Abstract
Cross-priming is essential for generating cytotoxic T lymphocytes to viral, tumor, and tissue antigens that are expressed exclusively in parenchymal cells. In this process, the antigen-bearing parenchymal cells must somehow transfer their antigens to bone marrow-derived professional antigen-presenting cells. Although intact proteins, small peptides, or peptide-heat shock protein complexes can all be acquired and presented by antigen-presenting cells, the physiologically relevant form of antigen that is actually transferred from parenchymal cells and cross-presented in vivo is unknown and controversial. To address this issue we have investigated the ability of fibroblasts stably expressing chicken ovalbumin constructs targeted to different subcellular compartments to cross-prime cytotoxic T lymphocytes. Although these transfectants generated similar amounts of the immunogenic ovalbumin peptide, their cross-priming activity differed markedly. Instead, the cells cross-priming ability correlated with their steady-state levels of ovalbumin protein and/or the physical form/location of the protein. Moreover, in subcellular fractionation experiments, the cross-priming activity colocalized with antigenic protein. In addition, depletion of intact protein antigen from these cell fractions eliminated their cross-priming activity. In contrast, the major heat shock protein candidates for cross-presentation were separable from the cell's main sources of cross-priming antigen. Therefore, cellular proteins, rather than peptides or heat shock protein/peptide complexes, are the major source of antigen that is transferred from antigen-bearing cells and cross-presented in vivo.
In all tissues, bone marrow-derived antigen-presenting cells (APCs), such as dendritic cells, generate peptides from antigens they have acquired from the local environment, a fraction of which are then bound by their MHC class I molecules (1-3). These APCs then migrate to secondary lymphoid organs, where they present their MHC class I-peptide complexes to CD8+ T cells. This allows the immune system to detect viral infections and tumors in the peripheral tissues and mobilize a specific CD8+ T cell response. This process is essential for the immune surveillance of tumors and viral infections because in the absence of antigen presentation by bone marrow-derived APC, no cytotoxic T lymphocyte (CTL) response is generated (1, 3).
There are two different mechanisms by which bone marrow-derived APCs acquire antigens in the tissues for presentation on MHC class I molecules. Like all other cells, the bone marrow-derived APCs generate class I-presented peptides from proteins they have synthesized (4). Therefore, in the tissues, they may become directly infected by a virus and present viral antigens. However, in almost all other situations, the APCs must acquire antigens synthesized by other cells, e.g., tumors or those infected with tissue tropic viruses. The bone marrow-derived APCs uniquely have the capability of generating and presenting on MHC class I molecules peptides from antigens internalized from the extracellular milieu (5-7). This process is called cross-presentation or cross-priming and has been shown to be essential for generating CTL to viruses, tumors, and tissue antigens (1-3, 8, 9).
In cross-priming, the antigen-bearing cells must somehow transfer their antigens to the professional APCs. In this process, these antigens may be shed from living cells or released from dying ones. A major unresolved question is the nature of the cross-priming antigen. There are at least two competing, but non-mutually exclusive, models for the origin of the cross-presented antigen. One postulated mechanism is that the antigen is transferred as a peptide bound to cellular heat shock proteins (HSP) (10, 11). In support of this model, it has been shown that various forms of HSPs, including hsp70, hsp90, and grp94/gp96, isolated from tumors, infected, or transfected cells contain bound antigenic peptides (10). These HSP/peptide complexes can be taken up by APC in vitro and the bound peptides represented on MHC class I molecules (12-15). Moreover, when HSP/tumor peptide complexes are purified from cells and injected into animals, they cross-prime CTL immunity specific for the tumors from which the HSP/peptide complexes were isolated (16). It has also been reported that HSP incubated with peptides in vitro induce CTL responses when injected in vivo (17, 18). However, it is unknown whether the HSPs that are naturally released from cells contribute significantly to cross-presentation in vivo. Relatively large amounts of HSP/peptide complexes are needed to prime CTL responses, probably because, HSP association with peptide is a very inefficient process (19, 20). Furthermore, recent studies demonstrate that grp94/gp96, a hsp90 family member, can stimulate tumor immunity in the absence of bound peptide (21). Thus, it is unknown how physiologically important HSP-peptide complexes are in immune surveillance.
Another postulated mechanism for cross-priming is that antigens are transferred from cells to APCs as intact protein or protein fragments (1). There are numerous ways for APC to acquire intact antigens from their environment. The bone marrow-derived APC are phagocytic and efficiently internalize dying cells and their debris and shed exosomes, all of which contain intact antigens (22-25). It has also been reported that dendritic cells can actively “bite” off pieces of antigen-bearing cells, and this might provide another means of antigen transfer (26). In support of the model that the cross-priming antigen can be protein, it has been shown in vitro that intact protein antigens, both in particulate form and, less efficiently, in soluble form, can be cross-presented on MHC class I by professional APCs (27). Moreover, when particulate protein antigen is injected in vivo it stimulates CTL responses (27-29). Thus intact protein can be cross-presented under experimental conditions.
