Abstract
The cysteinyl leukotrienes (cys-LTs), leukotriene (LT) C4, LTD4, and LTE4, are smooth muscle constrictors that signal via the CysLT1 receptor. Here we report that the cys-LTs play an important role in chronic pulmonary inflammation with fibrosis induced by bleomycin in mice. Targeted disruption of LTC4 synthase, the pivotal enzyme for cys-LT biosynthesis, protected significantly against alveolar septal thickening by macrophages and fibroblasts and collagen deposition. In contrast, targeted disruption of the CysLT1 receptor significantly increased both the concentration of cys-LTs in the bronchoalveolar lavage fluid and the magnitude of septal thickening as defined by morphology, digital image analysis, and deposition of reticular fibers. These findings change our understanding of the pathobiology mediated by the cys-LTs by revealing their role in chronic inflammation with fibrosis, likely via the CysLT2 receptor, and by uncovering a dual role for the CysLT1 receptor, namely proinflammatory acute constriction of smooth muscle and antiinflammatory counteraction of chronic injury.
The cysteinyl leukotrienes (cys-LTs), originally termed slow reacting substance (SRS) because of the gradual progression of their contractile response in smooth muscle relative to the brisk action elicited by histamine (1-3), were viewed only as smooth muscle agonists after their structural definition (4) and chemical synthesis (5). When inhaled, cys-LTs constrict human normal or asthmatic airways with a thousand times the potency of histamine (6-8). They also are more active in eliciting an intradermal wheal and flare response (9). Their role in the pathobiology of bronchial asthma was established by the therapeutic efficacy of agents that block their biosynthesis or their action at a receptor defined by transmission of a smooth muscle response (10, 11).
The cys-LTs are generated after arachidonic acid is released from the outer nuclear membrane of cells by cytosolic phospholipase A2 and converted to the epoxide leukotriene (LT) A4 by 5-lipoxygenase (5-LO) in the presence of the 5-LO activating protein (12). An integral perinuclear membrane protein, LTC4 synthase (LTC4S), conjugates reduced glutathione to LTA4 to form LTC4 (13, 14). After carrier-mediated export (15), LTC4 is converted to additional receptor-active metabolites, LTD4 and LTE4, by the sequential cleavage from the tripeptide adduct of glutamate (16, 17) and glycine. Alternatively, LTA4 can be converted by LTA4 hydrolase to a dihydroxy LT, LTB4. Two receptors for the cys-LTs, CysLT1 receptor and CysLT2 receptor, have been cloned for the human (18-22) and the mouse (23-25).
The therapeutic agents developed for the management of bronchial asthma at the receptor level block the agonist activity of the cys-LTs at the CysLT1 receptor and not at the CysLT2 receptor. Targeted disruption of either LTC4S or the CysLT1 receptor elicits marked and comparable attenuation of IgE-dependent, mast cell-mediated passive cutaneous anaphylaxis and of zymosan-elicited i.p. plasma leakage (26, 27). These findings indicate that these adaptive and innate immune acute inflammatory responses, respectively, reflect signaling of the cys-LTs via the CysLT1 receptor, again without a recognized role for the CysLT2 receptor. Nonetheless, by in situ hybridization and by immunodetection, both receptors are present on human airways and microvasculature (18-22) and also on bone marrow-derived cells, such as alveolar macrophages, CD34+ circulating mononuclear cells, and peripheral blood eosinophils (28, 29).
Homogenates of open lung biopsy specimens from patients with idiopathic pulmonary fibrosis have elevated levels of cys-LTs and LTB4 (30). In a mouse model of bleomycin-induced pulmonary fibrosis, targeted disruption of cytosolic phospholipase A2, which eliminates virtually all eicosanoid generation, significantly attenuates alveolar septal thickening and fibrosis (31). Furthermore, targeted disruption of 5-LO also attenuates bleomycin-induced injury on the basis of quantitative reductions in the lung content of hydroxyproline, a marker of collagen, and reduced total numbers of inflammatory cells counted in the collagenase digests of the lung tissue (32). Inasmuch as mouse peritoneal macrophages express both CysLT1 and CysLT2 receptors, as assessed by calcium flux and/or RT-PCR (25, 27), it seemed possible that the pulmonary macrophages involved in bleomycin-induced fibrosis might be responding to the cys-LTs.
We hypothesized that our LTC4S null mouse strain would be protected against bleomycin-induced pulmonary inflammation and fibrosis and that the parallel analysis of the CysLT1 receptor null strain would define the limits of the role for this receptor and likely uncover a function for the CysLT2 receptor. We found that cys-LT generation mediates chronic inflammation by macrophages and fibroblasts with fibrosis and that this pathobiology is counterbalanced by the CysLT1 receptor. These findings provide a paradigm with therapeutic considerations for the management of conditions in which cys-LTs are recognized in association with fibrosis.
Materials and Methods
Mice. We generated LTC4S null and CysLT1 receptor null mice as described (26, 27). We backcrossed the LTC4S null mutation onto the C57BL/6 background, and used N4, N6, and N8 generations with wild-type littermates for comparison. Because we established the CysLT1 receptor null mice into the C57BL/6 background, we used wild-type littermates or age- and sex-matched C57BL/6 mice (Charles River Laboratories) as controls. All animal studies were approved by the Animal Care and Use Committee of the Dana-Farber Cancer Institute.
