Abstract
It has been convincingly established, over the past decade, that the human insular cortices are involved in processing both body feelings (such as pain) and feelings of emotion. Recently, however, an interpretation of this finding has emerged suggesting that the insular cortices are the necessary and sufficient platform for human feelings, in effect, the sole neural source of feeling experiences. In this study, we investigate this proposal in a patient whose insular cortices were destroyed bilaterally as a result of Herpes simplex encephalitis. The fact that all aspects of feeling were intact indicates that the proposal is problematic. The signals used to assemble the neural substrates of feelings hail from different sectors of the body and are conveyed by neural and humoral pathways to complex and topographically organized nuclei of the brain stem, prior to being conveyed again to cerebral cortices in the somatosensory, insular, and cingulate regions. We suggest that the neural substrate of feeling states is to be found first subcortically and then secondarily repeated at cortical level. The subcortical level would ensure basic feeling states while the cortical level would largely relate feeling states to cognitive processes such as decision-making and imagination.
Keywords: cortices, insula, neural substrate, sentience, somatosensory
Introduction
Research on the neural basis of affective phenomena has established beyond doubt that the human insular cortices are involved in processing body feelings (such as pain, pleasure, and temperature) and feelings of emotions (Craig 2000; Damasio et al. 2000; Kupers et al. 2000; Brooks et al. 2002; Craig 2002). As part of this development, it has been suggested that the insular cortices are the necessary and sufficient platform for feeling states in humans and are, in effect, the sole source of their experience (Craig 2009, 2011). At first glance, given the traditional view that mental states are subserved mainly or exclusively by the cerebral cortex, the idea that the insular cortices would be the exclusive substrate for feelings may seem plausible. However, the neural and humoral body signals on the basis of which the insular cortices operate are not conveyed exclusively to the insula. Largely, the signals are first directed to subcortical structures in the spinal cord, brainstem, and hypothalamus, where they undergo extensive processing. The insular cortices are the recipients of information integrated in those subcortical stations and, at least in the case of primates, subsequently carried to the cerebral cortex via thalamic nuclei although some body information may be conveyed directly to the posterior insula in humans (Craig 2002). Moreover, besides brainstem structures and insula, other brain regions involved in affective processing (e.g., cingulate cortices, somatosensory cortices, basal forebrain nuclei, and basal ganglia nuclei in ventral striatum and pallidum) need to be considered in an attempt to provide a comprehensive picture of the neurophysiology of feelings.
Under the circumstances, it is important to put to the test the proposal that the insula is the exclusive platform for human feeling. We believe that the investigation of this hypothesis is best served by studies in human beings, who are able to report on their experiences, notwithstanding the indispensible contribution of evidence from pertinent studies in animals. Accordingly, we used a human lesion approach and aimed for the ideal test condition, one in which, in the very least, the insular regions would be destroyed bilaterally by acquired damage. Such instances have become extremely rare because, fortunately, the human disease most likely to cause bilateral insular destruction, “Herpes simplex” encephalitis (HSE), can be successfully treated, at least in part, with antiviral medications (Damasio and Van Hoesen 1985). Still, the patient registry of our long-standing lesion studies project contains suitable subjects, namely, a patient with complete bilateral insular damage and an intact brainstem. This is patient B., whom we studied extensively over a period of 2 decades and is now deceased. The observations noted in this article were made during laboratory experiments, field experiments, and visits to the care facility where B. lived. He was a willing and cooperative participant in numerous studies. The data were collected with his informed consent, in accordance with institutional and federal regulations.
The investigation considered the following sources of evidence: 1) neurological status; 2) neuroanatomical evidence obtained from structural neuroimaging analysis; 3) behavioral observations; 4) neuropsychological experiments; 5) psychological evaluations, including self-awareness tasks; and 6) ratings of the patient’s behavior by naïve observers.
Methods and Results
Neurological Status
Patient B. became acutely ill in October of 1975, at the age of 48, with high-fever, confusion, and seizures, a presentation typical of Herpes Simplex Type 1 encephalitis. After a 3 day period of coma, the patient regained consciousness and improved rapidly. The seizures did not recur. Within a month post-onset, he had reached a stable state. In this section, we provide the essentials of his neurological/neuropsychological status prior to documenting, in sections 2 through 6, evidence regarding neuroanatomy, feelings, and sentience.
As a result of the encephalitis, Patient B. developed major neuropsychological changes previously reported in studies from our group (e.g., Damasio et al. 1985; Damasio and Tranel 1990, 1993; Damasio, Damasio, et al. 1990; Damasio, Tranel, and Damasio 1990; Tranel et al. 1994, 2000). Patient B. had a severe declarative learning defect (he was unable to remember any new factual item for more than 45 s). He also had a dense retrograde memory defect relative to unique events having occurred in the previous 2 decades of his life, evident in both recall and recognition modes. He could recall many correct facts about his childhood and adolescence but recall of events in adult life was vague and nonspecific. However, his memory for nonunique objects and events was largely intact. He had no difficulty with the concept of an object or situation at generic level or with naming at generic level. In brief, he would not be able to recall his own wedding, but he knew exactly what a wedding was, what would be the typical steps in such a ceremony, and he could tell, given a photograph, if the picture depicted a wedding, a funeral, a baptism, or some other celebration. Likewise, shown a photograph of his house he could not tell it was his but he could offer a description of what kind of house it was, what it would be like inside, its approximate size, and so on. His procedural learning was intact. In tests of motor learning such as rotor pursuit, he showed a typical learning curve and the new skill retained its performance level even if he would not remember, from session to session, the verbal instructions he had been given to acquire it. He was fully oriented to person but not to place or date. His sensory perception was intact in the visual, auditory, and somesthetic domains but both taste and olfaction were compromised. After age 60, he developed a gradual hearing loss. Strength in all 4 limbs was always normal as was his gait. There was no tremor, bradykinesia, or rigidity. Language was intact with the exception of the recall of proper names (which was severely compromised in keeping with the retrograde memory defect for unique items) and of common nouns of certain categories (Damasio and Tranel 1990, 1993). The patient was affable and cooperative within the limits imposed by his retrograde amnesia and learning defect. When he was introduced to strangers who were not warned of his condition, he invariably came across as pleasant, engaging and obviously conscious. Only continued conversation revealed the shallowness of his mental contents and the tendency to confabulate and repeat, traits that typically accompany severe memory loss of this magnitude. In essence, other than for the neuropsychological defects described above and for the olfactory and taste changes, his neurological examination was normal and so it remained until he died in May of 2003 at age 75.
Neuroanatomical Evidence
When HSE Type 1 strikes adults it has a special affinity for the limbic sectors, namely, the periallocortices in insular, orbitofrontal, and temporal regions; the anterior cingulate cortex; the hippocampi and the entorhinal cortices; the amygdaloid nuclei; and an assortment of basal forebrain nuclei such as the septum and substantia innominata (Damasio and Van Hoesen 1985). Patient B. is a typical case of severe HSE and has evidence of damage in all these regions.
The neuroanatomical study of patient B. is based on computerized x-ray tomography and magnetic resonance imaging. All imaging studies were accomplished with great difficulty. Patient B was apprehensive about confined spaces and only out of desire to cooperate did he agree to go into the scanner. However, given his severe learning and memory defect, he had to be reminded at extremely short intervals of the need to remain motionless. For this reason, once we obtained reliable anatomical data, we abandoned any further attempts to have him participate in fMRI studies.
