Abstract
Toll is the founder of a group of pattern recognition receptors that play a critical role in the innate immunity in Drosophila. At least 10 distinct Toll-like receptors (TLRs), recognizing pathogen-associated molecular patterns, have now been identified in humans. Most investigations on TLRs have focused on cells of the innate system. We report here that naïve human T cells expressed high levels of cell-surface TLR2 after activation by anti-T cell receptor antibody and IFN-α. Activated cells produced elevated levels of cytokines in response to the TLR2 ligand, bacterial lipopeptide. Furthermore, CD4+CD45RO+ memory T cells from peripheral blood constitutively expressed TLR2 and produced IFN-γ in response to bacterial lipopeptide, which also markedly enhanced the proliferation and IFN-γ production by CD45RO+ T cells in the presence of IL-2 or IL-15. Thus, TLR2 serves as a costimulatory receptor for antigen-specific T cell development and participates in the maintenance of T cell memory. This suggests that pathogens, via their pathogen-associated molecular patterns, may contribute directly to the perpetuation and activation of long-term T cell memory in both antigen-dependent and independent manner.
Toll was originally found in Drosophila as a pattern recognition receptor associated with defense against fungal and bacterial infection (1, 2). Toll was subsequently found in mammals and was named Toll-like receptor (TLR). At least 10 distinct TLRs have now been identified in humans (3-9). TLRs are activated by pathogen-associated molecular patterns with target selectivity. The pathogen-associated molecular patterns are integral structural components of the pathogens and are thus expected to be essential to the survival of the infectious organisms. Therefore, pathogen-associated molecular patterns are expected to be conserved among a range of pathogens, including virus, bacteria, and fungus.
TLRs act as primary sensors of microbial products and activate signaling pathways that lead to the induction of immune and inflammatory genes (10). TLRs belong to a broader family of proteins, which include receptors for the proinflammatory cytokines IL-1 and IL-18 and the orphan receptor T1/ST2 (11). All members of this superfamily signal inflammation in a very similar manner because of the presence of a conserved protein sequence in the cytosolic domain called the Toll/IL-1 receptor domain, which activates common signaling pathways, most notably those leading to the activation of the transcription factor NF-κB and stress-activated protein kinases (11).
Most investigations on TLRs have focused on cells of the innate system, because TLRs are closely associated with innate response. Although innate immunity may constitute the primary functions of TLRs, there is no a priori reason why TLRs may not have a direct function on adaptive immunity. We have investigated the expression and functions of TLRs on T cells. We report here that TLR2 is expressed on the surface of activated and memory T cells. Furthermore, it functions as a costimulator receptor molecule for T cell activation and helps to maintain T cell memory. These data provide a role for TLR2 and may help to explain how memory T cells are sustained in an immune competent host. The finding should have important implications in our understanding of the host response to infections.
Methods
Cells and Cultures. Cord blood was obtained from the Yorkhill Maternity Hospital, Glasgow, from informed consented mothers. Peripheral blood was obtained from healthy laboratory donors. CD4+ T cells were purified by negative selection according to the manufacturers' instructions (Miltenyi Biotec, Auburn, CA) followed by double positive (CD3+ and CD4+) selection by fluorescence-activated cell sorting (FACS). Peripheral blood CD4+ T cells were further separated into CD45RA+ and CD45RO+ cells (12). The cells were cultured in RPMI medium 1640 + 10% FCS at 37°C in 5% CO2 (2 × 106 cells per ml) in 24-well plates for 72 h in the presence or absence of immobilized anti-CD3 antibody (1-5 μg/ml, BD Bioscience), IFN-α (100-1,000 units/ml, Insight Biotechnology, Wembley, U.K.), lipopolysaccharide (LPS, 1-10 μg/ml, Sigma), bacteria lipoprotein (BLP, Pam3Cys-SK4, 1-10 μg/ml, EMC, Tuebingen, Germany), or a combination of these reagents as indicated in the figures. For blocking experiments, cells were activated with anti-CD3 antibody ± IFN-α overnight followed by adding anti-human TLR2 antibody or control IgG (both 10 μg/ml, eBioscience, San Diego). BLP (2 μg/ml) was added 1 h later. In some experiments, cells were cultured with IL-2 (10-1,000 units/ml, GlaxoSmithKline, Stevenage, U.K.) or IL-15 (20-200 ng/ml, Immunex) added at the beginning of the cultures. We have titrated the concentrations of anti-CD3 antibody, IL-2, and IL-15 and found that there was no significant difference in the range of concentrations used above. For routine analysis, cell proliferation ([3H]thymidine incorporation) and cytokine production were determined at 72 h of culture (48 h for IL-2). Cytokine concentrations were determined by ELISA by using paired antibodies (BD Bioscience). Results are expressed as mean ± SEM (n = 3-5; *, P < 0.05; **, P < 0.01 by Student's t test). It should be noted that, because of the heterogeneity nature of human population, as expected there was considerable individual variation in response to T cell receptor (TCR) activation and BLP. All experiments were performed at least three times from three individuals. Results presented are representative from a single blood donor.