Bleomycin Injections. We anesthetized the mice with pentobarbital (50 mg/kg) by i.p. injection. We then injected bleomycin sulfate (2.5 units/kg, Nippon Kayaku, Tokyo) dissolved in saline, or saline intratracheally as a single dose at a volume of 2.5 μl per gram through a 24-gauge i.v. canula in a vertical position. All experiments were carried out three times with three to six mice for each strain per group.
Harvesting of Lungs and Histological Assessment. Mice in each group were killed by an overdose of pentobarbital by i.p. injection 7 and 12 days after treatment with bleomycin or saline. We harvested the lung tissues, isolated the lower lobes, fixed them for 4 h at room temperature in 4% paraformaldehyde in 0.1 M sodium phosphate (pH 7.6), washed them twice with PBS containing 2% DMSO, and suspended them in 50 mM NH4Cl overnight at 4°C. The samples were dehydrated and embedded in accordance with the JB-4 kit from Polysciences (Warrington, PA). Sections were cut on a Reichert-Jung Supracut microtome (Leica) with glass knives and picked up on glass slides. These samples were stained by using the chloroacetate esterase reaction or Congo red with counterstaining by hematoxylin to depict neutrophils or eosinophils, respectively (33). We stained a portion of each sample for reticular fibers by using Gomori's periodic acid-methenamine silver method (34) modified for JB-4 sections by doubling the times for the silver reaction, the prescribed washes, and the methyl-green counter stain. Another portion of each sample was embedded in paraffin and stained by using Masson's trichrome method (34). Because preliminary experiments revealed the greatest lung pathology in the lower lobes, we focused our experiments on these lobes.
Histological Assessment Using Digital Imaging. We took at least five pictures of randomly selected areas of the lower lobes of each lung at low power (×10 magnification) stained with chloroacetate esterase and hematoxylin and developed them into 3.5 × 5 inch photographs. We examined these photographs for septal thickening, defined as macrophage proliferation in the alveolar septa accompanied by fibroblasts, giant cells, and increased extracellular matrix, and outlined these areas in red. The photographs were converted to digital images (JPEG format) with a flatbed computer scanner (UMAX PowerLook III) and software (photoshop 7.0, Adobe Systems, San Jose, CA). The number of pixels contained within the areas of the digital images that had septal thickening, identified in red, and the number of pixels contained within the image of the entire lung field was determined with the histogram function. We divided the total number of pixels outlined in red by the total number of pixels in the entire lung field and multiplied the result by 100 to generate a percentage of area with septal thickening for each animal.
Analysis of Cells Recovered from Bronchoalveolar Lavage (BAL). BAL fluid was obtained by lavage of euthanized mice with 2.25 ml of PBS with 1 mM EDTA (0.75 ml three times) through a 22-gauge i.v. canula. After centrifugation, the supernatants were retained for measurement of eicosanoids. The cell pellets were resuspended in 100 μl of PBS, and cell counts were obtained manually at ×100 magnification with a Reichert hemacytometer. Differential cell counts were obtained after cells were cytospun onto glass slides and stained with a Diff-Quik staining kit (Dade-Behring, Deerfield, IL).
Measurement of Eicosanoid Levels in BAL Fluids. Assays were carried out on 2 ml of each BAL fluid supernatant to which 10,000 cpm of [3H]LTC4 (Perkin-Elmer) was added to calculate percentage recovery. After precipitation of the proteins and centrifugation, the supernatants were purified by a C18 Sep-Pak cartridge (Waters, Milford, MA) and the levels of cys-LTs, LTB4, and prostaglandin E2 (PGE2) were measured with enzyme immunoassay kits (Amersham Pharmacia) according to the manufacturer's instructions.
Statistical Analysis. Data are expressed as means ± SE. Student's t test was used for the statistical analysis in cases in which the variance was homogeneous, and Welch's test was used when the variance was heterogeneous. A value of P < 0.05 was considered significant.