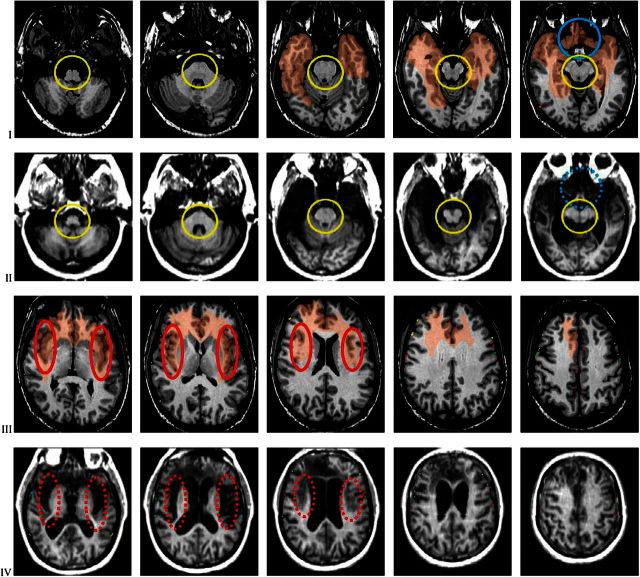
The best source of evidence is a study obtained in 1991 in a 1.5 Tesla GE scanner. Analysis was guided by relevant neuroanatomical information available in Mesulam and Mufson (1982), Mufson and Mesulam (1982), Damasio (2005), and Heimer and Van Hoesen (2006). As shown in Figure 1, both temporal lobes are extensively damaged. The temporal pole cortices are destroyed, as are the cortices in the mesial temporal gyri—parahippocampal, temporo-occipitel, and inferior temporal. In the right hemisphere, the damage extends further posteriorly involving the cortices in the mesial temporo-occipital region. The posterior orbito-frontal cortices are damaged bilaterally, as are the anterior cingulate cortices. The damage entirely covers the so-called affective sector of the anterior cingulate bilaterally. On the right side, the damage also extends to the mid cingulate sector; while on the left, the mid cingulate sector is partly but possibly not completely damaged. The remainder of the frontal lobes cortices (dorsolateral, mesial, and anterior orbital sectors), parietal, and occipital are intact in both hemispheres. In Figures 2–4, it is evident that the damage also involves both the left and right insular cortices that are entirely destroyed. No insular cortex remains, on either side, and the same applies to the underlying white matter (extreme and external capsules), to the claustrum, and to the limbic cortices that are closest to the insular regions anteriorly, namely, the medial and middle sections of the orbitofrontal cortices and the polar and mesial temporal cortices. The entorhinal cortex, the hippocampus proper, and the amygdala are also entirely destroyed.
Figure 1.
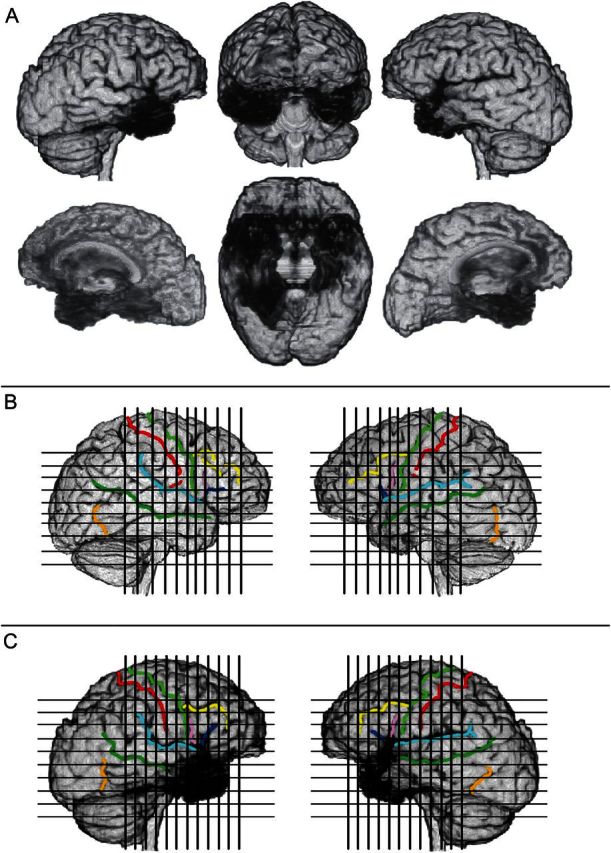
(A) Three-dimensional reconstruction of patient B’s brain, using Brainvox. The right hemisphere is shown on the left (lateral view on top; mesial view below). The same 2 views of the left hemisphere are depicted on the right. The middle column shows the brain seen from the front (top) and a ventral view of the 2 hemispheres after removal of the cerebellum and brainstem (bottom). The black shaded areas reveal the extensive damage involving a large sector of both temporal lobes, the posterior aspect of the orbital frontal region, and the anterior cingulate. (See Figs 2–4 for details). (B and C) Markings of major sulci (central sulcus = red; precentral sulcus = light green; inferior frontal sulcus = yellow; horizontal branch of the Sylvian fissure = dark blue; ascending branch of the Sylvian fissure = pink; Sylvian fissure = light blue; superior temporal sulcus = dark green; anterior occipital sulcus = light brown); and positioning of coronal and axial slices as shown in Figures 2 and 3, in the brain of a normal subject, the comparison brain (B), and in Patient B. (C) The comparison brain was obtained in the same scanner used for Patient B.
Figure 2.
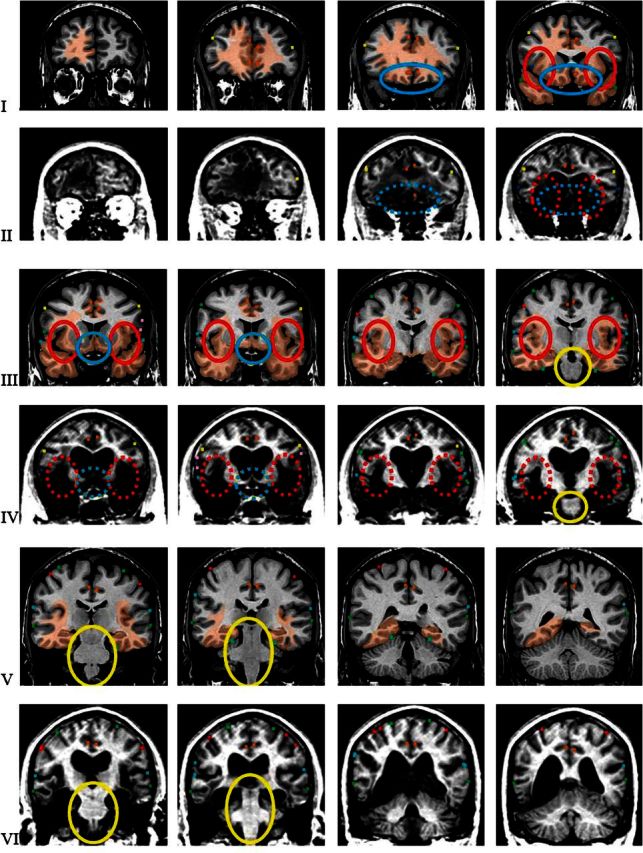
Coronal slices through the comparison brain and through patient B’s brain. The images are presented in radiological convention (right is on left and left is on right). The orientation and level of the slices in the comparison brain were matched to those in patient B. (see Fig. 1B,C). The first, third, and fifth rows (I, III, and V) show slices of the comparison brain; the second, fourth, and sixth (II, IV, and VI) those of patient B. The relevant sulci marked on the 3D reconstructed brains (see Fig. 1B,C) were automatically transferred to each slice that intersects them using Brainvox. The lesion seen in Patient B was manually transferred onto each corresponding slice of the comparison brain (areas in light brown), using the marked sulci as reference. Slice setting as seen in Figure 1. The coronal slices reveal complete damage of the insula, from its anterior-most edge (panel 4, row I and II) to its most posterior (panel 1, row V and VI), highlighted within the red ovals (continuous line for the comparison brain; dashed line for Patient B’s brain). The damage involves all tissue between the lateral insula surface and the outer limit of the lenticular nucleus destroying all insular cortex, extreme capsule, claustrum, and external capsule. The damage continues without interruption into the orbital sector adjacent to the insula (panels 4 and 5 in row I and II, and panels 1 and 2 in row III and IV), highlighted by blue ovals and circles. For additional detail of posterior orbito-frontal damage, see Figure 4. The lesion extends into both temporal lobes where it destroys the polar region (panels 3 and 4 in rows I and II), the amygdalae (panels 2 and 3 in rows III and IV), the hippocampuses and the parahippocampal gyri (panels 3 and 4 in rows III and IV and panel 5 and panels 1, 2, and 3 in rows V and VI). In the right hemisphere, the damage extends further posteriorly in the dorsolateral and inferior aspects of the temporal lobes. There is also partial damage to the frontoparietal operculum (panels 1–3 in rows III and IV). The brainstem is intact (see panel 5 in rows III and IV and panels 1 and 2 in rows V and VI; the brainstem is highlighted in continuous yellow circles); the cerebellum is also intact.
Figure 3.