Quantitative PCR. This was carried out as described (13). The primers and probes used are as follows: for TLR2, GGTTCAAGCCCCTTTCTTCTTTA (forward), TGTGAGATGAGAAAAAAGAGATGTTTC (reverse), and CATTCTTAAACTTACTGGGAAATCCTTACAAAACCCTAGG (probe); for TLR4, CAGAGTTTCCTGCAATGGATCA (forward), TGCTTATCTGAAGGTGTTGCACAT (reverse), and CCATTCGTTCAACTTCCACCAAGAGCTG (probe); for CD14, CCGCTGTGTAGGAAAGAA (forward), GCGCTCCATGGTCGATAA (reverse), and TTCCAGAGCCTGTCCGGA (probe); and for MD2, TCCATATTTACTGAAGCTC (forward), ATTGCATTTTATCACAGTA (reverse), and TTGGGTCTGCAACTCATCC (probe).
Flow Cytometry. Purified ex vivo T cells or activated T cells were stained with directly conjugated antibodies: αCD4(PE) and αHLA-DR(FITC) from Sigma, αCD3(ApC), αCD45RA(PE), and αCD45RO(PE) from BD Bioscience, and normal IgG control (PE or ApC) from BD Bioscience. All samples were preincubated for 30 min with human IgG (Sigma) to block FcR. The cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson). All results are representative of at least three independent experiments. The anti-TLR2 antibody (clone TL2.1) and anti-TLR4 antibody (clone HTA125) used here have been extensively characterized (14-17), and similar results were obtained with a different source of antibody.
Immunofluorescence Microscopy. CD4+ T cells were cytocentrifuged at 400 × g for 5 min (Cytospin-3, Shandon, Pittsburgh) and then fixed with 1% paraformaldehyde (Sigma) for 10-15 min. Cells were subsequently processed for analysis of CD3, CD4, TLR2, and TLR4 expression as described above for flow cytometry (except αCD3-PE, which was obtained from Sigma). Cells were analyzed with a Zeiss Axiovert microscope (Zeiss) by using an FITC and Texas red specific filter set (Glen Spectra, Middlesex, U.K.). Fluorescent images were captured by using an ORCA cooled CCD camera (Hamamatsu Photonics, Hamamatsu City, Japan) and processed by openlab software (Improvision, Coventry, U.K.).
Tetramer and TLR Analysis of Epstein-Barr Virus (EBV)-Specific Cell Line. Complexes were synthesized as described (18). Briefly, purified HLA-A*0201 heavy chain and b2 microglobulin were synthesized by means of a prokaryotic expression system, with the heavy chain modified by deletion of the transmembrane/cytosolic domain and C-terminal addition of a sequence containing the BirA enzymatic biotinylation site. HLA heavy chain, b2 microglobulin, and peptide (EBV BMLF1 protein 280-8 GLCTLVAML) (19) were refolded by dilution. The 45-kDa refolded product was isolated by FPLC and then biotinylated by BirA (Avidity, Denver) in the presence of biotin, ATP, and Mg2+ (Sigma). Streptavidin-phycoerythrin conjugate (Sigma) was added in a 1:4 molar ratio, and the tetrameric product was concentrated to 1 mg/ml.
Culture of Cells from TLR2-/- Mice. Spleen and lymph node cells were harvested from WT or TLR2-/- mice (20). CD4+ T cells were purified by negative selection. The purity was 95%. The cells (2 × 106 per ml) were cultured in 24-well plates with CD3+ cell-depleted spleen cells (2 × 105 per ml, without detectable CD4+CD3+ cells) from WT mice. Cell purification was carried out with kits from Miltenyi Biotec and purity analyzed by FACS. The cells were cultured for 3 days in the presence of immobilized anti-CD3 antibody (1 μg/ml, BD Bioscience). The activated CD4+ T cells were then collected and purified by positive selection (purity >99.5%) and cultured with immobilized anti-CD3 antibody with or without BLP (2 μg/ml) for 3 more days. Cell proliferation and IFN-γ concentration in the culture supernatant were then determined as above. This series of experiments, which ensured equal potential WT antigen-presenting cell (APC) contamination in the activated WT and TLR2-/- CD4+ T cell populations, was designed to test whether BLP acted directly on T cells or via APC. Experiments were also performed by deliberately adding WT APC into the cultures of CD4+ T cells from WT or TLR2-/- mice. Purified CD4+ T cells (1 × 106 per ml) from WT or TLR2-/- mice were cultured for 3 days with immobilized anti-CD3 antibody alone or with 5% WT APC. Cell proliferation and IFN-γ production were then determined as above.