Results
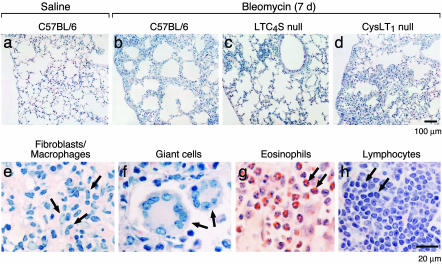
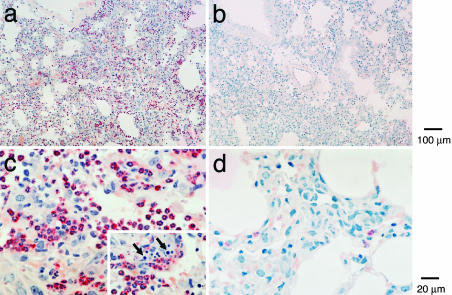
Histological Assessment of Bleomycin-Induced Pulmonary Inflammation and Fibrosis. To assess the role of cys-LTs in the development of bleomycin-induced lung injury, we examined histologically the lungs of LTC4S null mice compared to C57BL/6-backcrossed wild-type littermates and of CysLT1 receptor null mice compared to wild-type C57BL/6 mice on days 7 and 12 after the animals had received an intratracheal injection of saline or bleomycin. There were no apparent differences at baseline or in the bleomycin-induced inflammation between the wild-type littermates of the LTC4S null strain and the C57BL/6 controls for the CysLT1 receptor null strain. As compared to the saline-treated C57BL/6 controls, 7 days after treatment with bleomycin, approximately one-half of the alveolar septa in the lung were thickened because of an accumulation of monocyte/macrophages and an increase in the deposition of extracellular matrix (Fig. 1 a and b). The increased septal thickness was particularly prominent in peribronchial and subpleural locations. There was also an increase in foci of bronchial-associated lymphoid tissue (BALT). Occasional fibroblasts and Langhans-type giant cells infiltrated the monocyte/macrophage-thickened alveolar septa as well (Fig. 1 e, f, and h), whereas less than one-quarter of the mice had lung sections with focal aggregates of neutrophils (Fig. 2). These neutrophils lined and surrounded capillaries in the matrix of the thickened alveolar septa, but few entered alveolar spaces.
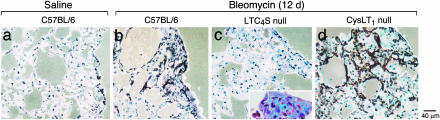
Fig. 1.
Histology of lungs 7 days after intratracheal injection of saline or bleomycin. Sections of lung from saline-treated wild-type mice (a) and bleomycin-treated wild-type (b), LTC4S null (c), and CysLT1 receptor null (d) mice are stained by chloroacetate esterase reaction with counterstaining with hematoxylin. High-power magnification views of cell types (e-h, arrow) observed in the pathobiologically condensed areas of the lung after bleomycin treatment. Eosinophils (g) are depicted by staining with Congo red.
Fig. 2.
Neutrophil infiltration 7 days after injection of bleomycin. Low-power (a and b) and high-power (c and d) magnification views of two different wild-type mice with comparable septal thickening with or without neutrophil infiltration 7 days after bleomycin treatment in the same experiment. Sections are stained by chloroacetate esterase reaction with counterstaining with hematoxylin. (Inset) For the lung of the mouse depicted in c, numerous apoptotic bodies in areas of neutrophil accumulation are shown (arrow).
After 7 days of treatment with bleomycin, the lungs of the LTC4S null mice exhibited the same qualitative lung tissue changes as the C57BL/6 mice, but to a lesser extent. There were fewer thickened alveolar septa with monocyte/macrophages, giant cells, fibroblasts, and aggregates of neutrophils, and less extracellular matrix (Fig. 1c). In contrast, after bleomycin treatment, the pathologic changes in the CysLT1 receptor null mice were exaggerated compared to their wild-type C57BL/6 controls. The areas of septal thickening were larger, the infiltration of monocyte/macrophages, giant cells and fibroblasts was greater, and the deposition of extracellular matrix was increased (Fig. 1d). However, the neutrophil aggregates, sequestered in capillaries and in the septal matrix, were about the same size as those in the bleomycin-treated C57BL/6 controls.
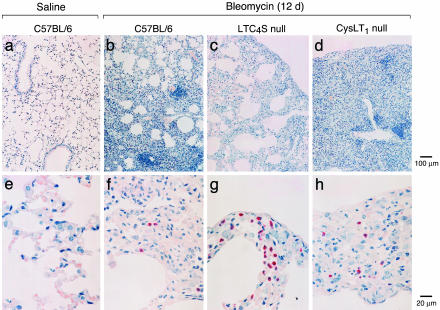
Twelve days after treatment with bleomycin as compared to the saline-treated controls, the septa of the C57BL/6 mice had increased in thickness because of further increments in monocyte/macrophages, giant cells, fibroblasts, and extracellular matrix and had assumed a nodular quality, closely resembling noncaseating sarcoid-type granulomas (Fig. 3 a, b, e, and f). The extracellular matrix and basement membranes stained blue with Masson's trichrome, indicating the presence of collagen. The nodular thickened septa appeared relatively avascular, with even fewer neutrophils per unit area than appear in normal lung. At this time, the granulocyte aggregates, primarily in large BALT-associated peribronchial clusters, were nearly exclusively eosinophils (Fig. 1g).
Fig. 3.
Histology of lungs 12 days after intratracheal injection of saline or bleomycin. Sections of lung from saline-treated wild-type mice (a, low power; e, high power) and bleomycin-treated wild-type (b, low power; f, high power), LTC4S null (c, low power; g, high power), and CysLT1 receptor null (d, low power; h, high power) mice are stained by chloroacetate esterase reaction with counterstaining with hematoxylin.