Axial slices of the comparison brain and of patient B’s brain, interleaved as in Figure 2. The comparison brain shows the transfer of the area damaged in Patient B. Slice setting as in Figure 1B,C. There is again evidence of the complete damage of the insula, the underlying white matter and the claustrum in both hemispheres, highlighted by the red ovals. See panels 1–3 in rows III and IV. The damage also encompasses both temporal lobes destroying the polar regions, the amygdalae, the hippocampuses, and the parahippocampal gyri (panels 3–5 in rows I and II). In the right hemisphere, the damage extends further posteriorly in the inferior and mesial aspects. The damage involves the posterior fronto-orbital region bilaterally (highlighted by the blue circles in panel 5, rows I and II); the damage extends dorsally to involve the anterior cingulate cortex subcalosally and beyond (panel 1 in rows III and IV), and the white matter in the core of both frontal lobes more extensively in the right hemisphere (all panels in rows III and IV). The brainstem and cerebellum are intact (see panels 1–5 in rows I and II; brainstem circled in yellow), as are all sensory and motor cortices, and the association cortices of the parietal and occipital lobes, and the primary visual regions.
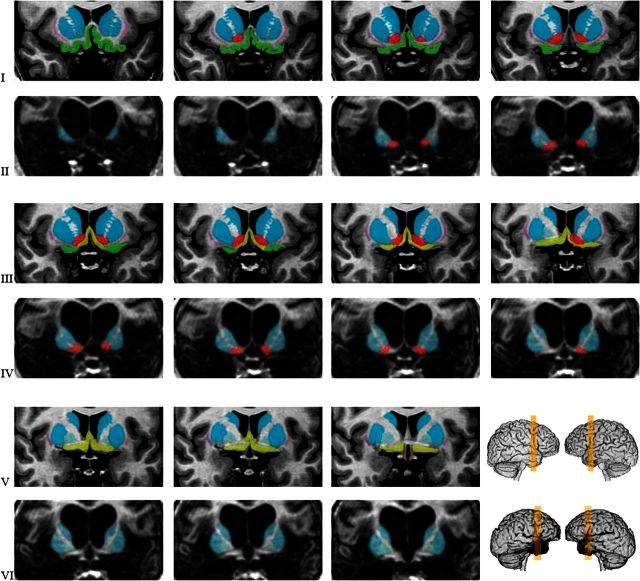
Figure 4.
Detail of the posterior orbital sector, basal forebrain region, and basal ganglia in the comparison brain and in patient B. The yellow colored slabs in the right lower corner represent the section of brain from which the panels of this figure were extracted. The slices in patient B. are 1.5-mm thick, and all consecutive cuts are shown. The slices in the comparison brain are anatomically matched to those of patient B. Rows of panels from the comparison brain are interleaved with those from Patient B., as in the previous figures. Several structures are colored. The same color is used for the comparison brain and Patient B’s brain. In Patient B., the dorsal striatum (putamen and caudate nuclei, in blue) appears intact. The ventral striatum/nucleus accumbens region (red), largely located below and in front of the anterior commissure is also seen in patient B. The whole basal ganglia complex appears to be diminished in size; partial damage is possible (see text). The basal forebrain nuclear masses (septal nuclei; diagonal band; substantia innominate), shown in greenish yellow in the comparison brain, are missing in Patient B. The posterior orbitofrontal cortices (green) and the claustrum (pink) are also missing in Patient B.
Medially, the damage stops at the boundary of the basal ganglia complex, on both sides, as is typical of severe cases of HSE (Fig. 4). Thus, right and left basal ganglia appear intact and symmetrical, with the typical shapes of caudate, putamen, and pallidus easily distinguishable but considerably reduced in volume. In all likelihood, the volume reduction is due to the loss of projections from the destroyed limbic cortices toward the ventral striatal component of the basal ganglia complex. The ventral pallidum appears intact but the basal forebrain components—the septal nuclei, the diagonal band of Broca, the substantia innominata—are not detectable. The cingulate cortices are damaged bilaterally, especially in the subcallosal and rostral sections of the anterior sector. The motor and premotor regions, the internal capsules, and the cerebellum are intact as are all primary and most early sensory association cortices in occipital, temporal, and parietal regions. Notably, the SI sector of the somatosensory cortices is intact bilaterally, as is the SII region (Fig. 5).
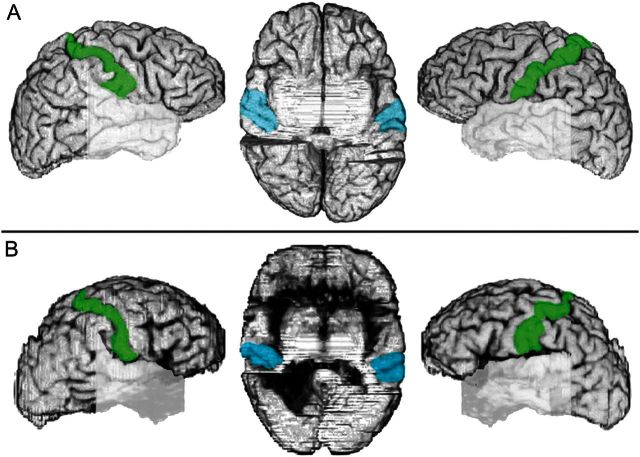
Figure 5.
(A) Right and left hemispheres of the comparison brain seen in lateral views with the postcentral gyrus (SI) shown in green. In the center, a view of the brain’s undersurface after the temporal lobes were removed to allow inspection of the lower surface of the fronto-parietal opercula. SII is shown in light blue. (B) Same as in A for the brain of Patient B. Both SI and SII areas are intact.
There is no evidence of damage to the brainstem, hypothalamus, or tectum. (Figs 2 and 3)
Behavioral Observations
We have samples of Patient B.’s behavior across a wide range of situations, and in connection to varied individuals and relationships, captured in testing protocols and videotape obtained between 1976 and 2003, a period during which he visited our laboratories at least yearly. Here, we report data extracted from the documented observations, relevant to the issue of feeling states.
The direct quotes included in the behavioral observations reported here are from B.’s own words, unless otherwise indicated.
B.’s behavior was consistent across the entire period of observation. In effect, B. was remarkably stable, in terms of affect and demeanor, and it is notable that many of his neuropsychologic tests yielded the same numerical results over more than 2 decades. In essence, B. got older and harder of hearing but the data that concern his feeling states were unchanged over the course of his post-disease life.
Besides his dense amnesia, the most striking and reliable feature of B.’s behavior was his frequent manifestation of likes and dislikes, comfort and discomfort, pleasure and pain. General activities of daily living and varied situations, including clinical visits, psychological testing, and formal experiments, were frequently accompanied by comments about how such activities were desirable or undesirable, pleasant or unpleasant, healthy or unhealthy. B. had distinct preferences for situations, activities, and persons and justified them on the basis of the associated feeling states. Those feeling states were often preceded by the deployment of an appropriate emotion. B. reported hunger and thirst. He also reported the feelings typically associated with a full bladder and a distended colon and, quite appropriately, during long testing sessions, would request to go to the bathroom. The same applies to itch, tickle, and changes in temperature. He reported pain in relation to needles, electrodes, and adhesive tape. He reported pleasure most notably in situations of exploration (as in going for walks) and play (as in checkers). He actively sought such situations along with the company of others and his enjoyment was obvious, as was the frustration when the situations were interrupted or terminated. In brief, the general emotive behavior and the general feeling reports of patient B. were easily recognizable, even to a casual observer. B. invariably engaged others in conventional social interactions and commonly evoked reactions of sympathy, empathy, and even friendship. As noted, unwarned strangers interacting with him for the first time had no inkling that he had major neurological damage, the fact only becoming apparent once his dense amnesia was exposed. To put it plainly, B. was a whole human being suffering from a very poor episodic memory. In fact, and at first glance, almost paradoxically, the shallowness of intellect caused by the memory defect made his affective life standout clearly and become the most salient aspect of his behavior.
In keeping with his amnesia, he registered no concerns about the future. He lived in a permanent present.
Positive Feelings
B. had frequent unequivocal manifestations of pleasure and reported feelings congruent with his behavior. For example, when interacting with the experimenters that he most enjoyed working with, he would pat them on the arm or even give them a hug and comment that he was feeling “very happy,” and was “very glad” to be with them. He would frequently observe that he was having a “wonderful time” and was feeling “very well and very happy.”