Results
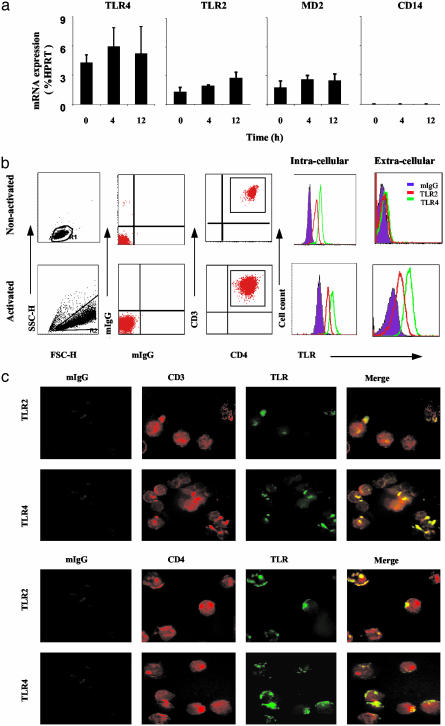
Expression of TLR2 and TLR4 on Activated Human CD4+ T Cells. To demonstrate the expression of TLRs on CD4+ T cells, cord blood (containing mostly naïve T cells) was collected and CD4+ T cells purified by negative selection followed by positive selection on a cell sorter (>99.9% pure). The cells were cultured with medium alone or immobilized anti-CD3 antibody, and the expression of TLR2 and TLR4 was determined by quantitative PCR and flow cytometry. Naïve CD4+ T cells expressed significant levels of mRNA and intracellular proteins of TLR2, TLR4, and MD2 but not CD14. This was not affected by anti-CD3 + IFN-α activation (Fig. 1a). In contrast, only activated T cells expressed appreciable levels of cell-surface TLR2 or TLR4 (Fig. 1b). Kinetic studies show that cell-surface TLR expression peaked between 12 and 72 h after activation and were still at a substantial level by 96 h (data not shown). The cell-surface expression of TLR2 and TLR4 on CD4+ T cells were also visualized by immunofluorescence microscopy, which clearly showed TLRs expressed in a polarized manner on CD3+ and CD4+ T cells (Fig. 1c). Similar results were obtained with cord blood CD8+ T cells (data not shown). Because the T cell preparation was >99.9% pure and the majority (but not all) of the cells stained positive for TLR2 or TLR4, it is unlikely that the staining was due to APC, such as macrophages and dendritic cells. The polarized staining pattern is intriguing. It is tempting to speculate that TLRs tend to aggregate forming lipid raft for effective function in a manner similar to TCR. We added IFN-α in our culture medium as it enhanced the expression of the TLRs. The mechanism for this synergistic effect of TCR engagement and IFN-α stimulation for TLR expression is currently under investigation. We have elected to use plate-bound anti-CD3 antibody to activate the T cells in our culture system instead of the more conventional soluble anti-CD3 plus irradiated APC, to avoid the potential contribution of APC, which carries TLRs and thus may potentially affect indirectly the activation of T cells by means of cytokines.
Fig. 1.
Naïve CD4+ T cells activated with anti-CD3 antibody and IFN-α expressed cell-surface TLR2 and TLR4. (a) Naïve CD4+ T cells purified from cord blood were activated with anti-CD3 antibody and IFN-α and analyzed for TLR2, TLR4, and MD2 mRNA at various time points by real-time PCR. (b) Cells activated with anti-CD3 antibody and IFN-α, or culture medium alone (nonactivated), for 72 h were washed extensively and triple stained with αTLR4-FITC, αTLR2-FITC, αCD3-ApC, and αCD4-PE antibodies or control isotype-match IgG. Cells were analyzed by FACS. (c) Cells were double stained with αCD-PE and αTLR-FITC or αCD3-PE and αTLR-FITC and analyzed by fluorescent microscopy. Results are representative of three experiments.
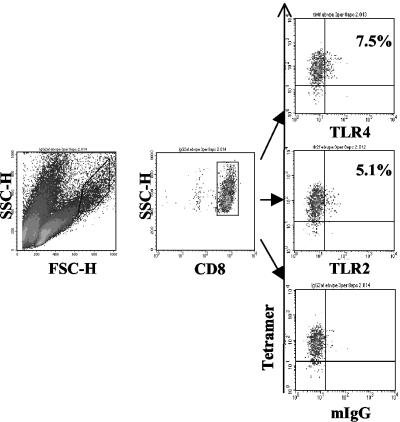
Expression of TLR2 and TLR4 on Activated Antigen-Specific EBV+ CD8+ T Cells. To demonstrate antigen specificity and relevance to infection of TLR expression, we generated CD8+ T cell lines from EBV-infected patients. The cell lines were analyzed by FACS by using MHC class I-EBV peptide tetramer and anti-TLR antibodies. Fig. 2 shows that 5.1% and 7.5% activated EBV-specific CD8+ T cells from a donor were TLR2 and TLR4 positive, respectively. The percent of TLR+ T cells varied among individuals ranging from 5% to 25%.
Fig. 2.
BMLF1-specific CD8+ T cells were expanded in vitro and stained with the BMLF1/HLA-A*0201 phycoerythrin-labeled tetramer (y axis) and FITC-labeled antibodies (x axis) directed to TLR4 (Top Right), TLR2 (Middle Right), and isotype control (Bottom Right). Of the CD8/tetramer double-positive cells, 7.5% were positive for TLR4, and 5.1% were positive for TLR2.