Twelve days after bleomycin treatment, the LTC4S null mice had smaller areas of septal thickening, with less accumulation of monocyte/macrophages, giant cells, and fibroblasts and less extracellular matrix deposition (Fig. 3 c and g) than their bleomycin-treated C57BL/6 controls (Fig. 3 b and f). Furthermore, the LTC4S null mice did not develop aggregates of eosinophils but did have small focal aggregates of neutrophils similar to those noted 7 days after bleomycin exposure. In contrast, 12 days after treatment with bleomycin, the CysLT1 receptor null mice had larger areas of septal thickening, more monocyte/macrophages, giant cells, and fibroblasts and more extracellular matrix deposition (Fig. 3 d and h) than the C57BL/6 mice. The BALT-associated clusters of eosinophils were comparable to those of C57BL/6 mice. Overall, pathologic changes after bleomycin treatment were less demonstrable in the LTC4S null strain and more demonstrable in the CysLT1 receptor null mice than in C57BL/6 mice.
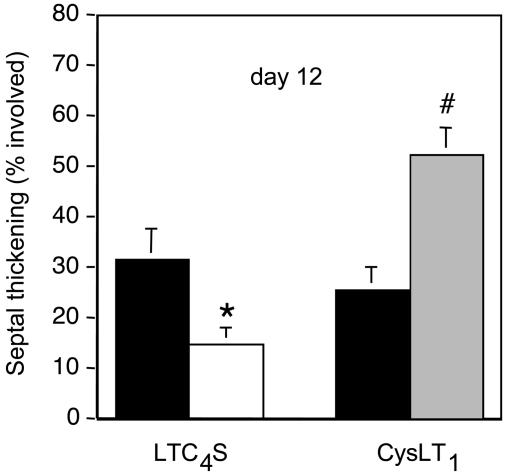
Area of Septal Thickening by Digital Imaging. To quantitatively assess bleomycin-induced inflammation and fibrosis, we analyzed by digital imaging the areas of alveolar septal thickening compared to the total area of lung in five or more photographs of the lower lung lobes of the mice 12 days after bleomycin injection. The area of lung with alveolar septal thickening in LTC4S null mice was one-half that of wild-type mice (14.5% vs. 31.3%; n = 13-14; P = 0.0242) (Fig. 4). In contrast, the area of alveolar septal thickening in CysLT1 receptor null mice was 2-fold more than in wild-type mice (52.1% vs. 25.5%; n = 10-11; P = 0.0008). This quantitative analysis revealed that there was no significant difference between the control groups used to contrast the responses of the null strains. None of the saline-treated mice had septal thickening, and all received a score of 0%.
Fig. 4.
Septal thickening 12 days after bleomycin treatment quantitated by digital imaging. The percent area of the lower lobes with septal thickening in C57BL/6-backcrossed littermates (black) and LTC4S null mice (white) (Left) and in wild-type C57BL/6 (black) and CysLT1 receptor null mice (gray) (Right) are shown. Data are combined from three experiments. *, P < 0.05; #, P < 0.001 versus bleomycin-treated wild-type group.
Reticular Fiber Staining. In the lungs of the saline-treated mice, the black periodic acid silver methenamine-stained reticular fibers beneath the pleura, around the bronchi, and in the alveolar septa were so fine that high magnification of ×50 objective was required for visualization. In the lungs of the bleomycin-treated C57BL/6 mice, the deposition of the stain in the nodular, thickened septa was sufficiently coarse to allow the fibers to be seen well at ×20 objective magnification (Fig. 5). The greatest depositions occurred in a fibrillar pattern in the thickened alveolar septa, particularly in subpleural and peribronchial locations.
Fig. 5.
Extracellular matrix protein deposition in the lungs 12 days after intratracheal injection of saline or bleomycin. Sections of lung from saline-treated wild-type mice (a) and bleomycin-treated wild-type (b), LTC4S null (c), and CysLT1 receptor null (d) mice are stained with modified periodic acid-methenamine silver. Reticular fibers (type III collagen) and basement membranes are stained dark brown to black. A representative lung section from a LTC4S null mouse is stained by Masson's trichrome staining (Inset).
Compared to lung tissue from the C57BL/6 mice (Fig. 5b), the amount of black fibrillar material was less in the LTC4S null mice (Fig. 5c) and greater in the CysLT1 receptor null mice (Fig. 5d) 12 days after bleomycin treatment. The differences in the degree of staining with periodic acid silver methenamine among the different groups of mice studied were greater than the differences in Masson's trichrome staining among the same groups (Fig. 5 Inset and data not shown). Masson's trichrome stains the septa blue in proportion to their thickness, whereas the deposition of the black silver methenamine, even among septa of the same thickness, was minimal in the LTC4S null mice, readily apparent in the C57BL/6, and markedly increased in the CysLT1 receptor null mice.