Negative Feelings
B. also had numerous manifestations of negative feelings, including physical and psychological pain, and he reported feelings congruent with his behavior. For example, whenever B. was being hooked up for psychophysiological recordings, he flinched and withdrew, and would complain when an electrode pinched his skin or the removal of tape inadvertently pulled off some hair. He was leery of blood pressure cuffs. B. had a fear of needles and flinched and withdrew at the sight of them. As noted earlier, he was afraid of the MRI scanner and was strongly averse to lying on the table and going into the magnet bore. He avoided experiments that might conceivably lead to discomfort and had pained expressions during unpleasant tasks, commenting that these were things that he “didn’t like” and wished to discontinue. On those situations, he unmistakably declared, “I do not care for that,” or “I would rather not do that.”
Observations Made by the Spouse
B.’s spouse completed a questionnaire that presented 148 items covering topics such as feelings, emotions, independent activities, cognitive functioning, and movement. For each item, she rated B. “before” and “after” the onset of his neurological condition, using a 4-point rating scale. As expected, the spouse rated B. as having changed dramatically on items having to do with memory and with independent decision making, indicating that her ratings were accurate and valid. There were 25 items on the questionnaire that dealt directly and explicitly with emotions and feelings (e.g., “Gets angry easily”; “Shows his feelings”; “Seems very cheerful”). On 18 of those items, the spouse rated B. as the same before and after his neurological condition. On 3 items, B. was rated as having changed in the upward direction (having more emotion or feeling after damage), and on 4 items, B. was rated as having changed in the downward direction (having less emotion or feeling after damage). The degree of change was one level (on the 4-point scale) for all “changed” items, up or down. The spouse noted a complete absence of libido as a notable change from before illness, although we note that Patient B. appeared especially pleased in the company of attractive women and behaved in what is best described as a respectfully flirtatious manner.
Interpretation.
Overall, the data from this questionnaire give no indication of a post-morbid lack of emotions and feelings. B. had an average range of emotions and feelings, before and after his illness. He became angry at appropriate levels of provocation; predictably enjoyed certain activities and the company of other people; and his mood changes were conventional. Of note, one item on which B. was rated as having changed in the upward direction post-morbidly was “Seems very cheerful.” Pre-morbidly this was rated “sometimes;” post-morbidly as “often.”
Neuropsychological Experiments
Learning of Affective Valence
Description of the experiment.
This experiment involved a week-long exposure of B. to 3 persons, 2 of whom were paired with a strong affective valence. The Good Guy was very pleasant and nice toward B., gave him many compliments, granted requests for treats (gum, pop) and was always extremely positive in demeanor and interpersonal manner. The Bad Guy was systematically negative and actively refused requests for treats, required B. to perform tedious and unrewarding experiments that B. found unpleasant and often attempted to discontinue and always had a disagreeable demeanor. In addition, there was a Neutral Guy, who was businesslike and with no systematic or strong positive or negative affective valence. Exposures of B. to these 3 persons took place in the course of regular daily interactions between the 3 experimental confederates (Good Guy, Neutral Guy, Bad Guy) and patient B. (Tranel and Damasio 1993).
Results.
At the sight of the person to whom he had been conditioned with negative valence (the “Bad Guy”), down the hallway or across the room, B. would frown and recoil. He would not smile at the Bad Guy, let alone shake hands. On seeing the person to whom he had been conditioned with positive valence (the “Good Guy”), B. would comment that he felt very happy and very well and wanted to continue working with the person. Asked “who would be the person that you would go to for a treat,” B. reliably chose the person who had rewarded him repeatedly (the Good Guy). Moreover, he reliably did not choose the person who had been unpleasant and unrewarding (the Bad Guy). We established these results using a 2-alternative forced-choice paradigm, in which B. was tested with pairs of face pictures, each pair containing one of the experimental confederates (the Good Guy, Neutral Guy, or Bad Guy) and a stranger. B. reliably selected the Good Guy (15/18 trials, 83%), reliably did not select the Bad Guy (4/18 trials, 22%) and was not different from chance for the Neutral Guy (10/18 trials, 56%). This outcome was replicated in a follow-up experiment, in which B. reliably selected a new Good Guy (18/19 trials, 95%) and was at chance level for a new “Neutral Guy” (12/25 trials, 48%), conditioned as in the initial experiment (we did not include a Bad Guy in this follow-up).
Interpretation.
B.’s repeated exposure to rewarding or aversive situations produced pleasant or unpleasant feelings whose association with the competent stimuli (the experimental confederates, Good Guy, and Bad Guy) appears to have been learned covertly. The representation of the competent stimuli provoked a reinstatement of the feeling states, on the basis of which B.’s preferences became manifest.
Experiments on Taste Preference
Herpes simplex encephalitis commonly compromises taste as a result of damage to the gustatory cortex that in humans appears to be located in the midinsular regions (Small 2010). Such patients often eat inedible products (e.g., toothpaste) and easily drink poorly tasting liquids. Patient B. exhibited both manifestations.
As reported in Adolphs et al. (2005), B. was provided drinks, either sucrose or saline. Given each beverage in isolation, he drank both readily until asked to stop. However, when he was provided the 2 beverages side by side and with coloring to distinguish them (red vs. green, counterbalanced across repeated sessions), B. first sampled each beverage and then reliably and strongly preferred sucrose and rejected saline. Specifically, on 19 trials pairing sucrose and saline, B. preferred sucrose on 18 of the trials. In 6 additional trials, after B. had selected which drink he preferred in the red-green forced-choice paradigm (he chose sucrose on all 6 trials), we encouraged him to sip the other drink and he vehemently refused to drink the saline in this situation (on all 6 trials). This happened no matter which drink he had sampled first—if sampling sucrose after having just sampled saline, he would refuse to switch back to drinking saline; if sampling saline after just having sampled sucrose, he immediately rejected the saline and “grabbed the sucrose.” In sum, when given a choice between sucrose and saline, B. reliably chose sucrose over saline, independently of the order of presentation. Asked about the reason for his preference, he simply stated that he “liked” the chosen solution better even though he did not know its name and did not explicitly call it pleasant or unpleasant. Likewise, he expressed his “dislike” for the saline solution, a dislike that was accompanied by a firm refusal to drink it.
Interpretation.
The patient’s taste processing was profoundly compromised as a result of damage to the taste-related sector of the insular cortices. However, by relying on subcortical processors of taste, and by being forced to attend to 2 alternatives and chose one, he appeared to detect the different chemical nature of the solutions and to detect pleasant or unpleasant feelings. On the basis of those feelings, he elected to drink one solution rather than the other. The feelings were manifest in facial expressions and in verbal labeling.
In brief, we propose that the experimental situation forced B. to notice the different feeling that resulted from drinking one or the other liquid. When he was not forced to choose he overlooked the unpleasantness of saline, possibly because the subcortical processing of taste generates a weaker sensory representation than the one that would have been provided by the insular cortices. If our interpretation is correct, this would be a curious example of residual generation of feeling in the absence of fully intact perception. Overall, the situation echoes that of decerebrate rats who are given saline or sucrose and reject saline purely on the basis of a subcortically processed negative feeling (Flynn and Grill 1988).
Psychological Evaluations
Several psychological evaluations were used to characterize B.’s personality, emotions, and feelings: conventional tests (“projective” measures); varied rating scales and questionnaires; and open-ended procedures designed to probe B.’s capacity to generate evidence of emotions and feelings, as described below. B.’s responses were recently interpreted by a licensed clinical psychologist with extensive training and experience in personality assessment, who was blind to the identity of the patient and the objectives of the current study.
Projective Tests: Responses to Items from the Thematic Apperception Test, Rorschach Inkblot Test, and Other Stimuli
We used items from the Thematic Apperception Test (TAT; Murray 1938), Rorschach Inkblot Test (Beck et al. 1961; Exner 1993), and other stimuli, to elicit from B. narrative descriptions of projective visual stimuli. The administration followed standard procedures, and we recorded and transcribed B.’s narratives. Samples from his responses are noted below.
TAT card 4.
B.: That looks like a married couple. They are just standing there. She looks like she wants a kiss. She wants some loving, affection, or something. She has the look on her face that she wants some affection. They are very satisfied being together, and they feel very well about each other. She feels like she wants to kiss him. They might go to bed, and before they go to sleep, they might do something natural.
TAT card 12M.
B.: That looks like a man and his son. Something may have happened to the son, and the man feels very bad. He is a good father, and he is very concerned for his son. He is checking on the son to see if he is alright. They care very deeply about each other, and they have a very warm relationship.
TAT card 3BM.
B.: That gentleman is sitting there, thinking about what he is going to be doing. There might be something that is bothering him quite a lot. Something may have happened. He may feel very weak and has to sit down.
TAT card 14.