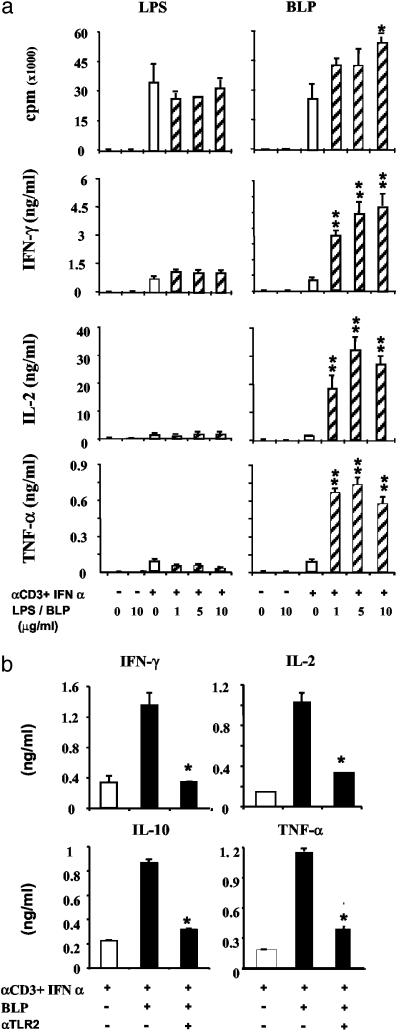
BLP, but Not LPS, Acts as a Costimulatory Molecule for T Cell Activation. TLRs have been associated principally with Th1 cell response (21). To determine the functional significance of TLR expression on T cell differentiation, purified naïve CD4+ T cells were cultured with anti-CD3 antibody and IFN-α in the presence or absence of BLP (Pam3Cys-SK4) or LPS, the ligands for TLR2 and TLR4, respectively (8, 9). The proliferation of activated T cells was not affected by LPS but modestly enhanced by BLP. However, BLP markedly enhanced the production of IFN-γ, IL-2, and tumor necrosis factor α by activated CD4+ T cells (Fig. 3a). Similar results were also obtained when the cells were cultured with two other TLR2 ligands, zymosan A, and peptidoglycan (data not shown). In contrast, LPS had little or no effect on the cytokine production by the T cells. We therefore focused on the function of TLR2. Fig. 3b shows that the cytokine-enhancing effect of BLP was specifically blocked by an anti-TLR2 antibody, further demonstrating the functional specificity of BLP as TLR2 dependent. It should be noted that because of heterogeneity of human population, as expected, there was considerable variation in the degree of enhancement of cellular proliferation and cytokine production between individual cord blood donors. Nevertheless, the pattern of elevation by BLP was consistent.
Fig. 3.
The TLR2 ligand (BLP) but not the TLR4 ligand (LPS) acts as a costimulatory molecule for the activation of CD4+ T cells. (a) CD4+ T cells were purified from cord blood and cultured with immobilized anti-CD3 and IFN-α in the presence of graded concentrations of LPS or BLP. T cell proliferation and cytokine production were determined at 72 h. Vertical bars represent SEM (n = 4) of three experiments. *, P < 0.05; **, P < 0.01 (vs. culture with anti-CD3 + IFN-α without BLP). (b) The costimulatory effect of BLP is blocked by anti-TLR2 antibody. Cord blood CD4+ T cells were cultured with immobilized anti-CD3 antibody + IFN-α with or without BLP (2 μg/ml). The enhancing effect of BLP was significantly blocked by the presence of an anti-TLR2 antibody (10 μg/ml) but not by an isotype-matched normal IgG2a (not shown). Results are representative of three experiments. *, P < 0.05 vs. column without BLP.
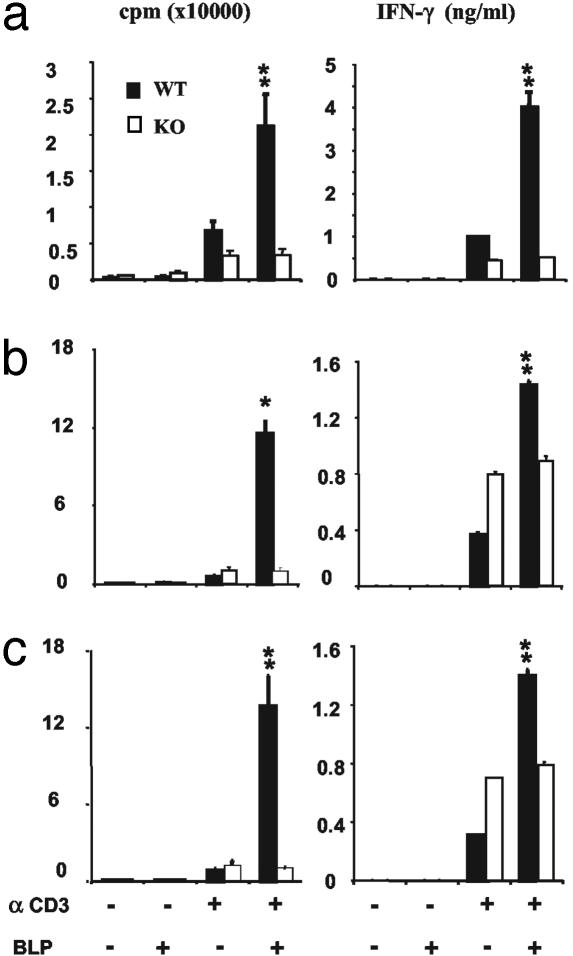
BLP Acts Directly on T Cells and Not via APC. To further exclude the possibility that the observed effect of BLP on T cell activity may be due to an indirect effect on contaminating APCs, we performed similar experiments with T cells from TLR2 knockout mice. Purified (>95%) CD4+ T cells from TLR2-/- or WT mice were cultured in vitro with plate-bound anti-CD3 antibody and CD3+ T cell-depleted WT APC for 3 days. CD4+ T cells were then harvested, purified, and cultured with immobilized anti-CD3 for a further 3 days in the presence or absence of BLP. In such situations, T cells from both TLR2-/- and WT mice should contain similar level of contaminating WT APC. Whereas T cells from WT mice produced enhanced proliferation and IFN-γ production in response to BLP, T cells from the TLR2-/- mice failed to respond to BLP (Fig. 4a). We then cultured purified CD4+ T cells from TLR2-/- or WT mice with immobilized anti-CD3 and deliberately added WT APC in the presence or absence of BLP. Although T cells from WT mice produced increased proliferation and IFN-γ production in the presence of BLP, T cells from TLR2-/- failed to do so even in the presence of up to 10% deliberately added CD3-depleted APC from WT mice. It should be noted that, unlike human T cells, murine T cells proliferated significantly in response to BLP. Furthermore, IFN-α did not influence murine T cell activation by anti-CD3 antibody (data not shown).
