (using 10μl serum) were performed with Rluc-Pdx1 fusion protein lysate. The healthy normal donor control sera (n = 10) were also added to this panel for additional analysis. RLU are expressed as fold relative to control mean.
Discussion
We have successfully established a liquid-phase, non-radioactive LIPS assay for GADA and IA-2A using 54 clinical samples with known results by RIPA and showed that the LIPS assays for GADA and IA-2A have comparable sensitivity and specificity for detection of T1D-realted autoantibodies in human sera. We also developed a LIPS assay to detect PAA in human sera and confirmed the presence of PAA in 7 out of 54 clinical serum samples, with higher prevalence in autoantibody triple-positive samples than triple-negative ones. Using the established LIPS assay, we screened 100 RO-T1D serum samples with 100 age-matched control samples as well as 50 LS-T1D serum samples. Consistent with the results reported in literature, we found that even in RO-T1D samples, many samples do not contain any detectable T1D-related autoantibodies (GADA, IA-2A, and IA-2βA) and a large portion of these sera have either one or two, but not all three antiantibodies that we tested.
In order to develop the PAA LIPS assay, we also performed LIPS assays to detect GADA and IA-2A. If RIPA is held as the gold standard, one would conclude that LIPS assay has nearly identical sensitivity but a reduced specificity for detection of these autoantibodies. This would suggest that we identified several false positive sera for GADA or IA-2A. However, another interpretation of the data is that our assay is more sensitive than RIPA and that these “false positive” samples are actually true positive samples that are undetectable by RIPA due to the possibility of recognition of different epitopes in the designed assays. Because of the advantages of the LIPS assay, (not involving the use of radioisotopes, sensitive, specific, and more convenient) it may be valuable in the clinical setting for detecting Pdx1 autoantibodies as well as other T1D-related autoantibodies.
Our data did not show a significantly higher prevalence of PAA in sera from patients who were triple-positive for ICA, GADA, and IA-2A than in those who were negative for all three autoantibodies (p = 0.10 by Fisher’s exact test). However, there was a trend toward an association and the numbers of patients evaluated here were relatively low, suggesting that larger studies might reveal an association either with patients who are triple-positive or perhaps with single- or double-positive patients. There also could be an association with other T1D-related autoantibodies such as ZnT8 islet autoantibodies (ZnT8A) [31] or IAA [27]. In addition, T1D patients who are negative for all other known T1D-related autoantibodies might be more likely to have PAA.
We observed a much higher prevalence of GADA, IA-2A, and IA-2βA in RO-T1D sera compared to LS-T1D sera. This suggests a decline of these particular autoantibodies at clinical onset and over the course of disease development with progressive loss of pancreatic beta cells, in agreement with previous reports [16,30,32,34]. Regarding PAA, we have identified no high signal positives from either the RO-T1D or LS-T1D groups. The small but higher prevalence in the non-T1D groups suggests that PAA could be an early autoantibody related to T1D and that these non-T1D PAA positive patients may be prediabetic patients. Since we have no clinical data for these individuals beyond age and sex, we cannot determine if they are prediabetic or possibly have other diseases which may elicit a PAA response. It is interesting to note that we obtained two pancreatic cancer patient serum samples, and using the LIPS assay, we detected one as a high (12-fold above control mean) signal PAA positive (unpublished observation). This indicates that PAA may be associated with diseases other that T1D and this may explain the higher prevalence of PAA in our non-T1D serum group. We speculate that the resulting lesion from pancreatic cancer could cause leaking of Pdx1 protein and subsequent presentation of Pdx1 to the immune system, perhaps by over production of Pdx1 from pancreatic cancer cells [17,28,29], leading to high titer PAA production by B-cells. If PAA is directly associated with pancreatic cancer, detection of PAA could be useful for screening and diagnosing pancreatic cancer which remains one of the most deadly cancers with a poor prognosis and 5 year survival rate less than 5% due to the delayed diagnosis [14]. Obviously, future studies are required to determine if there is an association between developing PAA and ongoing pancreatic injuries and regeneration resulting from acute and chronic pancreatitis as well as pancreatic or other gastrointestinal cancers.