B.: That person just had a long sleep. He probably slept 8 or possibly even 10 h. (E: How would he feel when he woke up?) Much more alive; fresh and alive.
TAT card 15.
B.: That man looks like he is very lonely. Something may have happened to his wife. She may be sick. He feels quite alone, evidently.
TAT card 13MF.
B.: That looks like a husband and wife. She is ill and maybe she is dead. The gentleman is standing there with his hand on his head, and he is probably feeling very bad. That is too bad. He feels very badly. Maybe by tomorrow, she may be feeling better, and she may feel very good and he would feel very happy that she is recovered. They would both feel well and they would go somewhere together. She may just be very tired and if she sleeps for 12 h or so, she will feel much better.
Rorschach—Card III.
B.: This one looks like a woman, and this looks like a man. They are looking at each other. (Examiner: If they are married, how do they feel about each other?) B.: They may be delighted to be together.
Alternative outcomes procedure.
In this procedure, the examiner provided B. with a brief scenario and asked him to complete the story with whatever ending or endings he deemed appropriate.
Examiner: Jim saw a good looking girl that he had never seen before, while eating in a restaurant. He was immediately attracted to her. The story ends when they get married.
B.: He obviously appreciated her very much. That was very nice. He felt very attracted to her, and they got along very well. He felt very friendly toward her, and she felt very friendly toward him. They went on a date and had a very nice time together and felt very well about each other. They had such a good time together that they decided to live together.
Interpretation.
The expert concluded that B.’s responses consistently began with a reasonably realistic perception of the basic, concrete features of the stimuli, after which one of 2 patterns played out, with the first being more frequent: 1) responses developed increasingly positive emotionality that went beyond the information in the cards; 2) responses came around to acknowledging, in muted terms, the very negative circumstances suggested by the scenes, but the degree of negativity was then reduced so as to generate a positive outcome that was not suggested by the scene (e.g., TAT card 13MF, “She may just be very tired and if she sleeps for 12 h of so, she will feel much better”).
The pattern of responses to TAT cards and Rorschach card III indicate a perception of negative themes but a bias toward emotionally positive responses and muted negatively valenced responses. Additionally, there was a tendency to focus on a man’s relationship with his wife (primarily) or son (secondarily) with highly warm, affectionate, and concerned feelings. This is not of itself pathological and fits a salient tendency: generally intact perception of emotionally relevant themes, followed by a dampening of negative feelings and an imposition of positive feelings as B. continues to process the stimuli cognitively. The overall pattern suggests a diminished influence of negative feelings in the elaboration of the meaning of complex social situations.
Writings
On several occasions, B. was asked to write a letter to the examiner and told that he was free to select the content. His writing effusively described the happiness and good feelings he shared with the examiner.
Self-awareness Tasks
B. was given various tasks that assess different aspects of self-awareness, including procedures that tap into basic self-recognition, self-agency, and self-concept. A self-awareness interview was also conducted, as a means of measuring his meta-cognitive, reflective, and introspective abilities.
Core Self-awareness
Basic self-recognition.
1. Mirror self-recognition: Following a well-established protocol that has been used to document self-recognition in animals and humans (cf. Reiss and Marino 2001), we observed B.’s reactions to mirror images in which he had been covertly “marked” in some fashion (e.g., with an item of clothing, pencil smudge on his face) the mark was unbeknownst to him or had been forgotten due to his severe amnesia. On all occasions, when he confronted his image in the mirror, he immediately recognized himself, showed surprise at the “change” in his image—for example, the clothing item that had been added or the pencil smudge on his face. He attempted to rub off the smudge and then proceeded with other self-grooming behaviors typical of persons in front of a mirror. On a number of informal occasions, when he passed by a mirror, B. recognized himself and adjusted his clothes accordingly.
2. Self-recognition from photographs: B. was presented with various pictures of himself and asked to identify the person in the photograph. We had 20 such pictures, taken from the epoch before the onset of B.’s neurological illness (mainly from B.’s early adulthood years), some featuring him alone and others in which he was pictured with family members, Army buddies, or other friends. He accurately recognized himself in most of these pictures (16/20) and immediately pointed out, “That’s me.” On 3 occasions, he mistook himself for his father; on one other occasion, he said that he didn’t know who the person was. The failures occurred in relatively recent pictures. Overall, he clearly recognized himself quickly and accurately in photographs, especially older ones. He also demonstrated discriminatory skin conductance responses (SCRs) to pictures of himself. As reported in Tranel and Damasio (1989), B. showed reliable high-amplitude SCRs to personally relevant visual stimuli (including pictures of himself), comparable to those produced by healthy comparison participants (for B., mean SCR amplitude = 0.15 μSiemens, averaged across the right and left hands; for 7 comparison participants, mean SCR amplitude = 0.16 μSiemens, averaged across the right and left hands). The SCR findings indicate a preserved autonomic response to stimuli with “signal value,” which in this case was conferred by the familiarity of the face pictures.
Basic self-agency—Tickle Task.
B. was tickled by an experimenter on several occasions, as a means of measuring his ability to discriminate between self-initiated touch as compared with touch initiated by an external source. The tickles were to the palms, soles of the feet, or sides of the ribs. On 5 occasions, the tickles elicited a strong behavioral and verbal response (laughing, squirming, withdrawing). He never had such responses to self-initiated touching. His responses to tickles were normal and clearly reflective of the classic difference between “self-touching” and “other-initiated touching” (e.g., Blakemore et al. 2000).
Extended and Introspective self-awareness
The varied observations elaborated above are born out by an analysis of records in which B.’s own words describe his feelings, including transcribed interviews and written descriptions of his feeling states. Specifically, B. was asked questions during the course of our many interviews and conversations with him that probed various conceptual and “metacognitive” aspects of self-awareness. Excerpts from transcriptions of his responses are provided below, grouped according to the general domains of “basic concepts” and “introspection”:
Basic concepts.
E: What does consciousness mean?
B.: To be alive and feeling well, and knowing who you are.
E. What does it mean to be unconscious?
B.: Nothing; you would have no moving and nothing would be happening.
E: What does free will mean?
B.: The ability to do all the wonderful things you want to.
E: What does the self mean?
B.: Who you are at the time, and feeling very satisfied and happy.
E: What does selfishness mean?
B.: Wanting things for yourself and not sharing them with others.
E: What part of the body is important for the self ?
B.: Probably the brain and maybe the heart a little bit. You would not feel very well if your brain wasn’t working.
E: Do dogs and cats and squirrels have self-awareness?
B.: I think dogs used to.
E: What does emotion mean?
B.: A strong desire; you would feel something very strong and wonderful and you would be extremely happy.
E: What does loneliness mean?
B.: That isn’t very nice; that’s too bad. The person should try to get some friends and not be alone.
E: What does sympathy mean?
B.: When someone is feeling tired or sad and you try to help them get more rest.
E: What does compassion mean?
B.: To try to help people feel happy and rested.
E: What is beauty?
B.: Any person or any thing that is taken care of regularly. People, music, places where people live, can be beautiful. Beauty is lovely, anything lovely.
E: What is desire?
B.: People have desires; when they want something, they desire it.
E: What is ambition?
B.: Strong feelings for work and living.
E: What is generous?
B.: Generous is large feelings; generosity; very willing; being wonderful, to give something you want.
Introspection.
E: Are you aware of yourself?
B.: I have a strong feeling of happiness, that we are here together working on these wonderful games and feeling happy together. I am glad to be here with you.
E: Is that bed over there (pointing across the room) aware of itself?
B.: I never knew any beds that could really do anything like that.
E: Am I aware of myself?
B.: You look very handsome. I think you know what to do here.
E: Are you the same person when you are awake and when you are asleep?
B.: I like to get rested and sleep at least 8 or 9 or 10 h every night, and then I feel fresh and rested. I am happy to be awake and I am the same person when I am awake and when I am asleep.
E: Have you changed at all during the many years we have been working together?
B.: Very little; I am very happy and delighted to be with my friends and to be working together. I am the same. I have more trouble hearing things than I used to.
E: Do you think other people can control your thoughts?
B.: I did not know anyone who was like that.
E: Do you think you could get a brain transplant?
B.: I would not like to do that; I think I am happy to be here and would like to stay here until we are done. I don’t really care for that.
E: What does it mean to die? What happens?
B.: Some of the people died, and I think they went to heaven. We used to cry when the people died. A lot of friends came and they cried for a long time.
E: Are you afraid of dying?
B.: I do not like to think about that; I am happy to be here with you today. I don’t care too much for that.