Although both HSP-peptide complexes and intact proteins can cross-prime CTL, it is unclear which form of antigen is more physiologically important for the cross-priming of cell associated antigens. Elucidating these issues should provide insight into a major mechanism of immune surveillance. In the current study, we analyze the nature of the antigen that is responsible for the cross-priming of a cellular antigen in vivo. We find that CTL are cross-primed by injection of cell lines stably transfected with chicken ovalbumin (OVA) constructs that are targeted to different subcellular compartments. These transfectants generate similar amount of antigenic ovalbumin peptides; however, they have vastly different activities in cross-priming CTL responses. Thus, their cross-priming activity does not correlate with the peptides available to HSP. Instead, we find that the cell's cross-priming ability correlates with their steady-state levels of ovalbumin and/or the physical form/location of the protein. Furthermore, subcellular fractionation experiments demonstrate that HSP and presumably HSP/peptide complexes are largely dispensable for cross-presentation in vivo. In contrast, depletion of protein antigen from cell fractions almost completely abrogated their cross-priming activity, indicating that protein antigen, but not HSP/peptide complexes, is the major cross-priming material from the antigen expressing cells.
Experimental Procedures
Mice and Reagents. B6C3F1 (H-2bxk) mice were purchased from The Jackson Laboratory and housed in a pathogen-free facility at the University of Massachusetts Medical School. Four- to 8-week-old male mice were used in experiments.
Polyclonal IgG goat or rabbit anti-OVA and control IgG Ab were purchased from ICN (Irving, CA). The purified anti-OVA mAb (all IgG1), HYB 99-01, 99-02, and 99-09, were obtained from AntibodyShop (Copenhagen). Rabbit polyclonal anti-hsp70 (SPA-812) and anti-hsp90α (SPA-771) and rat monoclonal anti-grp94/gp96 (SPA-850) Ab were purchased from Stressgen (Victoria, BC, Canada). 25D1.16 supernatants were obtained from hybridoma cells (kindly provided by R. Germain of the National Institutes of Health, Bethesda). 1F10 2.2 anti-SIINFEKL peptide mAb was described (30).
Generation of OVA Stable Transfectants. Full-length OVA (FL-OVA) cDNA in pBluescript SK-OVA was digested with HindIII and NotI and then ligated into pcDNA3.1. To generate a cytosolic form of OVA (Cyto-OVA), we deleted the sequence encoding the N-terminal 50 aa by PCR. The PCR product was directly cloned into pCR3.1 plasmid by using TA cloning kit (Invitrogen) and later also subcloned into pcDNA3.1. TfR-OVA was described (31) and subcloned into pcDNA3.1.zeocin. We also generated enhanced GFP (EGFP) fusion constructs with Cyto-OVA, FL-OVA, and TfR-OVA attached to the C terminus of EGFP by PCR. The PCR products were ligated in-frame into pEGFP C1 (Invitrogen) and then subcloned into pcDNA3.1. DNA sequences were confirmed by sequencing.
DAP cells were transfected by using FuGENE 6 (Roche Diagnostics) and cloned by limiting dilution. OVA expression was determined by ELISA and/or Western blotting of Nonidet P-40 detergent lysates of cells. For EGFP-OVA fusion constructs, flow cytometry was used to identify positive clones. Endotoxin levels in cells was below the level of detection of a Limulus Amebocyte Lysate assay (BioWhittaker, Walkersville, MD).
ELISA. OVA in cell lysates and cytosol fractions was measured by ELISA. Cells were lysed at 2-4 × 106 per ml in lysis buffer as described above. Briefly, 96-well enzyme immunoassay plates were coated with goat anti-OVA polyclonal Ab overnight at 4°C. Fractions with or without antibody depletion were serially diluted into wells, incubated at room temperature for 1 h, and then washed and blocked. The wells were then incubated with rabbit anti-OVA Ab, washed, and incubated with horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch). Finally, a perioxidase substrate was added and OD405 nm was read in a plate reader. By comparison to an OVA (Sigma) standard, it was estimated that 1.0, 0.6, and 0.1 ng of OVA protein was present in 1 × 106 FL-, TfR-, and Cyto-OVA transfected cells, respectively (data not shown).
To measure the binding of poly- and monoclonal Ab to OVA SIINFEKL peptide, plates were coated with the peptide and then incubated with various antibodies. The Ab binding to peptides immobilized on plates was determined by ELISA using HRP-conjugated secondary Ab and color development as described above.
Antigen Presentation Assay and Flow Cytometry. OVA transfectants were first infected with 1 × 106 plaque-forming units (pfu) of rVacKb for 16 h and then were fixed with 2% paraformaldehyde. Washed cells were titrated on 96-well plates, cocultured with 1 × 105 per well RF33.70 T-T hybridomas, and incubated for 16 h. Subsequently, supernatant was removed and its content of IL-2 was determined by using CTLL2 cells. Results are expressed as the cpm of [3H]thymidine incorporated into DNA.
rVac Kb-infected OVA transfectants were also assessed for surface Kb/SIINFEKL complexes by incubation with 25D1.16 supernatant or isotype control mAb, followed by FITC-conjugated anti-mouse IgG and then analysis by flow cytometry.
35S Metabolic Labeling and Pulse-Chase Immunoprecipitation. OVA transfected cells in six-well plates were starved in methionine- and cysteine-free RPMI medium 1640 for 1 h. Cells were labeled with 100 μCi of [35S]methionine/cysteine for 5 min, washed at 4°C, and then incubated in chase medium (complete RPMI) for 30-120 min (1 Ci = 37 GBq). Subsequently, cells were lysed in 1% Nonidet P-40/0.5% deoxycholate/150 mM NaCl/1 mM EDTA/50 mM Tris, pH 8.0, containing a mixture of protease inhibitors. Filtered lysates were then incubated with Protein A-agarose beads prebound with rabbit anti-OVA antibody for 1 h at 4°C. After wash, the samples were then boiled with reducing sample buffer and separated on SDS/10% PAGE gels. The gels were treated with Autofluor (Natural Diagnostics), dried, and exposed to film at -80°C.