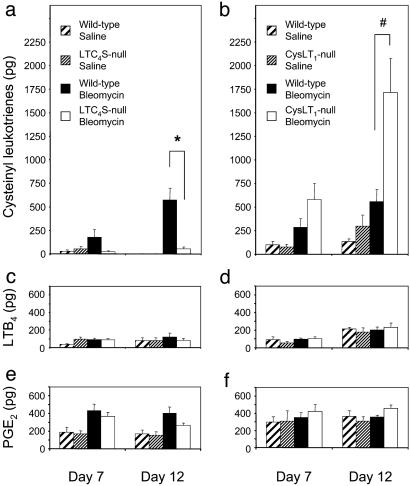
Analysis of BAL Fluids. In both the C57BL/6-backcrossed wild-type littermates of the LTC4S null mice and the wild-type C57BL/6 mice, bleomycin treatment significantly increased the levels of cys-LTs in BAL fluid as compared to the saline-treated group at day 12 (571 ± 128 pg vs. 0 ± 0 pg; n = 16 and 7; P = 0.0005 for LTC4S wild-type littermates as a representative) and this trend was also observed at day 7 (Fig. 6 a and b). These levels are consistent with observations reported by others (32). Bleomycin-treated LTC4S null mice had significantly reduced levels of cys-LTs at day 12 compared to the bleomycin-treated wild-type group at day 12 (571 ± 128 pg vs. 53 ± 19 pg; n = 16 and 16; P = 0.0011), and this trend was apparent at day 7 (175 ± 81 pg vs. 23 ± 7 pg; n = 16 and 18; P = 0.0795) (Fig. 6a). In contrast, the BAL fluid of bleomycin-treated CysLT1 receptor null mice had a significantly greater amount of cys-LTs than that of bleomycin-treated wild-type mice at day 12 (1717 ± 355 pg vs. 558 ± 132 pg, n = 10 and 12; P = 0.0104), and this trend was apparent at day 7 (581 ± 169 pg vs. 283 ± 95 pg, n = 17 and 16; P = 0.1355) (Fig. 6b).
Fig. 6.
Levels of cys-LTs, LTB4, and PGE2 in BAL fluids. The amounts of cys-LTs (a and b), LTB4 (c and d), and PGE2 (e and f) are shown 7 and 12 days after saline or bleomycin treatment of controls and LTC4S null mice (Left) and of controls and CysLT1 receptor null mice (Right). *, P < 0.005; #, P < 0.05 versus bleomycin-treated wild-type group.
Bleomycin treatment did not significantly increase the levels of LTB4 in wild-type, LTC4S null, or CysLT1 receptor null mice at days 7 and 12, and there were no differences in LTB4 levels between bleomycin-treated wild-type and bleomycin-treated LTC4S null mice, or between bleomycin-treated wild-type and bleomycin-treated CysLT1 receptor null mice at days 7 and 12 (Fig. 6 c and d). Similarly, there were no significant differences in PGE2 levels between bleomycin-treated wild-type and bleomycin-treated LTC4S null mice, or between bleomycin-treated wild-type and bleomycin-treated CysLT1 receptor null mice at days 7 and 12 (Fig. 6 e and f). These levels of LTB4 and PGE2 in bleomycin-treated wild-type mice are essentially consistent with observations reported by others (32).
Bleomycin treatment increased the numbers of macrophages, neutrophils, lymphocytes, and eosinophils in the BAL fluid compared to saline treatment in wild-type, LTC4S null, and CysLT1 receptor null mice. LTC4S null and wild-type mice had comparable total cell counts at days 7 and 12, and no significant differences in their cell profiles. Bleomycin-treated CysLT1 receptor null mice had significantly greater total cell counts in their BAL fluid than wild-type controls at day 12 (1.26 ± 0.19 × 106 vs. 7.82 ± 1.28 × 105; n = 10 and 13; P = 0.05) with significantly more neutrophils (1.15 ± 0.30 × 105 vs. 2.35 ± 0.54 × 104; n = 10 and 12; P = 0.015).
Discussion
We have found that bleomycin-induced pulmonary inflammation with fibrosis is significantly attenuated in LTC4S null mice, which cannot produce cys-LTs, and is markedly aggravated in CysLT1 receptor null mice, which overproduce cys-LTs. This finding provides evidence that cys-LTs have mediator functions beyond smooth muscle constriction. The understanding that arachidonic acid metabolites mediate the interstitial thickening by monocyte/macrophages and fibroblasts depositing collagen about the alveolar spaces in bleomycin injury was revealed by the protection afforded with deletion of the upstream enzymes cytosolic phospholipase A2 (31) and 5-LO (32). The results of the current studies showing significant protection in the LTC4S null mice (Figs. 3 and 5) define the end-product as cys-LTs. Unexpectedly, the signal for chronic inflammation and fibrosis was not mediated by the CysLT1 receptor, which is responsible for bronchoconstriction and microvasculature leakage. This finding is apparent from the increased septal thickening by macrophages, fibroblasts, and extracellular matrix at days 7 (Fig. 1) and 12 (Fig. 3) in the CysLT1 receptor null mice, which was 2-fold greater than that of the C57BL/6 controls at day 12 as assessed by the proportion of total lung area affected by septal thickening (Fig. 4). This finding not only implicates the CysLT2 receptor as the likely receptor providing the signal for chronic inflammation, but also reveals an additional unexpected role for the CysLT1 receptor, namely, counteracting the chronic inflammation.