Ratings of the Patient by Naïve Observers
We asked 10 naïve observers to watch a videotape of B. and then to rate him on various dimensions of feeling and awareness.
Experiment
The observers were 10 normal, healthy persons (4 men, 6 women) between the ages of 20 and 36, with an average of 15.6 years of education, who had never seen or heard of B. before (live or in video). The videotape they watched was a short series of unstructured interviews (9 min total duration), where B. was being asked questions (by A.D. or D.T.) about his whereabouts, the time, the weather, and his general situation. After watching the video, the observers rated B. on the following 10 characteristics, using a 7-point Likert scale for each: general feelings, specific feelings, general emotions, specific emotions, self-awareness, other-awareness, consciousness, human beingness, robotness, and animation. As an example of the method, the human beingness item was: To what extent does this person appear to be a normal, intact human being?: “1”—No human beingness at all; “7”—Normal human beingness. (The robotness item was reverse keyed so that “1” corresponded to “exactly like a robot or machine” and “7” corresponded to “not at all like a robot or machine.”)
Results
The observers assigned B. high ratings on emotions, feelings, and awareness, with the mean ratings for these scales ranging from 3.9 to 6.1, all of which are on the high end of the scale and clearly indicative of perceived normalcy on these dimensions (Table 1). They rated him as not being like a robot or machine, and by contrast, rated him high on both human beingness and animation.
Table 1.
Ratings of B. by naïve observers
| Mean | Standard deviation | Range | |
| General feelings | 4.9 | 1.0 | 3–6 |
| Specific feelings | 6.1 | 1.1 | 4–7 |
| General emotions | 4.2 | 1.2 | 3–6 |
| Specific emotions | 3.9 | 1.4 | 2–6 |
| Self-awareness | 4.3 | 1.8 | 2–7 |
| Other awareness | 5.2 | 1.6 | 2–7 |
| Consciousness | 4.4 | 1.9 | 2–7 |
| Human beingness | 4.5 | 1.1 | 3–6 |
| Robotness | 5.1 | 1.2 | 3–7 |
| Animation | 5.1 | 1.2 | 3–6 |
Interpretation
Naïve observers watching B. interact socially in an unstructured situation perceive him to be a normal person, to have normal emotions and feelings, and to have a normal degree of animation. In Behavioral Observations, we emphasized that our observations of B. over many years of interacting with him support the conclusion that he has a wide range of emotions and feelings. The data from the naïve observers corroborate and extend those impressions and support the idea that B. comes across as a whole human being, to any manner of observation.
Discussion
Patient B., whose insular cortices were entirely destroyed, experienced body feelings as well as emotional feelings. He reported feeling pain, pleasure, itch, tickle, happiness, sadness, apprehension, irritation, caring and compassion, and he behaved in ways consonant with such feelings when he reported experiencing them. He also reported feelings of hunger, thirst, and desire to void, and behaved accordingly. He yearned for play opportunities, for example, playing checkers; visiting with others; going for walks, and registered obvious pleasure when engaged in such activities as well as disappointment or even irritation when the opportunities were denied.
Because Patient B.’s profound amnesia limited the recall of unique events and persons, the network of associations linked to a given feeling was limited and tended toward the repetitive, especially in regard to emotional feelings. Patient B. did not engage spontaneously on reflections over his situation or on meditations on the human condition. As expected of someone who lived in a continuous present, he was not concerned with the future. Intriguingly, however, these limitations allowed feelings to dominate his mental life and influence the most salient aspect of his behavior. Given the impoverishment of his imagination, Patient B.’s existence was a virtually continuous “affective” reaction to his own body states and to the modest demands posed by the world around him, undampened by high-order cognitive controls.
Thus, on the basis of the neuroanatomical and neuropsychological findings reported here, the insula, along with its extensions into orbitofrontal and medio-anterior temporal cortices, should not be regarded as the exclusive basis for the experience of feelings. Moreover, it is apparent that in spite of bilateral insular destruction Patient B. was conscious and had a robust sense of self. He fulfilled the criteria for protoself, core self, and autobiographical self and he had an unwavering sense of identity, although, in keeping with his profound defect in episodic memory, the scope of his autobiographical self was limited (Damasio and Meyer 2008). According to Craig (2011), the tell-tale sign of self-awareness is the ability to recognize oneself in a mirror, an ability that in his words “can only be provided by a functional, emotionally valid neural representation of self.” Patient B. passed this test consistently and repeatedly. In brief, these findings run counter to the proposal that human self-awareness, along with the ability to feel, would depend entirely on the insular cortices and, specifically, on its anterior third (Craig 2009, 2011).
In the absence of insular cortices, we need to entertain neuroanatomical alternatives to explain the basis for B.’s feeling abilities and sentience.
Structures in Cerebral Cortex
There is reliable evidence that the somatosensory cortices (SI and SII) and the cingulate cortices are functionally engaged by nociceptive stimulation (see Shackman et al. 2011; also Roy et al. 2009; Piché et al. 20009; Craig 2011) We also know that SI is engaged in the processing of emotions such as disgust when disgust is caused by viewing body mutilations (Harrison et al. 2010). The neuroanatomical analysis reveals that SI and SII are spared in Patient B. Activity in these cortices might thus contribute to his preserved feelings. Given that the overwhelming component of standard emotional feelings is based on visceral and humoral information, whose main target is the insula, the SI and SII regions are not the ideal candidates to explain the full range of Patient B’s feelings. However, we note that plastic changes may have led to a reorientation of visceral and humoral inputs to the SI/SII complex, even though plastic changes of such a caliber are likely to take a considerable time to develop and there is no evidence that patient B. was deprived of feeling experiences in the period immediately following his acute illness.
What about the cingulate cortices? There is no doubt that the anterior sector of the cingulate cortices is involved in varied aspects of affect and pain-related processing (Bush et al. 2000; Devinsky et al. 1995). Thus, it is important to ask if it might contribute to the feeling states of Patient B. On the right hemisphere this does not appear possible since the entire anterior sector is damaged. On the left, however, part of rostral midcingulate sector may have survived. Given that this midcingulate sector is consistently activated by nociceptive stimuli, the possibility of a cingulate role must be considered. Still, we note that the functional correlations comprehensively reviewed in Shackman et al. (2011), and the neurophysiological evidence concerning this region (see Dum et al. 2009), suggest a motor control role in reaction to stimuli of negative valence rather than a role in feeling experience. This is in keeping with the idea that the cingulate cortex would provide a general motor counterpart to the insula’s sensory role (Craig 2009).
Subcortical Structures
There are 3 distinct subcortical sectors to consider in relation to feeling states: 1) the amygdaloid complex; 2) the ventral striatum and the adjoining nuclear formations of the basal forebrain and the ventral pallidum; and 3) the brainstem tegmentum and hypothalamus. We are leaving out of consideration the thalamus, whose role we assume, for the sake of this discussion, to be primarily that of information conduit from brainstem to telencephalon.
We can say with confidence that the amygdaloid complex is entirely destroyed and cannot play any role in patient B.’s emotive and feeling states and the same appears to apply to the basal forebrain nuclei in the septum, the diagonal band of Broca, and the substantia innominata. Curiously, Patient B. exhibited fear in relation to enclosed spaces such as the MR scanner and to medical procedures involving needles and electrodes. There is good reason to believe that such fear reactions do not require the amygdala and can be generated, in all probability, from hypothalamus and brainstem nuclei (Motta et al. 2009; Damasio 2011; Feinstein et al. 2010). However, the possibility that the ventral striatum, namely the nucleus accumbens, and the ventral pallidum could have made a contribution to Patient B.’s emotions and feelings must be entertained given the involvement of these structures in a variety of affective states (Smith and Berridge 2005, 2007; Kringelbach and Berridge 2010; Smith et al. 2010). These structures could still receive projections from association cortices in the dorsolateral prefrontal, parietal, and posterior temporal regions, and it is likely that their output connections remained patent. In brief, basal ganglia nuclei could have triggered appetitive and emotive processes on the basis of which feeling states would have been generated elsewhere. We suspect that they contributed importantly to the pleasure component of the feelings that B. experienced in his playful social interactions.
The ensemble of brainstem and hypothalamus survived intact in Patient B. HSE normally spares this entire sector and the data in Patient B. suggest that they are indeed intact, having suffered only a loss of volume corresponding, in all likelihood, to the loss of connections to and from damaged areas in the telencephalon. Accordingly, we suggest that this brain sector is likely to have sustained the bulk of Patient B.’s actual feeling states along with possible contributions from the somatosensory cortices. We now turn our attention to this possibility.