SDS/PAGE and Western Blot. Whole cell lysates or subcellular fractions were boiled in sample application buffer containing 2-mercaptoethnol. Proteins were separated by SDS/10% PAGE, and transferred to a poly(vinylidene difluoride) membrane. The membranes were probed with various antibodies followed by appropriate secondary Ab. The proteins were visualized by enhanced chemiluminescence.
Cell Fractionation. Membrane and cytosol was prepared from 2-4 × 108 OVA-transfected cells as described (32) with modifications. Cells at 25 × 106 per ml in PBS at 4°C were disrupted by N2 cavitation at 500 psi for 6 min and then centrifuged at 5,500 rpm (3,650 × g) for 15 min to remove nuclei and any intact cells. The resulting supernatants were further centrifuged at 13,500 rpm (22,000 × g) for 30 min to separate membrane and cytosol. The pellet (membrane fraction) was resuspended in PBS at a cell equivalent of 25 × 106 per ml. In some experiments, the cytosol fraction (supernatant) was further ultracentrifuged at 35,000 rpm (120,000 × g) for 1 h or 7 h.
Depletion of OVA Using Antibodies. The cytosol fractions were depleted of OVA protein by incubating 500 μl of cytosol fractions with 5-10 μg of various antibodies at 4°C for 1 h followed by an incubation with 20-40 μl of Protein A/G beads (Pierce) for 1 h. Alternatively, 10 μg antibodies were prebound to protein A/G beads, and then used for immunodepletion. Finally, the beads were removed by centrifugation at 10,000 × g for 10 min, and supernatants were used for assays.
CTL Assay. Mice were immunized s.c. with 2-5 × 106 OVA transfectants, or 2.5 × 106 cell equivalent subcellular fractions in 100 μl PBS. Seven days later, spleens were harvested and restimulated with 10-7 M SIINFEKL peptide. On day 5 or 6 of the restimulation, a 51Cr release assay was performed to determine the CTL cytotoxicity. EL4 cells were labeled and pulsed with or without SIINFEKL peptide. Effector cells were incubated with the target cells (5 × 103) at the indicated effector-to-target cell ratio for 5 h. Percent specific killing was calculated as: (experimental release - spontaneous release)/(total release - spontaneous release) × 100%. In all experiments, the spontaneous release is <15% of the total release. All experiments were repeated at least three times, and representative results are shown. Statistical analysis of 51Cr release assay results was performed by using ANOVA.
Results and Discussion
The models in which either protein or HSP/peptide complexes are the source of the cross-priming antigen from cells make distinct and testable predictions. If protein is the primary source, then cross-priming will be influenced by the steady-state level, subcellular location, and/or physical form (e.g., membrane-associated versus soluble) of the protein. In contrast, if HSP-peptide complexes are the source of the cross-presented antigen, then cross-priming should depend solely on the amount of antigenic peptide that is generated in cells. In this model, the level or state of the antigenic protein in cells would only impact cross-presentation to the extent that it influences the amount of peptide generated. Another prediction is that depleting the intact protein antigen from cells, e.g., with antibodies, should inhibit cross-priming if protein is the source of the cross-presented antigen but not if it is HSP/peptide complexes (which will not react with antibody to intact protein).
To test these predictions, we used a classical cross-priming experimental system in which F1 mice are injected with “parental” cells that express a foreign antigen. In this situation, the antigen bearing cells lack the appropriate MHC class I molecules needed to present their antigens to the host's CD8+ T cells. Instead, immune responses can only be generated when antigen from the immunizing cell is cross-presented by host APCs. For the antigen-bearing cell, we used DAP cells (L cells), a fibroblast cell line originally generated from a C3H/An (H-2k) mouse, that were transfected with OVA constructs. These OVA transfectants were used to immunize H-2bxk F1 mice in which the only mechanism available to induce Kb/SIINFEKL-specific CD8+ T cell responses was cross-priming by endogenous APC (because the DAP cells lack the H-2 Kb that presents SIINFEKL).
We made stable transfectants expressing three different forms of OVA antigen that varied in their subcellular distribution. (i) Unmodified, full-length OVA (FL-OVA), which is transported into the endoplasmic reticulum (ER) and exocytic compartment of cells. (ii) Cytosolic OVA (Cyto-OVA). In this construct, the N-terminal 50 aa, which include a signal sequence, were deleted so that the expressed protein resides in the cytosol. (iii) Transferrin receptor-OVA fusion protein (TfR-OVA). In this construct, ovalbumin is fused in frame with the transferrin receptor to produce a membrane-bound form of OVA. This protein is transported into the ER and exocytic compartments of cells and then localizes to the plasma membrane and early endosomes (31). In addition, to visualize their intracellular location and for the ease of selection and quantitation by flow cytometry, we also fused these forms of ovalbumin to EGFP.