Fig. 4.
APC from WT mice failed to modify the lack of effect of BLP on CD4+ T cells from TLR2-/- mice. (a) CD4+ T cells from WT or TLR2-/- mice were cultured for 3 days with CD3+ cell-depleted APC from WT mice (T:APC, 10:1) in the presence of immobilized anti-CD3 antibody. CD4+ T cells were then harvested, purified, and recultured with anti-CD3 antibody for a further 3 days. Cell proliferation and IFN-γ production were then analyzed. (b and c) CD4+ T cells from WT or TLR2-/- mice were cultured for 3 days either alone (b) or with 5% APC from WT mice (c) in the presence of anti-CD3 antibody. Cell proliferation and IFN-γ production were then determined. Data are mean ± SEM (n = 3) from three experiments. *, P < 0.05; **, P < 0.01 (vs. figures of WT cells cultured without BLP).
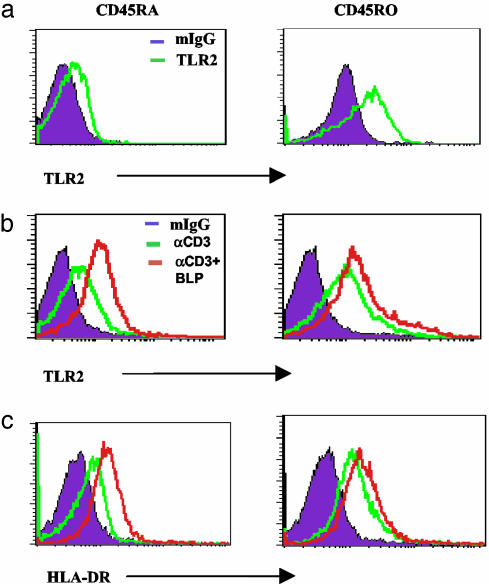
TLR2 Is Constitutively Expressed on Memory T Cells. We then investigated the relative expression and function of TLR2 on memory and naïve CD4+ T cells. CD4+ T cells were purified from peripheral blood of healthy donors and separated into CD45RA+ (naïve) and CD45RO+ (memory) subsets. Cell-surface TLR2 expression was then analyzed by flow cytometry. As expected, naïve CD45RA+ cells did not express appreciable amounts of TLR2 in the absence of activation. In contrast, CD45RO+ memory T cells expressed significant levels of TLR2 ex vivo (Fig. 5). The high level of TLR2 expression on CD45RO+ cells could not be further enhanced by stimulation with anti-CD3 or anti-CD3 + BLP. In contrast, TLR2 expression by the naïve CD45RA+ cells was significantly increased by the stimulation with anti-CD3, and this was further enhanced by the presence of BLP. The expression of TLR2 paralleled the activation status of the cells as evident by their coexpression of HLA-DR antigen, an activation marker.
Fig. 5.
Expression of TLR2 on naïve (CD45RA+, Left) and memory (CD45RO+, Right) peripheral blood CD4+ T cells. Flow cytometry was carried out on CD4+ T cells by using anti-CD45RO (PE), anti-CD45RA (PE), and anti-TLR2 (FITC) antibodies. (a) Cells were stained for TLR2 ex vivo; mIgG denotes murine IgG isotype control. (b) Anti-CD3 activation induced TLR2 expression on naïve cells. This was further enhanced by the presence of BLP. The constitutively expressed TLR2 by memory cells was not significantly increased further by anti-CD3 and BLP stimulation. (c) TLR2 expression paralleled HLA-DR expression on these cells. Results are representative of three donors.