Insulin is considered to be an early AAg in T1D and it has been proposed to be the primary AAg related to T1D [5,13,35]. This is based on the early detection of IAA in people later developing T1D and by the fact that insulin is uniquely expressed and secreted by the beta cell whereas other AAgs are not unique to the beta cell (e.g., GAD or IA-2). Like insulin, Pdx1 could be an early AAg with respect to T1D development and some evidence suggests that Pdx1 could be a primary AAg related to T1D. In our previous report [20], we detected PAA in NOD mice by ELISA, western blotting, and RIPA. We also followed the PAA titer of several female NOD mice during the development of diabetes and found that PAA titers tended to peak prior to the onset of hyperglycemia and then dropped to undetectable levels after T1D onset. This may be due to a lack of Pdx1 antigen stimulation following destruction of the beta cells. To determine whether this is the case for humans will require longitudinal evaluation of PAA in human T1D patients prior to and during disease development. Serum from high-risk individuals or first-degree relatives of T1D patients might be measured for PAA at various time points during disease development, from pre-diabetic to clinical manifestation of diabetes.
Several questions remain unanswered regarding PAA. In particular, large scale systematic, longitudinal studies will be required to determine the prevalence and clinical significance of PAA in the normal population, T1D patients at various stages of disease, high-risk or prediabetic populations, and in other diseases, as well as to identify whether the detection of PAA is useful for prediction, diagnosis, and/or monitoring of T1D patients or patients with other diseases.
Acknowledgments
We thank Dr. Peter Burbelo, NIH for providing plasmids (Rluc-GAD65, Rluc-IA-2, and Rluc-IA-2β) for our validation of LIPS assay.
Declaration of interest
There is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work was supported by R01 DK071831 (L.J.Y).
References
- 1.Diabetes Prevention Trial–Type Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–1691. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 4.Baekkeskov S, Aanstoot HJ, Christgau S, Reetz A, Solimena M, Cascalho M, Folli F, Richter-Olesen H, De CP. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 5.Bonifacio E, Atkinson M, Eisenbarth G, Serreze D, Kay TW, Lee-Chan E, Singh B. International Workshop on Lessons From Animal Models for Human Type 1 Diabetes: identification of insulin but not glutamic acid decarboxylase or IA-2 as specific autoantigens of humoral autoimmunity in nonobese diabetic mice. Diabetes. 2001;50:2451–2458. doi: 10.2337/diabetes.50.11.2451. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacio E, Lampasona V, Genovese S, Ferrari M, Bosi E. Identification of protein tyrosine phosphatase-like IA2 (islet cell antigen 512) as the insulin-dependent diabetes-related 37/40K autoantigen and a target of islet-cell antibodies. J Immunol. 1995;155:5419–5426. [PubMed] [Google Scholar]
- 7.Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–1283. doi: 10.1016/s0140-6736(74)90140-8. [DOI] [PubMed] [Google Scholar]
- 8.Burbelo PD, Ching KH, Mattson TL, Light JS, Bishop LR, Kovacs JA. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems) Biochem Biophys Res Commun. 2007;352:889–895. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 9.Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbelo PD, Groot S, Dalakas MC, Iadarola MJ. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem Biophys Res Commun. 2008;366:1–7. doi: 10.1016/j.bbrc.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burbelo PD, Hirai H, Issa AT, Kingman A, Lernmark A, Ivarsson SA, Notkins AL, Iadarola MJ. Comparison of radioimmunoprecipitation with luciferase immunoprecipitation for autoantibodies to GAD65 and IA-2beta. Diabetes Care. 2010;33:754–756. doi: 10.2337/dc09-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbelo PD, Hirai H, Leahy H, Lernmark A, Ivarsson SA, Iadarola MJ, Notkins AL. A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes Care. 2008;31:1824–1826. doi: 10.2337/dc08-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen W, Bergerot I, Elliott JF, Harrison LC, Abiru N, Eisenbarth GS, Delovitch TL. Evidence that a peptide spanning the B-C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol. 2001;167:4926–4935. doi: 10.4049/jimmunol.167.9.4926. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 15.Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146:129–141. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman DL, Erlander MG, Clare-Salzler M, Atkinson MA, Maclaren NK, Tobin AJ. Autoimmunity to two forms of glutamate decarboxylase in insulin-dependent diabetes mellitus. J Clin Invest. 1992;89:283–292. doi: 10.1172/JCI115573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koizumi M, Doi R, Toyoda E, Masui T, Tulachan SS, Kawaguchi Y, Fujimoto K, Gittes GK, Imamura M. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery. 2003;134:260–266. doi: 10.1067/msy.2003.231. [DOI] [PubMed] [Google Scholar]