It seems likely that macrophages are the source of the cys-LTs in bleomycin-induced chronic inflammation with fibrosis because the macrophage accumulation with septal thickening and the level of cys-LTs in BAL fluid are increased in the absence of the CysLT1 receptor and are decreased with the disruption of LTC4S (Figs. 4, 5, 6). In earlier studies, the fibroblast proliferation generated in in vitro cocultures of macrophages and human skin fibroblasts could also be achieved by direct stimulation of the fibroblasts with cys-LTs in the absence of macrophages (35). In another study, again before the specific receptors were defined, cys-LTs stimulated collagen biosynthesis by rat lung fibroblasts (36). One might speculate that in the context of innate host defense, the cys-LTs generate a microvascular leak as part of the acute response and then a typical chronic inflammatory cellular profile. The latter could be attenuated by the same receptor, the CysLT1 receptor, that provided the initial response, but would progress to fibrosis through the other receptor if the insult was persistent or severe.
Bleomycin-induced, cys-LT-mediated chronic inflammation caused no difference in the BAL fluid concentration of LTB4, the alternative end product of the 5-LO pathway that attracts cells such as neutrophils and effector T cells (37, 38), in LTC4S null or CysLT1 receptor null mice compared to their wild-type controls (Fig. 6 c and d). There was also no difference in the BAL fluid concentration of the antifibrotic prostanoid, PGE2, between these null mice and their controls (Fig. 6 e and f). PGE2 had been implicated in the bleomycin model because granulocyte/macrophage colony-stimulating factor null mice experience greater lung inflammation and fibrosis accompanied by a reduced capacity of the harvested alveolar macrophages to generate PGE2 compared to their controls (39). In contrast, 5-LO null mice with attenuation of the bleomycin-induced fibrosis exhibit elevated concentrations of PGE2 in their BAL fluid relative to their controls (32).
The margination of neutrophils with focal infiltrates accompanied by apoptotic cells in occasional thickened alveolar septa at day 7 in bleomycin-treated normal strains did not progress to involvement of the alveolar space (Fig. 2). There were fewer neutrophil aggregates in the LTC4S null strain, and they were not increased in the CysLT1 receptor null strain. By day 12, in the controls and CysLT1 receptor null mice, granulocytes were primarily in peribronchial infiltrates with eosinophils as the dominant cell, whereas in the LTC4S null strain there were occasional foci of neutrophils without eosinophils at this location. Importantly, the granulocytes were minor components at each time point in all strains. In syndecan-1 null strains, a dominant effect of bleomycin injury is the accumulation and retention of neutrophils extravascularly in the septa with attendant hypoxia attributed to their failure to migrate out of intraseptal space in response to a neutrophil chemokine, KC (40). In the CD44 null strain, lacking the receptor function by which macrophages ingest apoptotic neutrophils, the impaired clearance of the apoptotic neutrophils in the thickened septa and alveolar spaces leads to death by hypoxic respiratory failure (41). The neutrophil cycle was neither prominent nor aberrant in peribronchial regions or septa of the LTC4S and CysLT1 receptor null strains.
The findings of this study that the cys-LTs mediate chronic inflammation and fibrosis and that signaling through the CysLT1 receptor counterregulates this response have practical implications. The histologic pattern of fibrosis elicited in a compressed time course by bleomycin in the mouse model is similar to that of humans with active progressive idiopathic pulmonary fibrosis (42). Both circumstances are associated with an increase in cys-LTs shown for the BAL fluid in the mouse (Fig. 6) and for lung homogenates of humans with idiopathic pulmonary fibrosis (30). Both conditions show early transcripts for type III collagen (43, 44) and an increased ratio of type III to type I collagen protein (45). We have shown the bleomycin-induced fibrotic change by staining the deposition of reticular fibers, whose major component is type III collagen (46, 47). Type III collagen is also increased in the skin of children during early scar formation, whereas type I collagen is dominant in old scars (48). These findings suggest that agents that prevent the generation of cys-LTs may be useful in the early management of patients with idiopathic pulmonary fibrosis.
Acknowledgments
We thank Weili Chang for technical assistance and Drs. Joshua Boyce, Howard Katz, and Michael Brenner for critical reading of the manuscript. This work was supported by National Institutes of Health Grants AI-07306, AI-31599, and HL-36110.
Abbreviations: LT, leukotriene; cys-LT, cysteinyl LT; LTC4S, LTC4 synthase; 5-LO, 5-lipoxygenase; BAL, bronchoalveolar lavage; BALT, bronchial-associated lymphoid tissue; PGE2, prostaglandin E2.