Emotions and Feelings Based on the Brain Stem Complex
Is it reasonable to consider that feelings do not arise “first” in the cerebral cortex but actually have their foundation at brainstem level? We believe it is entirely reasonable. It is an established fact that basic homeostasis—hunger, thirst, other drives, metabolic regulation, cardiovascular function—is controlled from the sector that includes the brain stem and the hypothalamus (Swanson 1986, 1987, 1989, 2000; Saper 2002). Moreover, basic emotions are also executed at this level, from fear and panic to joy (Panksepp 1998). In fact, telencephalic structures either in the cerebral cortex or in the amygdala, ventral striatum and ventral pallidum, trigger emotions and execute homeostatic regulation by acting on brain stem level devices such as the periaqueductal gray and the hypothalamus. What we are suggesting here is that besides serving as executors of the machinery of life regulation and emotions, the brain stem region also contains structures capable of generating neural maps of the physiologic states that result from regulatory and emotive responses, thus serving as a platform for the experience of feelings. In brief, in this article, we subscribe to the hypothesis that feelings, ranging from feelings of pain and pleasure to feelings of emotions, first emerge from the integrated operation of structures in the brain stem, hypothalamus and the deep layers of the nearby superior colliculi (hereafter we refer to this set of structures as the brain stem complex). The activity patterns unequivocally present in the insular cortex during feeling states would constitute a second-order mapping of activity patterns first assembled subcortically. The second-order insular maps would be enriched by signals hailing from other regions (e.g., somatosensory cortices) thus producing the most detailed maps underlying feelings. Moreover, the information contained in insular maps would be suitable for interaction with information in cortical systems involved in other sensory processing (e.g., visual, auditory, and tactile) and in higher order functions such as episodic memory, imagination, decision making, and language. In brief, the role we envision for the insula is akin to the role one usually assigns to early sensory cortices of a modality such as vision. As far as feelings are concerned, the insular cortices would stand to the brain stem complex, as the early visual cortices stand to superior colliculi and lateral geniculate nuclei.
Non-human species, certainly mammals but also birds and even simpler species, clearly exhibit appetitive behaviors and emotions. They also exhibit signs compatible with the idea that they experience such behaviors even if they cannot report the experience to the observers, an idea that has been advocated by Panksepp (1998), Denton (2006), and ourselves (Damasio et al. 2000; Damasio 2010). The brains of these species are equipped with brain stem complexes whose general design is comparable to that of humans although their cerebral cortices are not as sophisticated as those of mammals, let alone humans in particular. While it is obvious that non-human minds are not capable of intellectual feats of the human variety, it does not follow that non-human minds are deprived of body feelings, emotional feelings, and sentience. In fact, the initiation, execution, and the regulation of adaptive behaviors can only benefit from the perception of physiologic states (e.g., hunger), and the perception of the behaviors used to modify such states, that is, from feelings. Moreover, human children born without a cerebral cortex or thalamus but with intact brain stem complexes reveal specific emotive reactions and behaviors suggestive of feeling states (Shewmon 1999; Steiner et al. 2001; Merker 2007).
The hypothesis we advance here draws on a fundamental distinction between emotive behaviors and emotional feelings that has been presented in several publications from our research group (for a brief review see Damasio 2011). Emotive behaviors encompass drives, motivations, and emotions proper and consist of a large range of action programs aimed at life regulation in the broadest sense, from the variety of homeostatic regulatory actions that are normally triggered by favorable or unfavorable changes in the organism’s internal state and are carried out by the autonomic and endocrine systems, to the typical emotions that are usually triggered by threats or opportunities (actually presented to the organism or mentally represented in the organism’s brain, even in the absence of an external object). Emotional feelings, on the other hand, are based on sensory representations of the collection of “emotive” regulatory actions executed in varied visceral sectors of an organism along with local and global humoral changes. We have hypothesized that the representations are made in the form of neural maps in brain structures endowed with topographical organization and thus equipped to carry out mappings related to the mental state we call feeling. Such neural mappings, as noted below, are likely to occur in suitably designed subcortical brain structures, as well as the insula and somatosensory regions. It is apparent that the most primordial emotive behaviors are generated at brain stem level (Panksepp 1998) and we hypothesize that the corresponding feelings are mapped at brain stem level as well.
The remarkable progress in affective neuroscience of the past 2 decades has revealed that insular activity and to some extent activity in somatosensory cortices is correlated with feeling states (see Damasio et al. 2000; Craig 2002; Wicker et al. 2003). The structure of such cortices certainly lends itself to the sort of mapping operations that corresponds to our understanding of emotion and feeling processes. Our group has been instrumental in gathering evidence in support of that idea, and we are on record as interpreting the insular cortices aided by nearby orbital and temporal cortices and SI/SII as a platform for feeling states (Adolphs et al. 2000). We have even shown that in certain situations, such as the cravings associated with smoking addiction, damage to the insula can suspend such feelings (Naqvi et al. 2007). This finding is in keeping with the high-level role we assign to the insula as a basis for connecting feeling states with the representation of complex situations in other sensory modalities, at cortical level, and enabling their conscious access. Of note, studies of extensive but unilateral insular damage do not reveal a reduction in feelings. On the contrary, insular damage impairs the modulation of pain such that patients report more intense pain than age-matched controls (Starr et al. 2006).
Assigning an important relational function to the cortex and having the insula play a lead role in that function does not require denying subcortical structures a participation in feeling processes. On the contrary, we propose that brain structures such as the nucleus tractus solitarius (NTS) and the parabrachial nucleus (PBN), assisted by the area postrema (AP) and hypothalamus, also constitute platforms for feeling states. Of necessity, the mappings they provide are simpler than those that can be formed at cortical level and are not as richly connected with other cortical maps and functions as insular maps are. But these nuclei have a fine topographical organization and are capable, for example, of integrating varied aspects of body sensation in relation to different areas of the body and different functions (e.g., cardiac functions, respiratory functions, blood pressure regulation), as shown in the classic neuroanatomical and physiologic experiments of Saper and colleagues (Fulwiler and Saper 1984; Cechetto et al. 1985; Herbert et al. 1990; Moga et al. 1990; Chamberlin and Saper 1992, 1994) and Blessing (1997). The NTS, the PBN, the AP and the hypothalamus are richly interconnected among themselves and this integrated ensemble is interconnected with the PAG, which also has topographical organization and which, besides playing an unequivocal role in emotional responses, is possibly involved in feelings as well (Panksepp 1998; Parvizi and Damasio 2001). Moreover, the PAG is interconnected with the deep layers of the superior colliculus which are also endowed with topographically mapped capabilities and are capable of cross-integrating visual, auditory, and somesthetic information (Huerta and Harting 1984; May 2000; Strehler 1991).
In brief, we are not denying the insular cortices (and its close neighbors in orbitofrontal and temporal regions), a role in the processing of feelings. We are simply resisting, on the basis of the data from Patient B. and on the basis of evolutionary reasoning, the assigning of that role in exclusivity. We suggest that when the insula is absent bilaterally, the brain stem complex and the somatosensory cortices, can still generate feeling maps, and the ventral basal ganglia nuclei can still enrich the resulting feeling states with a hedonic component.
Nor are we denying the fact that the insula provides the main platform to relate feeling states to imagination, reasoning, and language. This is something that the brain stem complex could possibly supply but perhaps less efficiently. Finally, we note that based on Patient B.’s evidence, the insula cannot be regarded as the sole provider of sentience, although the insula would contribute refinement to the process and possibly serve as a preferred platform for conscious access to feeling states.
Funding
National Institute of Neurological Disorders and Stroke P50 NS19632 and The Mathers Foundation.
Acknowledgments
Conflict of Interest : None declared.