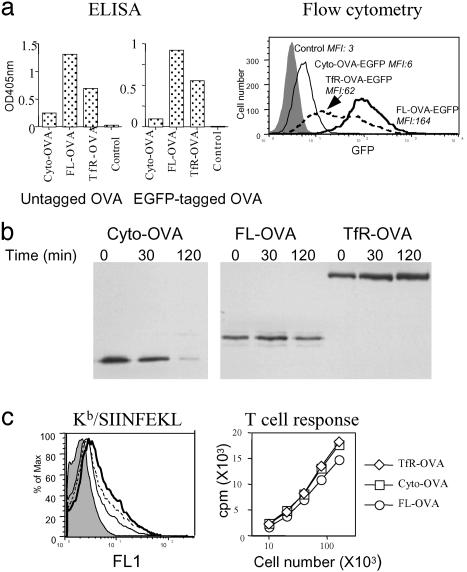
We first quantified the steady-state levels of OVA protein in the various transfectants. These cells were solublized in detergent, and their content of OVA was measured by ELISA. The FL-OVA transfected cells contained the highest steady-state level of OVA. In comparision, the TfR-OVA transfectants expressed half the amount, and Cyto-OVA transfectants only 1/10 the amount, of OVA (Fig. 1a). Similar results were obtained for the Cyto-, FL-, and TfR-OVA constructs fused to GFP (Fig. 1a). For these latter cells, we also quantified the steady-state levels of the fusion proteins by measuring the amount of GFP fluorescence by flow cytometry. The results of this analysis were consistent with the ELISA data (Fig. 1a). These data were further confirmed by measuring the content of OVA in the various transfectants by Western blot, although in these assays the amount of OVA protein in cells transfected with the Cyto-OVA construct was near the limit of detection for the assay (data not shown). These differences in the steady-state levels of OVA were caused by difference in the rates of synthesis and degradation of the various constructs. In pulse-chase experiments, the cytosolic version of OVA was found to be rapidly degraded with a half life of ≈40 min. In contrast, the FL-OVA had a half life of ≈280 min and the TfR-OVA constructs were stable over the time period that was analyzed (Fig. 1b).
Fig. 1.
OVA protein steady-state level, degradation, and antigenic peptides in cells transfected with different OVA constructs. (a) OVA protein steady-state level. DAP cells stably transfected with Cyto-, FL-, TfR-OVA, and untransfected control cells were lysed, and their content of OVA was determined by sandwich ELISA (Left and Center). For cells transfected with EGFP OVA fusion constructs, live cell GFP intensity (as a direct measurement for OVA levels) was also determined by flow cytometry (Right). Mean fluorescent intensity (MFI) is shown in the figure. (b) OVA protein degradation. The indicated stable transfectants were metabolically labeled with [35S]Met/Cys for 5 min (pulse) and chased for 30 and 120 min. Cell lysates were immunoprecipitated with rabbit anti-OVA antibody bound to protein A agarose beads. The beads were then washed and boiled in reducing sample buffer, and proteins were separated by SDS/PAGE. (c Left) DAP cells untransfected (shaded, MFI 2.3) or transfected with Cyto- (dotted line, MFI 5.6), FL- (thin solid line, MFI 4.3), and TfR- (thick solid line, MFI 6.3) OVA were infected with rVac Kb, and the levels of Kb/SIINFEKL complexes were determined by flow cytometry with 25D1.16 mAb. (Right) Similar to Left, except the cells were fixed and tested for their ability to stimulate IL-2 production of T-T hybridoma RF33.70.
We next sought to determine the amounts of SIINFEKL peptide generated in the transfected cells from these OVA constructs. SIINFEKL-containing peptides are generated during the degradation of ovalbumin constructs in the cytosol by proteasomes. A fraction of these peptides are then transported into the ER, where they bind to H-2Kb class I molecules. We have previously shown that the amount of SIINFEKL presented on MHC class I molecules is proportional to the amount of SIINFEKL generated in the cytosol of cells (33). Therefore, we infected the antigen transfected DAP cells with a recombinant vaccinia expressing H-2 Kb, and then measured the relative amounts of SIINFEKL peptide on Kb molecules by using either a mAb (25D1.16) (34) or a T cell hybridoma (RF33.70) (35) specific for Kb/SIINFEKL complexes. As shown in Fig. 1c, all three transfectants showed similar levels 25D1.16 staining (Fig. 1c Left) and stimulated RF33.70 hybridoma to produce comparable amounts of IL-2 (Fig. 1c Right) with the FL-OVA transfectant showing a slightly lower response. In control experiments, we verified that amount of Kb in these cells was not limiting, and could bind and present more SIINFEKL if present (data not shown). These results indicate that the amounts of antigenic peptides generated in the cytosol of cells transfected with the Cyto-, FL-, or TfR-OVA constructs are very similar and, therefore, the HSP proteins in the cytosol and ER of these cells will be exposed to similar amounts of SIINFEKL-containing peptides.
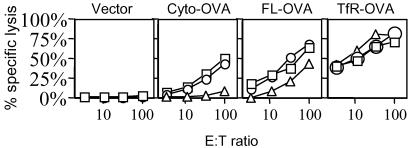
Having characterized the OVA steady-state levels and peptide pools in the transfectants, we could test how these variables affected the ability of the transfected cells to cross-prime CTL. B6C3F1 (H-2bxk) mice were immunized s.c. with the various DAP (H-2k) OVA transfectants or untransfected controls, and the cross-priming of SIINFEKL/Kb-specific CTL was determined by 51Cr release assay after restimulation of splenocytes in vitro. There were marked differences in the ability of the various transfectants to cross-prime CTL. The Cyto-OVA constructs consistently induced much lower levels of CTL activity (0.8 lytic units per 106 cells) as compared to the other forms of OVA (6.5 lytic units per 106 cells and 58 lytic units per 106 cells for FL-OVA and TfR-OVA, respectively) (Fig. 2). The differences in CTL priming induced by these various constructs were statistically significant (P < 0.05, ANOVA). This finding shows that cross-priming activity does not correlate with the amount of antigenic peptides generated in the cytosol of cells (which is similar between the transfectants), but rather is influenced by the nature of the protein antigen in cells.