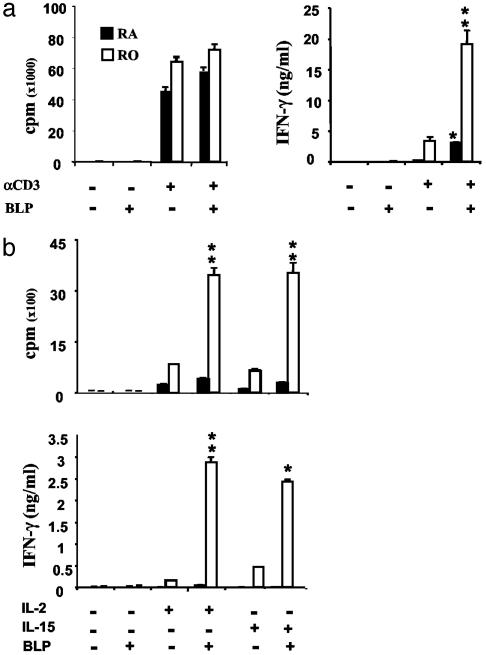
BLP Enhances Proliferation and IFN-γ Production by Memory T Cells. We then investigated the functional role of TLR2 expression in activated naïve CD4+CD45RA+ T cells and memory CD4+CD45RO+ T cells in response to BLP in vitro. The cells were cultured with plate-bound anti-CD3 antibody ± BLP, and cellular proliferation and cytokine production was determined. Naïve and memory CD4+ T cells did not show any detectable response when cultured with BLP alone (Fig. 6). Both cell types produced high levels of proliferation when cultured with anti-CD3 antibody, which was not affected by BLP. Naïve CD4+ T cells cultured with anti-CD3 produced a low level of IFN-γ, which was modestly enhanced by BLP. In contrast, memory CD4+CD45RO+ cells activated with anti-CD3 produced significant amounts of IFN-γ, which was markedly enhanced by BLP (Fig. 6a). IL-4 production was not detected (data not shown). We further compared the role of BLP on naïve and memory CD4+ T cells under a bystander activation condition with IL-2 and IL-15, two cytokines closely associated with memory cell expansion (12, 22-25). Naïve CD4+CD45RA+ cells did not produce appreciable levels of proliferation or cytokines when cultured with IL-2 or IL-15, and this was not affected by BLP. In contrast, CD4+CD45RO+ memory T cells produced significant levels of proliferation and IFN-γ when cultured with IL-2 or IL-15, and these functions were markedly enhanced by the presence of BLP (Fig. 6b). IL-4 or IL-10 was not detected in the cultures (data not shown).
Fig. 6.
BLP increases the proliferation and IFN-γ production by memory CD4+ T cells in response to anti-CD3 activation or IL-2 or IL-15 stimulation. (a) CD45RA+ and CD45RO+ T cells from peripheral blood were cultured with anti-CD3 antibody. Proliferation and IFN-γ production was determined. *, P < 0.05; **, P < 0.01 (vs. culture with immobilized anti-CD3 alone). (b) CD45RA+ or CD45RO+ cells were cultured with IL-2 or IL-15 in the presence or absence of BLP. *, P < 0.05; **, P < 0.01 (vs. cultures of respective cytokine).
Discussion
TLR messages have been reported to be present in T cells (26-32). We have now directly demonstrated the expression and function of TLRs on the cell surface of antigen-specific T cells. The following lines of evidence support the functional expression of TLR2 on T cells: (i) the T cell population tested had no detectable CD14 message (Fig. 1a); (ii) the majority of a 99.9% pure population of CD4+CD3+ cells stained positive for TLR2 (Fig. 1 b and c); (iii) antigen-specific tetramer-positive cells also stained positive for TLR2 (Fig. 2); (iv) BLP alone did not induce a detectable level of cytokine or proliferation (Fig. 3a); and (v) in contrast to WT T cells, anti-CD3-activated T cells from TLR2-/- mice did not respond to BLP even in the presence of up to 5% of WT APC (Fig. 4).
Several features reported here are noteworthy. (i) Surface expression (and hence function) of TLR2 was evident only after TCR activation, and this was enhanced by the presence of the TLR2 ligand, BLP. Although the mechanism for the induction of TLR migration from the cytoplasm to cell surface is currently unknown, the effect of BLP in this process may represent an important self-amplification loop for TLR functions. (ii) TLR2 and TLR4 are also expressed on subsets of activated EBV-specific CD8+ T cells derived from EBV-positive patients. This result extends our finding to clinically relevant T cells beyond the CD4+ subset. (iii) Although activated CD4+ T cells expressed similar levels of TLR4 and TLR2, the cells did not respond to LPS, a recognized ligand for TLR4 (33). This is consistent with a large number of reports in the literature (34-36). LPS has recently been reported to activate CD4+CD25+ T cells in mice (32). However, this finding remains to be confirmed. In the CD4+CD25+ T cell system, the potential involvement of cell types other than T cells could not be excluded. Using highly purified T cells, we were not able to activate murine CD4+CD25+ T cells to respond consistently to LPS (data not shown). The reason for the failure of human T cells, in contrast to cells of the monocytic lineage, to respond to LPS is currently unclear, but it may be due to the lack of coreceptors (such as CD14 shown in Fig. 1a) on these T cells essential for LPS-induced activation. (iv) Activated or memory human T cells also failed to respond to PolyI:C (a ligand for TLR3) or CpG (a ligand for TLR9) (data not shown), suggesting that TLR2, whose ligands are perhaps the most conserved among all of the known TLRs, may be unique in its ability to function as a costimulator receptor of T cells. (v) Induction of TLR expression on T cells is TCR activation-dependent. Once induced, TLR2 signaling further enhances the T cell functions. This TCR-TLR interaction pathway suggests that TLR2 may be essential for optimal antigen-specific T cell response. (vi) TLR expression is cell activation-dependent and is enhanced by the presence of IFN-α, another innate cytokine. This demonstrates the networking role of TLR2 with other innate mediators in enhancing adaptive immune response.