References
- 1.Kellaway, C. H. & Trethewie, W. R. (1940) Q. J. Exp. Physiol. 30, 121-145. [Google Scholar]
- 2.Brocklehurst, W. E. (1953) J. Physiol. 120, 16-17. [PubMed] [Google Scholar]
- 3.Brocklehurst, W. E. (1960) J. Physiol. 151, 416-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy, R. C., Hammarstrom, S. & Samuelsson, B. (1979) Proc. Natl. Acad. Sci. USA 76, 4275-4279.41240 [Google Scholar]
- 5.Lewis, R. A., Drazen, J. M., Austen, K. F., Clark, D. A. & Corey, E. J. (1980) Biochem. Biophys. Res. Commun. 96, 271-277. [DOI] [PubMed] [Google Scholar]
- 6.Dahlen, S. E., Hedqvist, P., Hammarstrom, S. & Samuelsson, B. (1980) Nature 288, 484-486. [DOI] [PubMed] [Google Scholar]
- 7.Weiss, J. W., Drazen, J. M., Coles, N., McFadden, E. R., Jr., Weller, P. F., Corey, E. J., Lewis, R. A. & Austen, K. F. (1982) Science 216, 196-198. [DOI] [PubMed] [Google Scholar]
- 8.Griffin, M., Weiss, J. W., Leitch, A. G., McFadden, E. R., Jr., Corey, E. J., Austen, K. F. & Drazen, J. M. (1983) N. Engl. J. Med. 308, 436-439. [DOI] [PubMed] [Google Scholar]
- 9.Soter, N. A., Lewis, R. A., Corey, E. J. & Austen, K. F. (1983) J. Invest. Dermatol. 80, 115-119. [DOI] [PubMed] [Google Scholar]
- 10.Israel, E., Dermarkarian, R., Rosenberg, M., Sperling, R., Taylor, G., Rubin, P. & Drazen, J. M. (1990) N. Engl. J. Med. 323, 1740-1744. [DOI] [PubMed] [Google Scholar]
- 11.Manning, P. J., Watson, R. M., Margolskee, D. J., Williams, V. C., Schwartz, J. I. & O'Byrne, P. M. (1990) N. Engl. J. Med. 323, 1736-1739. [DOI] [PubMed] [Google Scholar]
- 12.Samuelsson, B., Dahlen, S. E., Lindgren, J. A., Rouzer, C. A. & Serhan, C. N. (1987) Science 237, 1171-1176. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimoto, T., Soberman, R. J., Spur, B. & Austen, K. F. (1988) J. Clin. Invest. 81, 866-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam, B. K., Penrose, J. F., Xu, K., Baldasaro, M. H. & Austen, K. F. (1997) J. Biol. Chem. 272, 13923-13928. [DOI] [PubMed] [Google Scholar]
- 15.Lam, B. K., Owen, W. F., Jr., Austen, K. F. & Soberman, R. J. (1989) J. Biol. Chem. 264, 12885-12889. [PubMed] [Google Scholar]
- 16.Anderson, M. E., Allison, R. D. & Meister, A. (1982) Proc. Natl. Acad. Sci. USA 79, 1088-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi, Z. Z., Han, B., Habib, G. M., Matzuk, M. M. & Lieberman, M. W. (2001) Mol. Cell. Biol. 21, 5389-5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lynch, K. R., O'Neill, G. P., Liu, Q., Im, D. S., Sawyer, N., Metters, K. M., Coulombe, N., Abramovitz, M., Figueroa, D. J., Zeng, Z., et al. (1999) Nature 399, 789-793. [DOI] [PubMed] [Google Scholar]
- 19.Sarau, H. M., Ames, R. S., Chambers, J., Ellis, C., Elshourbagy, N., Foley, J. J., Schmidt, D. B., Muccitelli, R. M., Jenkins, O., Murdock, P. R., et al. (1999) Mol. Pharmacol. 56, 657-663. [DOI] [PubMed] [Google Scholar]
- 20.Heise, C. E., O'Dowd, B. F., Figueroa, D. J., Sawyer, N., Nguyen, T., Im, D. S., Stocco, R., Bellefeuille, J. N., Abramovitz, M., Cheng, R., et al. (2000) J. Biol. Chem. 275, 30531-30536. [DOI] [PubMed] [Google Scholar]
- 21.Takasaki, J., Kamohara, M., Matsumoto, M., Saito, T., Sugimoto, T., Ohishi, T., Ishii, H., Ota, T., Nishikawa, T., Kawai, Y., et al. (2000) Biochem. Biophys. Res. Commun. 274, 316-322. [DOI] [PubMed] [Google Scholar]
- 22.Nothacker, H. P., Wang, Z., Zhu, Y., Reinscheid, R. K., Lin, S. H. & Civelli, O. (2000) Mol. Pharmacol. 58, 1601-1608. [DOI] [PubMed] [Google Scholar]
- 23.Maekawa, A., Kanaoka, Y., Lam, B. K. & Austen, K. F. (2001) Proc. Natl. Acad. Sci. USA 98, 2256-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui, Y., Yang, G., Galczenski, H., Figueroa, D. J., Austin, C. P., Copeland, N. G., Gilbert, D. J., Jenkins, N. A. & Funk, C. D. (2001) J. Biol. Chem. 276, 47489-47495. [DOI] [PubMed] [Google Scholar]