Fig. 2.
Cross-priming ability of cells expressing various forms of OVA antigen. B6C3F1 (H-2bxk) mice were injected s.c. with DAP cells stably transfected with EGFP Cyto-, FL-, TfR-OVA, and EGFP vector control cells. Three mice were used in each group, and each line represents an individual mouse. Seven days later, splenocytes were restimulated with SIINFEKL peptide for 5 days, and a 51Cr release assay was performed to determine the CTL activity. EL4 cells pulsed with SIINFEKL peptide were used as targets. Effector cells were incubated with the target cells at the indicated effector-to-target cell (E/T) ratio for 5 h. Lysis of EL4 cells without peptide pulsing was <15% (data not shown). ANOVA analysis showed that the P values at E/T ratio 100:1, 33:1, 11:1, and 3.6:1 were 0.037, 0.009, 0.02, and 0.0001, respectively.
The membrane-bound TfR-OVA generated stronger CTL activity than the FL-OVA transfectant (Fig. 2), although the transfected cells contained about half the amount of TfR-OVA as compared to FL-OVA (Fig. 1 a and b). This suggests that the steady-state level of antigen is not the only factor in determining the efficiency of cross-priming. Earlier studies showed that particulate forms of antigen are more efficiently cross-presented than soluble ones (27, 28). Therefore, another factor that is likely to influence the efficiency of cross-presentation is the physical form of the antigen. Much of the membrane-bound form of TfR-OVA would be expected to be associated with cell debris (which contains cell membranes) and therefore would be particulate; this prediction is confirmed below.
The protein and the peptide models of cross-priming also make distinct predictions about how cross-presentation will be affected by the subcellular location of the protein in cells. In the protein model, changing the subcellular location of the mature protein should change where the cross-priming activity is found in cells. For example, the cytosol should contain cross-priming activity in cells expressing cytosolic antigen, whereas membrane fractions should contain cross-priming activity in cells expressing membrane-associated antigen. In contrast, if HSP peptides are the source of the cross-priming antigen, then the subcellular location of the cross-priming activity should be independent of where the mature protein antigen is found. This is because cytosolic and ER HSPs (which are the ones shown to have cross-priming activity) will get charged with peptides that are generated in the cytosol during the degradation of all of the forms of OVA and some of which are also transported into the ER by transporter associated with antigen processing (TAP).
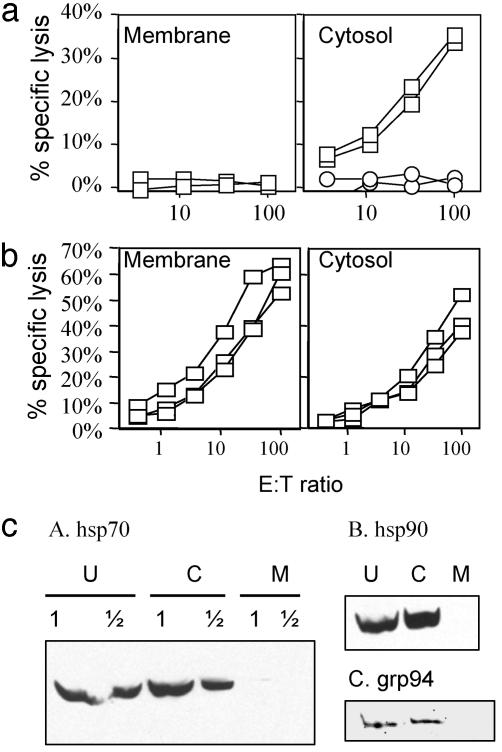
To examine this prediction, we disrupted the OVA-transfected cells by nitrogen cavitation, and separated them by differential centrifugation into membrane and soluble fractions (which we will refer to as cytosol). Western blots showed that the major HSPs (hsp70, hsp90, and grp94/96) that have previously been implicated in cross-priming were in the soluble fraction and were undetectable in the membrane fraction (Fig. 3c). We presume that the grp94/96, which is a soluble protein, was released from the ER lumen when the ER was disrupted. These fractions were then tested for their ability to stimulate CTL responses when injected into B6C3F1 (H-2bxk) mice. In cells transfected with cytosolic constructs of OVA, only the cytosol fraction (where the antigen is expressed) and not the membrane fraction stimulated CTL responses (Fig. 3a). In contrast, in cells transfected with the TfR-OVA construct, in which OVA antigen is transmembrane, the membrane fraction strongly primed CTL responses (Fig. 3b). Thus, these results demonstrate that the cross-priming activity in cells correlates with the subcellular location of the protein antigen. This strongly supports the model that protein is the major source of cross-priming antigen.
Fig. 3.
Cross-priming by subcellular fractions. B6C3F1 (H-2bxk) mice were injected s.c with membrane and cytosol fractions of DAP cells transfected with EGFP Cyto-OVA (a) and TfR-OVA (b). CTL activity was determined by using a 51Cr release assay. Each line represents one mouse. EL4 with (open squares) or without (open circles) SIINFEKL peptide pulsing were used as targets. (c) Immunoblotting of HSPs. Western blots of membrane (M), cytosol (C) fractions, and unfractionated whole cell lysates (U) of DAP cells transfected with TfR-OVA were carried out with anti-hsp70 (A), hsp90 (B), and grp94 (C) Ab followed by appropriate HRP-conjugated secondary Ab, and visualized by ECL. In A, a 2-fold dilution of samples was loaded.