References
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio A. A role for somatosensory cortices in the visual recognition of emotion as reveled by three-dimensional lesion mapping. J Neurosci. 2000;20:2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Koenigs M, Damasio AR. Preferring one taste over another without recognizing either. Nat Neurosci. 2005;8:860–861. doi: 10.1038/nn1489. [DOI] [PubMed] [Google Scholar]
- Beck SJ, Beck AG, Levitt EE, Molish HB. Rorschach’s test. I: basic processes. 3rd ed. New York: Grune & Stratton; 1961. [Google Scholar]
- Blakemore SJ, Wolpert D, Frith C. Why can’t you tickle yourself? Neuroreport. 2000;11:R11–16. doi: 10.1097/00001756-200008030-00002. [DOI] [PubMed] [Google Scholar]
- Blessing WW. The lower brainstem and bodily homeostasis. New York: Oxford University Press; 1997. [Google Scholar]
- Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:53–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol. 1992;326:245–262. doi: 10.1002/cne.903260207. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of respiratory responses to glutamate microstimulation of the parabrachial nucleus in the rat. J Neurosci. 1994;14:6500–6501. doi: 10.1523/JNEUROSCI.14-11-06500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- Craig AD. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225:72–82. doi: 10.1111/j.1749-6632.2011.05990.x. [DOI] [PubMed] [Google Scholar]
- Damasio A. Neural basis of emotions. Scholarpedia [Internet] 2011. Available from: http://www.scholarpedia.org/article/Neural_basis_of_emotions. [Google Scholar]
- Damasio A. Self comes to mind. New York: Pantheon/Vintage; 2010/2012. [Google Scholar]
- Damasio AR, Damasio H, Tranel D, Brandt J. Neural regionalization of knowledge access: preliminary evidence. Sympos Quant Biol. 1990;55:1039–1047. . doi: 10.1101/sqb.1990.055.01.098. Cold Spring Harbor Laboratory Press. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Eslinger P, Damasio H, Van Hoesen GW, Cornell S. Multimodal amnesic syndrome following bilateral temporal and basal forebrain damage. Arch Neurol. 1985;42:252–259. doi: 10.1001/archneur.1985.04060030070012. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, Damasio H, Ponto LLB, Parvizi J, Hichwa RD. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Meyer K. 2008 Consciousness: an overview of the phenomenon and of its possible neural basis. In: Laureys S, Tononi G, editors. The neurology of consciousness. London: Elsevier. p. 3–14. [Google Scholar]
- Damasio AR, Tranel D. Knowing that “Colorado” goes with “Denver” does not imply knowledge that “Denver” is in “Colorado”. Behav Brain Res. 1990;40:193–200. doi: 10.1016/0166-4328(90)90076-q. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci. 1993;90:4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Face agnosia and the neural substrates of memory. Ann Rev Neurosci. 1990;13:89–109. doi: 10.1146/annurev.ne.13.030190.000513. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Van Hoesen GW. The limbic system and the localization of herpes simplex encephalitis. J Neurol Neurosurg Psychiatry. 1985;48:297–301. doi: 10.1136/jnnp.48.4.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. New York: Oxford University Press. 2005 [Google Scholar]
- Denton D. The primordial emotions: the dawning of consciousness. New York: Oxford University Press. 2006 [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL. The spinothalamic system targets motor and sensory areas in the cerebral cortex in monkeys. J Neurosci. 2009;29:14223–14235. doi: 10.1523/JNEUROSCI.3398-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner JE., Jr . The Rorschach: a comprehensive system. Basic foundations. 3rd ed. Vol. 1. New York: Wiley; 1993. [Google Scholar]
- Feinstein JS, Rudrauf D, Khalsa SS, Cassell MD, Bruss J, Grabowski TJ, Tranel D. Bilateral limbic system destruction in man. J Clin Exp Neuropsychol. 2010;32:88–106. doi: 10.1080/13803390903066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn FW, Grill HJ. Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci. 1988;102(6):934–941. doi: 10.1037//0735-7044.102.6.934. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Gray MA, Gianaros PJ, Critchley HD. The embodiment of emotional feelings in the brain. J Neurosci. 2010;30:12878–12884. doi: 10.1523/JNEUROSCI.1725-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Behav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Connectional organization of the superior colliculus. Trends Neurosci. 1984;7:286–289. [Google Scholar]
- Kringelbach ML, Berridge KC. The neuroscience of happiness and pleasure. Social Res. 2010;77:659–678. [PMC free article] [PubMed] [Google Scholar]
- Kupers RC, Gybels JM, Gjedde A. Positron emission tomography study of a chronic pain patient successfully treated with somatosensory thalamic stimulation. Pain. 2000;87:295–302. doi: 10.1016/S0304-3959(00)00295-5. [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2000;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- Merker B. Consciousness without a cerebral cortex. Behav Brain Sci. 2007;30:63–81. doi: 10.1017/S0140525X07000891. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I: architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yukihiko Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol. 1990;295:624–661. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Motta S, Goto M, Gouveia FV, Baldo MFV, Cateras NS, Swanson L. Dissecting the brain’s fear system reveals the hypothalamus is critical for responding in subordinate conspecific intruders. Proc Natl Acad Sci. 2009;106:4870–4875. doi: 10.1073/pnas.0900939106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II: afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Murray HA. Explorations in personality. New York: Oxford University Press; 1938. [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: the foundations of human and animal emotions. New York: Oxford University Press; 1998. [Google Scholar]
- Parvizi J, Damasio AR. Consciousness and the brainstem. Cognition. 2001;79:135–160. doi: 10.1016/s0010-0277(00)00127-x. [DOI] [PubMed] [Google Scholar]
- Piché M, Arsenault M, Rainville P. Dissection of perceptual, motor and autonomic components of brain activity evoked by noxious stimulation. PAIN. 2009;149:453–462. doi: 10.1016/j.pain.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Reiss D, Marino L. Mirror self-recognition in the bottlenose dolphin: a case of cognitive convergence. Proc Natl Acad Sci. 2001;98:5937–5942. doi: 10.1073/pnas.101086398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Piché M, Chen J, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proc Natl Acad Sci. 2009;106:20900–20905. doi: 10.1073/pnas.0904706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Ann Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewmon AD. Consciousness in congenitally decorticate children: developmental vegetative state as a self-fulfilling prophecy. Dev Med Child Neurol. 1999;41:364–374. doi: 10.1017/s0012162299000821. [DOI] [PubMed] [Google Scholar]
- Small DM. Taste representation in the human insula. Brain Struct Funct. 2010;214:551–561. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neurosci. 2005;25:8637–8649. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Berridege KC. Opioid limbic circuit for reward: interaction between hedonic hotspots of nucleus accumbens and ventral pallidum. J Neurosci. 2007;27:1594–1605. doi: 10.1523/JNEUROSCI.4205-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic hotspots: generating sensory pleasure in the brain. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford: Oxford University Press; 2010. pp. 27–49. [Google Scholar]
- Starr S, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, Coghill RC. Roles of the insular cortex in the modulation of pain: insights from brain lesions. Eur Neurol. 2006;55:160–165. doi: 10.1523/JNEUROSCI.5173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neuroscience Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- Strehler BM. Where is the self? A neuroanatomical theory of consciousness. Synapse. 1991;7:44–91. doi: 10.1002/syn.890070105. [DOI] [PubMed] [Google Scholar]
- Swanson LW. 1986 Organization of mammalian neuroendocrine system. In: Bloom FE, editor. Handbook of physiology, the nervous system, vol. IV. Baltimore (MD): Waverly Press. [Google Scholar]
- Swanson LW. 1987 The hypothalamus. In: Hokfelt T, et al. editors. Handbook of chemical neuroanatomy, vol 5, Integrated systems of the CNS, Part I. Amsterdam, the Netherlands: Elsevier. p. 1–124. [Google Scholar]
- Swanson LW. The neural basis of motivated behavior. Acta Morphopol. Neer.-Scand. 1989;26:165–176. [PubMed] [Google Scholar]
- Swanson L. Cerebral hemisphere regulation of motivated behavior. Brain Research Initiative. 2000;886:113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR. The covert learning of affective valence does not require structures in hippocampal system or amygdala. J Cogn Neurosci. 1993;5:79–88. doi: 10.1162/jocn.1993.5.1.79. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio AR, Damasio H, Brandt JP. Sensorimotor skill learning in amnesia: additional evidence for the neural basis of nondeclarative memory. Learn Mem. 1994;1:165–179. [PubMed] [Google Scholar]
- Tranel D, Damasio H. Intact electrodermal skin conductance responses after bilateral amygdala damage. Neuropsychologia. 1989;27:381–390. doi: 10.1016/0028-3932(89)90046-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Damasio AR. Amnesia caused by herpes simplex encephalitis, infarctions in basal forebrain, and anoxia/ischemia. In: Boller F, Grafman J, editors. Handbook of neuropsychology. 2nd ed. 2000. Vol. 2 (L. Cermak, Section Editor). Amsterdam: Elsevier Science. p. 85–110. [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]