- 25.Ogasawara, H., Ishii, S., Yokomizo, T., Kakinuma, T., Komine, M., Tamaki, K., Shimizu, T. & Izumi, T. (2002) J. Biol. Chem. 277, 18763-18768. [DOI] [PubMed] [Google Scholar]
- 26.Kanaoka, Y., Maekawa, A., Penrose, J. F., Austen, K. F. & Lam, B. K. (2001) J. Biol. Chem. 276, 22608-22613. [DOI] [PubMed] [Google Scholar]
- 27.Maekawa, A., Austen, K. F. & Kanaoka, Y. (2002) J. Biol. Chem. 277, 20820-20824. [DOI] [PubMed] [Google Scholar]
- 28.Figueroa, D. J., Breyer, R. M., Defoe, S. K., Kargman, S., Daugherty, B. L., Waldburger, K., Liu, Q., Clements, M., Zeng, Z., O'Neill, G. P., et al. (2001) Am. J. Respir. Crit. Care Med. 163, 226-233. [DOI] [PubMed] [Google Scholar]
- 29.Figueroa, D. J., Borish, L., Baramki, D., Philip, G., Austin, C. P. & Evans, J. F. (2003) Clin. Exp. Allergy 33, 1380-1388. [DOI] [PubMed] [Google Scholar]
- 30.Wilborn, J., Bailie, M., Coffey, M., Burdick, M., Strieter, R. & Peters-Golden, M. (1996) J. Clin. Invest. 97, 1827-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagase, T., Uozumi, N., Ishii, S., Kita, Y., Yamamoto, H., Ohga, E., Ouchi, Y. & Shimizu, T. (2002) Nat. Med. 8, 480-484. [DOI] [PubMed] [Google Scholar]
- 32.Peters-Golden, M., Bailie, M., Marshall, T., Wilke, C., Phan, S. H., Toews, G. B. & Moore, B. B. (2002) Am. J. Respir. Crit. Care Med. 165, 229-235. [DOI] [PubMed] [Google Scholar]
- 33.Friend, D. S., Gurish, M. F., Austen, K. F., Hunt, J. & Stevens, R. L. (2000) J. Immunol. 165, 344-352. [DOI] [PubMed] [Google Scholar]
- 34.Sheehan, D. & Hrapchak, B. (1980) in Theory and Practice of Histotechnology (Battelle, Columbus, OH).
- 35.Baud, L., Perez, J., Denis, M. & Ardaillou, R. (1987) J. Immunol. 138, 1190-1195. [PubMed] [Google Scholar]
- 36.Phan, S. H., McGarry, B. M., Loeffler, K. M. & Kunkel, S. L. (1988) Biochemistry 27, 2846-2853. [DOI] [PubMed] [Google Scholar]
- 37.Ott, V. L., Cambier, J. C., Kappler, J., Marrack, P. & Swanson, B. J. (2003) Nat. Immunol. 4, 974-981. [DOI] [PubMed] [Google Scholar]
- 38.Tager, A. M., Bromley, S. K., Medoff, B. D., Islam, S. A., Bercury, S. D., Friedrich, E. B., Carafone, A. D., Gerszten, R. E. & Luster, A. D. (2003) Nat. Immunol. 4, 982-990. [DOI] [PubMed] [Google Scholar]
- 39.Moore, B. B., Coffey, M. J., Christensen, P., Sitterding, S., Ngan, R., Wilke, C. A., McDonald, R., Phare, S. M., Peters-Golden, M., Paine, R., III, et al. (2000) J. Immunol. 165, 4032-4039. [DOI] [PubMed] [Google Scholar]
- 40.Li, Q., Park, P. W., Wilson, C. L. & Parks, W. C. (2002) Cell 111, 635-646. [DOI] [PubMed] [Google Scholar]
- 41.Teder, P., Vandivier, R. W., Jiang, D., Liang, J., Cohn, L., Pure, E., Henson, P. M. & Noble, P. W. (2002) Science 296, 155-158. [DOI] [PubMed] [Google Scholar]
- 42.Izbicki, G., Segel, M. J., Christensen, T. G., Conner, M. W. & Breuer, R. (2002) Int. J. Exp. Pathol. 83, 111-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bateman, E. D., Turner-Warwick, M., Haslam, P. L. & Adelmann-Grill, B. C. (1983) Thorax 38, 93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shahzeidi, S., Mulier, B., de Crombrugghe, B., Jeffery, P. K., McAnulty, R. J. & Laurent, G. J. (1993) Thorax 48, 622-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirk, J. M., Heard, B. E., Kerr, I., Turner-Warwick, M. & Laurent, G. J. (1984) Coll. Relat. Res. 4, 169-182. [DOI] [PubMed] [Google Scholar]
- 46.Unsworth, D. J., Scott, D. L., Almond, T. J., Beard, H. K., Holborow, E. J. & Walton, K. W. (1982) Br. J. Exp. Pathol. 63, 154-166. [PMC free article] [PubMed] [Google Scholar]
- 47.Fleischmajer, R., Jacobs, L., II, Perlish, J. S., Katchen, B., Schwartz, E. & Timpl, R. (1992) Am. J. Pathol. 140, 1225-1235. [PMC free article] [PubMed] [Google Scholar]
- 48.Gay, S., Vijanto, J., Raekallio, J. & Penttinen, R. (1978) Acta Chir. Scand. 144, 205-211. [PubMed] [Google Scholar]