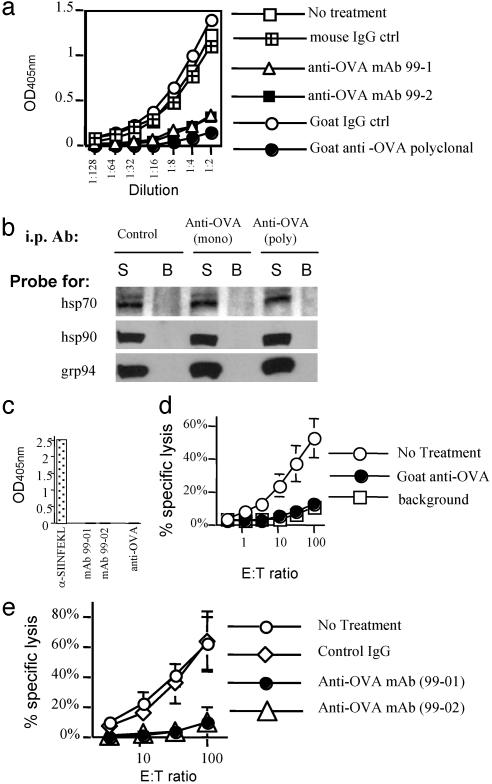
Interestingly, the cytosol fraction of TfR-OVA-transfected cells also had cross-priming activity (Fig. 3b). This could be due to the presence of OVA protein in the cytosol (e.g., from protein that failed to translocate, i.e., defective ribosomal products (DRiPs), or molecules not yet released from ribosomes) (36) or OVA in the ER that underwent retrograde translocation to the cytosol (37). Alternatively this activity could be caused by peptides, either free in the cytosol or associated with HSPs. To distinguish between these two possibilities, we depleted the OVA protein from the cytosol fraction of TfR-OVA transfected cells with antibodies. OVA protein in the cytosol could be readily detected by ELISA (Fig. 4a). Immunoabsorption on goat anti-OVA polyclonal Ab almost completed removed OVA from the cytosol fraction as assessed by ELISA (Fig. 4a). Two anti-OVA mAbs also effectively depleted OVA, although slightly less completely as compared to the polyclonal antibody (Fig. 4a). To determine whether this treatment might also have removed HSPs nonspecifically, or HSPs that were potentially associated with OVA, we assayed for hsp70, hsp90, and grp94 in the OVA-depleted supernatants and the OVA immunoprecipitated fractions by immunoblotting. Under conditions that completely removed OVA protein, there was no reduction of these HSPs in the OVA-depleted cytosol, and these HSPs were not detectable in the OVA-Ab-immune complexes (Fig. 4b). In addition, the antibodies that deplete OVA from the cytosol fractions did not bind to SIINFEKL peptide (Fig. 4c), whereas mAb 1F10 (30) specific for the peptide did. The fractions depleted of OVA were compared to the untreated or control antibody-treated fractions for their ability to induce CTL response when injected into mice. As seen in Fig. 4d, the goat anti-OVA antibody reduced the cytosol fraction cross-priming activity to background level. Consistent with the ELISA OVA depletion data, two anti-OVA mAbs depleted cytosol fractions showed significantly reduced levels of CTL priming as compared to the isotype control (Fig. 4e). Because the two anti-OVA mAbs are reported to recognize different nonoverlapping epitopes on native OVA protein (38) and do not react with SIINFEKL peptide, these results rule out the possibility that small peptides or peptides associated with HSP are the source of antigen for cross-priming. Because the depletion of OVA from the cytosol eliminates cross-priming activity but not hsp70, hsp90, and grp94 (Fig. 4b), we conclude that these HSPs cannot be responsible for OVA-specific cross-priming activity. Our data does not rule out a possible role for other HSPs in this process; however, it is unlikely that they were depleted by immunoprecipitation of OVA and hsp70, hsp90, and grp94 have been implicated as the major chaperones in binding and cross-priming antigenic peptides.
Fig. 4.
Depletion of OVA protein abrogates CTL priming activity. (a) OVA antigen was present in the cytosol fraction and could be immunodepleted. The cytosol fraction of TfR-OVA-transfected cells was untreated or treated with indicated anti-OVA or control Ab followed by removal of OVA-Ab complexes by using protein A/G beads. The supernatants were quantified by sandwich ELISA as described in Experimental Procedures. (b) OVA depletion did not remove HSPs from the cytosol fraction. After OVA depletion with anti-OVA mAb (99-1) (“mono”) or goat anti-OVA polyclonal Ab (“poly”), the supernatants (S) and protein A/G bead-bound fractions (B) were analyzed for the presence of hsp70, hsp90, and grp94 by Western blot. The amount of OVA in the cytosol was too low to be reliably detected by Western blot, but was determined by ELISA to be completely removed by the OVA immunoprecipitation (data not shown). (c) The anti-OVA antibodies did not bind SIINFEKL peptide. Indicated antibodies were incubated with plate-bound SIINFEKL peptide. The antibody binding to peptides were determined by addition of appropriate HRP-conjugated secondary antibodies and color development. (d and e) Cross-priming ability of the cytosol fraction after OVA protein depletion. Cytosol fraction was depleted of OVA protein with polyclonal anti-OVA Ab (d) and mAbs (e). Then the CTL priming ability was determined by using 51Cr release assay. Data were expressed as percent specific lysis of EL4 target cells pulsed with SIINFEKL peptide. Lysis of unpulsed EL4 cells was <10% (data not shown). Error bars are SD of three mice used in each group.