The present studies establish TLR2 as a costimulatory receptor for pathogen-derived ligand, as opposed to conventional T cell costimulation ligands expressed on autologous cells. TLR2 may play an important role in adaptive immunity by directly enhancing antigen-specific Th1 cell function, in addition to an indirect role through the activation of APC (9, 21). Our results therefore demonstrate the existence of a T cell activation pathway via TLR2 that recognizes the largest spectrum of pathogen-associated molecular patterns and stress proteins among the TLRs (9, 31, 37). The constitutive and sustained expression of TLR2 on memory T cells may represent an important host device to mount an immediate strong response on encountering a reinfecting pathogen. Because BLP is highly conserved among bacterial strains, results reported here may help to explain the often observed “flare up” of T cell-mediated autoimmunity during microbial infection. Another important feature for the expression of TLR2 on memory T cells could be the contribution to the expansion and maintenance of memory T cells in the absence of specific antigen but in the presence of memory-sustaining cytokines, including IL-2 and IL-15 produced by adaptive as well as innate immune cells. This mechanism could contribute to the perpetuation of long-term memory by infectious agents with shared TLR2 ligand but are otherwise unrelated to the antigen specificity of the memory T cells. It should also be noted that BLP alone did not activate naïve or memory T cells. It does so only in the presence of TCR activation or a bystander effect of cytokines such as IL-2 or IL-15. This dual-signaling mechanism should avoid excessive T cell proliferation by BLP alone. Recently, it was reported that B cell receptor triggering led to up-regulation of TLR9, which was constitutively expressed in memory B cells (38). Furthermore, human memory B cell proliferated and differentiated into plasma cells in response to CpG DNA (39). Our findings reported here suggest that there may be a parallel between T and B cells in that TLR signaling helps to maintain long-term memory of these cells. This observation may also help to resolve a current central controversy on the requirement for the continuous presence of specific pathogen for the maintenance of long-term specific T cell memory (40-42).
Acknowledgments
We thank Dr. James Brewer (University of Glasgow) for assistance with immunofluorescence microscopy. We also thank Dr. Sabine Totemeyer (University of Cambridge) for providing lymphoid cells from the TLR2-/- mice originally provided by Dr. Shizou Akira (University of Osaka). This work was supported by the Medical Research Council, the Wellcome Trust, the Chief Scientist's Office, Scotland, and the European Commission.
Abbreviations: APC, antigen-presenting cell; BLP, bacteria lipoprotein; EBV, Epstein-Barr virus; FACS, fluorescence-activated cell sorter; LPS, lipopolysaccharide; TCR, T cell receptor; TLR, Toll-like receptor.
References
- 1.Belvin, M. P. & Anderson, K. V. (1996) Annu. Rev. Cell Dev. Biol. 12, 393-416. [DOI] [PubMed] [Google Scholar]
- 2.Lemaitre, B., Nicolas, E., Michaut, L., Reichhart, J. M. & Hoffmann, J. A. (1996) Cell 86, 973-983. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov, R., Preston-Hurlburt, P. & Janeway, C. A., Jr. (1997) Nature 388, 394-397. [DOI] [PubMed] [Google Scholar]
- 4.Rock, F. L., Hardiman, G., Timans, J. C., Kastelein, R. A. & Bazan, J. F. (1998) Proc. Natl. Acad. Sci. USA 95, 588-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhary, P. M., Ferguson, C., Nguyen, V., Nguyen, O., Massa, H. F., Eby, M., Jasmin, A., Trask, B. J., Hood, L. & Nelson, P. S. (1998) Blood 91, 4020-4027. [PubMed] [Google Scholar]
- 6.Takeuchi, O., Kawai, T., Sanjo, H., Copeland, N. G., Gilbert, D. J., Jenkins, N. A., Takeda, K. & Akira, S. (1999) Gene 231, 59-65. [DOI] [PubMed] [Google Scholar]
- 7.Chuang, T. & Ulevitch, R. J. (2001) Biochim. Biophys. Acta 1518, 157-161. [DOI] [PubMed] [Google Scholar]
- 8.Aderem, A. & Ulevitch, R. J. (2000) Nature 406, 782-787. [DOI] [PubMed] [Google Scholar]
- 9.Akira, S., Takeda, K. & Kaisho, T. (2001) Nat. Immunol. 2, 675-680. [DOI] [PubMed] [Google Scholar]
- 10.Takeda, K., Kaisho, T. & Akira, S. (2003) Annu. Rev. Immunol. 21, 335-376. [DOI] [PubMed] [Google Scholar]
- 11.Brint, E. K., Fitzgerald, K. A., Smith, P., Coyle, A. J., Gutierrez-Ramos, J. C., Fallon, P. G. & O'Neill, L. A. (2002) J. Biol. Chem. 277, 49205-49211. [DOI] [PubMed] [Google Scholar]