- 18.Koya V, Lu S, Sun YP, Purich DL, Atkinson MA, Li SW, Yang LJ. Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: a novel protein transduction domain-based therapy. Diabetes. 2008;57:757–769. doi: 10.2337/db07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan MS, Wasserfall C, Maclaren NK, Notkins AL. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1996;93:6367–6370. doi: 10.1073/pnas.93.13.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li SW, Koya V, Li Y, Donelan W, Lin P, Reeves WH, Yang LJ. Pancreatic duodenal homeobox 1 protein is a novel beta-cell-specific autoantigen for type I diabetes. Lab Invest. 2010;90:31–39. doi: 10.1038/labinvest.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li SW, Sun Y, Donelan W, Yu H, Scian J, Tang D, Yang LJ. Expression, purification, and characterization of recombinant human pancreatic duodenal homeobox-1 protein in Pichia pastoris. Protein Expr Purif. 2010;72:157–161. doi: 10.1016/j.pep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Li Q, Xie H, Chen ZJ, Borovitskaya AE, Maclaren NK, Notkins AL, Lan MS. Identification of a second transmembrane protein tyrosine phosphatase, IA-2beta, as an autoantigen in insulin-dependent diabetes mellitus: precursor of the 37-kDa tryptic fragment. Proc Natl Acad Sci U S A. 1996;93:2307–2311. doi: 10.1073/pnas.93.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, Bingley PJ, Bonifacio E, Palmer JP, Eisenbarth GS, Wolfsdorf J, Skyler JS. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97–104. doi: 10.1111/j.1399-5448.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 24.Noguchi H, Kaneto H, Weir GC, Bonner-Weir S. PDX-1 protein containing its own antennapedia-like protein transduction domain can transduce pancreatic duct and islet cells. Diabetes. 2003;52:1732–1737. doi: 10.2337/diabetes.52.7.1732. [DOI] [PubMed] [Google Scholar]
- 25.Noguchi H, Matsumoto S, Okitsu T, Iwanaga Y, Yonekawa Y, Nagata H, Matsushita M, Wei FY, Matsui H, Minami K, Seino S, Masui Y, Futaki S, Tanaka K. PDX-1 protein is internalized by lipid raft-dependent macropinocytosis. Cell Transplant. 2005;14:637–645. doi: 10.3727/000000005783982648. [DOI] [PubMed] [Google Scholar]
- 26.Noguchi H, Matsushita M, Matsumoto S, Lu YF, Matsui H, Bonner-Weir S. Mechanism of PDX-1 protein transduction. Biochem Biophys Res Commun. 2005;332:68–74. doi: 10.1016/j.bbrc.2005.04.092. [DOI] [PubMed] [Google Scholar]
- 27.Palmer JP, Asplin CM, Clemons P, Lyen K, Tatpati O, Raghu PK, Paquette TL. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983;222:1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- 28.Park JY, Hong SM, Klimstra DS, Goggins MG, Maitra A, Hruban RH. Pdx1 expression in pancreatic precursor lesions and neoplasms. Appl Immunohistochem Mol Morphol. 2011;19:444–449. doi: 10.1097/PAI.0b013e318206d958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ Jr, Wright CV, Leach SD. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology. 1999;117:1416–1426. doi: 10.1016/s0016-5085(99)70292-1. [DOI] [PubMed] [Google Scholar]
- 30.Vaziri-Sani F, Oak S, Radtke J, Lernmark K, Lynch K, Agardh CD, Cilio CM, Lethagen AL, Ortqvist E, Landin-Olsson M, Torn C, Hampe CS. ZnT8 autoantibody titers in type 1 diabetes patients decline rapidly after clinical onset. Autoimmunity. 2010;43:598–606. doi: 10.3109/08916930903555927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104:17040–17045. doi: 10.1073/pnas.0705894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wenzlau JM, Walter M, Gardner TJ, Frisch LM, Yu L, Eisenbarth GS, Ziegler AG, Davidson HW, Hutton JC. Kinetics of the post-onset decline in zinc transporter 8 autoantibodies in type 1 diabetic human subjects. J Clin Endocrinol Metab. 2010;95:4712–4719. doi: 10.1210/jc.2010-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter WE, Schatz DA. Autoimmune markers in diabetes. Clin Chem. 2011;57:168–175. doi: 10.1373/clinchem.2010.148205. [DOI] [PubMed] [Google Scholar]
- 34.Yokota I, Matsuda J, Naito E, Ito M, Shima K, Kuroda Y. Comparison of GAD and ICA512/IA-2 antibodies at and after the onset of IDDM. Diabetes Care. 1998;21:49–52. doi: 10.2337/diacare.21.1.49. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Nakayama M, Eisenbarth GS. Insulin as an autoantigen in NOD/human diabetes. Curr Opin Immunol. 2008;20:111–118. doi: 10.1016/j.coi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]