Our results strongly argue against the notion that HSPs associated with antigenic peptides are the major source of cross-priming activity from cells. Instead, three lines of evidence indicate that cross-priming of cellular antigen involves the transfer of intact (or relatively intact) proteins from the antigen-bearing cells. First, cross-priming activity correlates with the form and/or levels of protein, but not the amounts of peptides generated in these cells. Second, the cross-priming activity correlates with protein antigen location in cells and not the location of peptides or the major HSP species. Third, and perhaps most importantly, depletion of protein antigen almost completely abrogates the cross-priming activity. It is possible that molecular chaperones released from cells participate in the cross-priming process, but not as a major source of antigen in the form of HSP/peptide complexes.
Our data do not completely exclude the possibility that partially cleaved OVA contributes to cross-presentation. However, the mAbs that deplete cross-priming antigen from cell lysates react with native but not denatured ovalbumin. Based on these findings, the cross-priming antigen appears to have retained tertiary structure. Therefore, our data suggests that much of the cross-priming activity comes from intact, native protein.
It is clear from the literature that HSPs purified from cells will, when injected in sufficient amounts, cross-prime CD8+ T cell immunity. Because they do not play an important physiological role in cross-priming in our experimental system, we presume that either the quantities of HSP-peptide complexes in cells is too low to be immunogenic or that, perhaps, other molecules released from dying cells compete and block their activity. We cannot rule out the possibility that HSP/peptide complexes play a more important role in the cross-priming of certain other antigens or in other cell types.
Our data lead to a model of immune surveillance where, as dying cells begin to disintegrate and release their antigens, bone marrow-derived APCs ingest the dying cells and their cell debris by phagocytosis and macropinocytosis. Through this mechanism, the APCs acquires intact (or possibly partially cleaved) cellular proteins, including viral and cancer antigens if present. These antigens are then presented on MHC class I molecules through the phagosome-to-cytosol and/or vacuolar pathways of cross-presentation. Our data suggest that key variables that will influence the immunogenicity of cross-presented antigen are the abundance of the antigen and its physical form.
Our findings have implications for the design of vaccines. For DNA-based (e.g., viral vectors or plasmids) vaccines (39), it is important to understand the underlying pathway of antigen presentation. If the antigen is directly presented by bone marrow-derived APCs, then it may be advantageous to have a rapidly degraded antigen (40). However, if the antigens are synthesized by other cells and cross-presented, then it should be advantageous to have more stable proteins that accumulate to higher steady-state levels. Similar considerations apply to the immunogenicity of cross-presented antigens from infections, tumors, and transplanted tissue.
Acknowledgments
We thank Drs. Ronald Germain and Jonathan Yewdell at the National Institutes of Health for providing 25D1.16 MAb and rVac Kb, respectively. Tom Vedvick of Corixa kindly provided SIINFEKL peptide. We also thank Drs. Larry Stern and Ian York at University of Massachusetts Medical School for critical reading of the manuscript. L.S. was supported by a fellowship from the Canadian Institutes of Health Research. This work was supported by National Institutes of Health grants (to K.L.R.).
Abbreviations: APC, antigen-presenting cell; CTL, cytotoxic T lymphocyte; HSP, heat shock protein; OVA, chicken ovalbumin; HRP, horseradish peroxidase; FL-OVA, full-length OVA; Cyto-OVA, cytosolic OVA; TfR-OVA, transferrin receptor OVA fusion protein; ER, endoplasmic reticulum.
References
- 1.Rock, K. L. (1996) Immunol. Today 17, 131-137. [DOI] [PubMed] [Google Scholar]
- 2.Watts, C. (1997) Annu. Rev. Immunol. 15, 821-850. [DOI] [PubMed] [Google Scholar]
- 3.Heath, W. R. & Carbone, F. R. (2001) Nat. Rev. Immunol. 1, 126-134. [DOI] [PubMed] [Google Scholar]
- 4.Rock, K. L., York, I. A., Saric, T. & Goldberg, A. L. (2002) Adv. Immunol. 80, 1-70. [DOI] [PubMed] [Google Scholar]
- 5.Heath, W. R. & Carbone, F. R. (2001) Annu. Rev. Immunol. 19, 47-64. [DOI] [PubMed] [Google Scholar]
- 6.Kovacsovics-Bankowski, M., Clark, K., Benacerraf, B. & Rock, K. L. (1993) Proc. Natl. Acad. Sci. USA 90, 4942-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen, Z., Reznikoff, G., Dranoff, G. & Rock, K. L. (1997) J. Immunol. 158, 2723-2730. [PubMed] [Google Scholar]