- 12.Geginat, J., Sallusto, F. & Lanzavecchia, A. (2001) J. Exp. Med. 194, 1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sweet, M. J., Leung, B. P., Kang, D., Sogaard, M., Schulz, K., Trajkovic, V., Campbell, C. C., Xu, D. & Liew, F. Y. (2001) J. Immunol. 166, 6633-6639. [DOI] [PubMed] [Google Scholar]
- 14.Lien, E., Sellati, T. J., Yoshimura, A., Flo, T. H., Rawadi, G., Finberg, R. W., Carroll, J. D., Espevik, T., Ingalls, R. R., Radolf, J. D. & Golenbock, D. T. (1999) J. Biol. Chem. 274, 33419-33425. [DOI] [PubMed] [Google Scholar]
- 15.Flo, T. H., Halaas, O., Lien, E., Ryan, L., Teti, G., Golenbock, D. T., Sundan, A. & Espevik, T. (2000) J. Immunol. 164, 2064-2069. [DOI] [PubMed] [Google Scholar]
- 16.Shimazu, R., Akashi, S., Ogata, H., Nagai, Y., Fukudome, K., Miyake, K. & Kimoto, M. (1999) J. Exp. Med. 189, 1777-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, Q., Akashi, S., Miyake, K. & Petty, H. R. (2000) J. Immunol. 165, 3541-3544. [DOI] [PubMed] [Google Scholar]
- 18.Altman, J. D., Moss, P. A., Goulder, P. J., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274, 94-96. [DOI] [PubMed] [Google Scholar]
- 19.Steven, N. M., Annels, N. E., Kumar, A., Leese, A. M., Kurilla, M. G. & Rickinson, A. B. (1997) J. Exp. Med. 185, 1605-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi, O., Hoshino, K., Kawai, T., Sanjo, H., Takada, H., Ogawa, T., Takeda, K. & Akira, S. (1999) Immunity 11, 443-451. [DOI] [PubMed] [Google Scholar]
- 21.Schnare, M., Barton, G. M., Holt, A. C., Takeda, K., Akira, S. & Medzhitov, R. (2001) Nat. Immunol. 2, 947-950. [DOI] [PubMed] [Google Scholar]
- 22.Unutmaz, D., Pileri, P. & Abrignani, S. (1994) J. Exp. Med. 180, 1159-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. (1998) Immunity 8, 591-599. [DOI] [PubMed] [Google Scholar]
- 24.Marrack, P., Bender, J., Hildeman, D., Jordan, M., Mitchell, T., Murakami, M., Sakamoto, A., Schaefer, B. C., Swanson, B. & Kappler, J. (2000) Nat. Immunol. 1, 107-111. [DOI] [PubMed] [Google Scholar]
- 25.Niedbala, W., Wei, X. & Liew, F. Y. (2002) Eur. J. Immunol. 32, 341-347. [DOI] [PubMed] [Google Scholar]
- 26.Muzio, M., Bosisio, D., Polentarutti, N., D'Amico, G., Stoppacciaro, A., Mancinelli, R., van't Veer, C., Penton-Rol, G., Ruco, L. P., Allavena, P. & Mantovani, A. (2000) J. Immunol. 164, 5998-6004. [DOI] [PubMed] [Google Scholar]
- 27.Mokuno, Y., Matsuguchi, T., Takano, M., Nishimura, H., Washizu, J., Ogawa, T., Takeuchi, O., Akira, S., Nimura, Y. & Yoshikai, Y. (2000) J. Immunol. 165, 931-940. [DOI] [PubMed] [Google Scholar]
- 28.Zarember, K. A. & Godowski, P. J. (2002) J. Immunol. 168, 554-561. [DOI] [PubMed] [Google Scholar]
- 29.Bendigs, S., Sallzer, U., Lipford, G. B., Wagner, H. & Heeg, K. (1999) Eur. J. Immunol. 29, 1209-1218. [DOI] [PubMed] [Google Scholar]
- 30.Matsuguchi, T., Takagi, K., Musikacharoen, T. & Yoshikai, Y. (2000) Blood 95, 1378-1385. [PubMed] [Google Scholar]
- 31.Zanin-Zhorov, A., Nussbaum, G., Franitza, S., Cohen, I. & Lider O. (2003) FASEB J. 17, 1567-1569. [DOI] [PubMed] [Google Scholar]
- 32.Caramalho, I., Lopes-Carvalho, T., Ostler, D., Zelenay, S., Haury, M. & Demengeot, J. (2003) J. Exp. Med. 197, 403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085-2088. [DOI] [PubMed] [Google Scholar]
- 34.Vilanova, M., Tavares, D., Ferreira, P., Oliveira, L., Nobrega, A., Appelberg, R. & Arala-Chaves, M. (1996) Scand. J. Immunol. 43, 155-163. [DOI] [PubMed] [Google Scholar]
- 35.Tough, D. F., Sun, S. & Sprent, J. (1997) J. Exp. Med. 185, 2089-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro, A., Bemer, V., Nobrega, A., Coutinho, A. & Truffa-Bachi, P. (1998) Eur. J. Immunol. 28, 488-495. [DOI] [PubMed] [Google Scholar]
- 37.Ohashi, K., Burkart, V., Flohe, S. & Kolb, H. (2000) J. Immunol. 164, 558-561. [DOI] [PubMed] [Google Scholar]
- 38.Bernasconi, N. L., Onai, N. & Lanzavecchia, A. (2003) Blood 101, 4500-4504. [DOI] [PubMed] [Google Scholar]
- 39.Bernasconi, N. L., Traggial, E. & Lanzavecchia, A. (2002) Science 298, 2199-2102. [DOI] [PubMed] [Google Scholar]
- 40.Zinkernagel, R. M., Bachmann, M. F., Kundig, T. M., Oehen, S., Pirchet, H. & Hengartner, H. (1996) Annu. Rev. Immunol. 14, 333-367. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed, R. & Gray, D. (1996) Science 272, 54-60. [DOI] [PubMed] [Google Scholar]
- 42.Dutton, R. W., Bradley, L. M. & Swain, S. L. (1998) Annu. Rev. Immunol. 16, 201-223. [DOI] [PubMed] [Google Scholar]