- 8.Huang, A. Y., Golumbek, P., Ahmadzadeh, M., Jaffee, E., Pardoll, D. & Levitsky, H. (1994) Science 264, 961-965. [DOI] [PubMed] [Google Scholar]
- 9.Sigal, L. J., Crotty, S., Andino, R. & Rock, K. L. (1999) Nature 398, 77-80. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava, P. (2002) Annu. Rev. Immunol. 20, 395-425. [DOI] [PubMed] [Google Scholar]
- 11.Baker-LePain, J. C., Reed, R. C. & Nicchitta, C. V. (2003) Curr. Opin. Immunol. 15, 89-94. [DOI] [PubMed] [Google Scholar]
- 12.Castellino, F., Boucher, P. E., Eichelberg, K., Mayhew, M., Rothman, J. E., Houghton, A. N. & Germain, R. N. (2000) J. Exp. Med. 191, 1957-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basu, S., Binder, R. J., Ramalingam, T. & Srivastava, P. K. (2001) Immunity 14, 303-313. [DOI] [PubMed] [Google Scholar]
- 14.Berwin, B., Rosser, M. F., Brinker, K. G. & Nicchitta, C. V. (2002) Traffic 3, 358-366. [DOI] [PubMed] [Google Scholar]
- 15.Singh-Jasuja, H., Toes, R. E., Spee, P., Munz, C., Hilf, N., Schoenberger, S. P., Ricciardi-Castagnoli, P., Neefjes, J., Rammensee, H. G., Arnold-Schild, D. & Schild, H. (2000) J. Exp. Med. 191, 1965-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suto, R. & Srivastava, P. K. (1995) Science 269, 1585-1588. [DOI] [PubMed] [Google Scholar]
- 17.Blachere, N. E., Li, Z., Chandawarkar, R. Y., Suto, R., Jaikaria, N. S., Basu, S., Udono, H. & Srivastava, P. K. (1997) J. Exp. Med. 186, 1315-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciupitu, A. M., Petersson, M., O'Donnell, C. L., Williams, K., Jindal, S., Kiessling, R. & Welsh, R. M. (1998) J. Exp. Med. 187, 685-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogen, S., Gidalevitz, T., Biswas, C., Simen, B. B., Stein, E., Gulmen, F. & Argon, Y. (2002) J. Biol. Chem. 277, 40742-40750. [DOI] [PubMed] [Google Scholar]
- 20.Flynn, G. C., Chappell, T. G. & Rothman, J. E. (1989) Science 245, 385-390. [DOI] [PubMed] [Google Scholar]
- 21.Baker-LePain, J. C., Sarzotti, M., Fields, T. A., Li, C. Y. & Nicchitta, C. V. (2002) J. Exp. Med. 196, 1447-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thery, C., Zitvogel, L. & Amigorena, S. (2002) Nat. Rev. Immunol. 2, 569-579. [DOI] [PubMed] [Google Scholar]
- 23.Bellone, M., Iezzi, G., Rovere, P., Galati, G., Ronchetti, A., Protti, M. P., Davoust, J., Rugarli, C. & Manfredi, A. A. (1997) J. Immunol. 159, 5391-5399. [PubMed] [Google Scholar]
- 24.Albert, M. L., Sauter, B. & Bhardwaj, N. (1998) Nature 392, 86-89. [DOI] [PubMed] [Google Scholar]
- 25.Inaba, K., Turley, S., Yamaide, F., Iyoda, T., Mahnke, K., Inaba, M., Pack, M., Subklewe, M., Sauter, B., Sheff, D., et al. (1998) J. Exp. Med. 188, 2163-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harshyne, L. A., Watkins, S. C., Gambotto, A. & Barratt-Boyes, S. M. (2001) J. Immunol. 166, 3717-3723. [DOI] [PubMed] [Google Scholar]
- 27.Kovacsovics-Bankowski, M. & Rock, K. L. (1994) Eur. J. Immunol. 24, 2421-2428. [DOI] [PubMed] [Google Scholar]
- 28.Li, M., Davey, G. M., Sutherland, R. M., Kurts, C., Lew, A. M., Hirst, C., Carbone, F. R. & Heath, W. R. (2001) J. Immunol. 166, 6099-6103. [DOI] [PubMed] [Google Scholar]
- 29.Falo, L. D., Jr., Kovacsovics-Bankowski, M., Thompson, K. & Rock, K. L. (1995) Nat. Med. 1, 649-653. [DOI] [PubMed] [Google Scholar]
- 30.Hilton, C. J., Dahl, A. M. & Rock, K. L. (2001) J. Immunol. 166, 3952-3956. [DOI] [PubMed] [Google Scholar]
- 31.Fernandes, D. M., Vidard, L. & Rock, K. L. (2000) Eur. J. Immunol. 30, 2333-2343. [DOI] [PubMed] [Google Scholar]
- 32.Shen, L. & Kane, K. P. (1995) J. Exp. Med. 181, 1773-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craiu, A., Akopian, T., Goldberg, A. & Rock, K. L. (1997) Proc. Natl. Acad. Sci. USA 94, 10850-10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porgador, A., Yewdell, J. W., Deng, Y., Bennink, J. R. & Germain, R. N. (1997) Immunity 6, 715-726. [DOI] [PubMed] [Google Scholar]
- 35.Rock, K. L., Rothstein, L. & Gamble, S. (1990) J. Immunol. 145, 804-811. [PubMed] [Google Scholar]
- 36.Princiotta, M. F., Finzi, D., Qian, S. B., Gibbs, J., Schuchmann, S., Buttgereit, F., Bennink, J. R. & Yewdell, J. W. (2003) Immunity 18, 343-354. [DOI] [PubMed] [Google Scholar]
- 37.Kopito, R. R. (1997) Cell 88, 427-430. [DOI] [PubMed] [Google Scholar]
- 38.Koch, C., Jensen, S. S., Oster, A. & Houen, G. (1996) APMIS 104, 115-125. [DOI] [PubMed] [Google Scholar]
- 39.Shedlock, D. J. & Weiner, D. B. (2000) J. Leukocyte Biol. 68, 793-806. [PubMed] [Google Scholar]
- 40.Tobery, T. W. & Siliciano, R. F. (1997) J. Exp. Med. 185, 909-920. [DOI] [PMC free article] [PubMed] [Google Scholar]