Abstract
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are mammalian neurodegenerative disorders characterized by a posttranslational conversion and brain accumulation of an insoluble, protease-resistant isoform (PrPSc) of the host-encoded cellular prion protein (PrPC). Human and animal TSE agents exist as different phenotypes that can be biochemically differentiated on the basis of the molecular mass of the protease-resistant PrPSc fragments and the degree of glycosylation. Epidemiological, molecular, and transmission studies strongly suggest that the single strain of agent responsible for bovine spongiform encephalopathy (BSE) has infected humans, causing variant Creutzfeldt-Jakob disease. The unprecedented biological properties of the BSE agent, which circumvents the so-called ”species barrier” between cattle and humans and adapts to different mammalian species, has raised considerable concern for human health. To date, it is unknown whether more than one strain might be responsible for cattle TSE or whether the BSE agent undergoes phenotypic variation after natural transmission. Here we provide evidence of a second cattle TSE. The disorder was pathologically characterized by the presence of PrP-immunopositive amyloid plaques, as opposed to the lack of amyloid deposition in typical BSE cases, and by a different pattern of regional distribution and topology of brain PrPSc accumulation. In addition, Western blot analysis showed a PrPSc type with predominance of the low molecular mass glycoform and a protease-resistant fragment of lower molecular mass than BSE-PrPSc. Strikingly, the molecular signature of this previously undescribed bovine PrPSc was similar to that encountered in a distinct subtype of sporadic Creutzfeldt-Jakob disease.
The transmissible spongiform encephalopathies (TSEs), or prion diseases (1), encompass a group of progressive neurodegenerative disorders, including Creutzfeldt-Jakob disease (CJD) in humans, scrapie in sheep, and bovine spongiform encephalopathy (BSE) (1-4). These disorders are characterized by brain deposition of an insoluble, protease-resistant isoform of the host-encoded cellular prion protein (PrPC), named PrPSc (1, 4, 5) In different TSE phenotypes, PrPSc exhibits disease-specific properties, including distinctive cleavage sites after proteolytic treatment, ratio of glycoforms, and deposition patterns, all features useful in providing a means of strain identification (6-10).
Although not contagious, TSEs are potentially infective, and in humans may present as sporadic, inherited, and acquired diseases. Human-to-human transmission of TSE is well documented and has occurred either through oral or mucocutaneous route of infection, as in kuru (11), or after medical and surgical procedures, as in iatrogenic CJD (12). Recently, animal-to-human transmission has also occurred. Epidemiological (13), experimental transmission (14), and biochemical PrPSc typing (8) have provided strong evidence that the single prion strain responsible for BSE has infected humans, causing variant CJD (vCJD), in addition to several animal species. In BSE and BSE-related disorders, including vCJD, the molecular typing of disease-associated PrPSc shows identical PrP fragment sizes and predominance of the high molecular mass glycoform both in natural hosts and in experimentally inoculated animals. To date, at variance with CJD in humans and scrapie in sheep, only a single strain and a single PrPSc type have been detected in BSE.
The spreading of the BSE agent across mammalian species barriers has aroused considerable concern for the following reasons: (i) the possible existence of new or previously unrecognized cattle TSE strains, potentially pathogenic for humans; and (ii) the occurrence of phenotypic variation of the BSE strain, with propagation of a new agent encoding distinctive molecular and biological properties.
In Italy, an active surveillance system on BSE in cattle was started in January 2001, and by August 2003 a total of 103 BSE cases had been diagnosed of 1,638,275 statutory tested brainstem samples. Confirmatory positive results have been obtained in all cases by immunohistochemical and Western immunoblot demonstration of disease-specific protease-resistant PrPSc.
To assess molecular and neuropathological characteristics in Italian BSE cases, we have over the last few months collected whole brains of eight Italian cattle that were PrPSc-positive in Western immunoblots. In two cattle, older than other affected bovines, the PrPSc glycotype was clearly different from the BSE-associated PrPSc molecule, and widespread PrP-amyloid plaques were seen in supratentorial brain regions. Unlike typical BSE, the brainstem was less involved and no PrP deposition was detected in the dorsal nucleus of the vagus nerve. Given the biochemical and pathological similarities with sporadic CJD (sCJD) cases linked to type-2 PrPSc (9) and methionine/valine (M/V) polymorphism at codon 129 in the prion protein gene (PRNP), these findings have prompted ongoing strain typing in inbred mice. Although the present findings dictate caution, here we show that a PrPSc type associated with sCJD and the previously undescribed bovine PrPSc show convergent molecular signatures.
Materials and Methods
Tissue Collection and Processing. Whole brains were collected from four Friesian, three Bruna Alpina, and one Piemontese cattle between 5 and 15 years old. All these animals were routinely slaughtered and resulted positive to the statutory rapid TSE test (Prionics, Zurich), which is based on the immunobiochemical detection of bovine PrPSc in brain samples. Brains were longitudinally cut into two halves; the left hemibrain was frozen and stored at -80°C until biochemical studies, whereas the right part was fixed in 10% buffered formaldehyde solution and dissected in 5-mm-thick sections that were embedded in paraffin after decontamination with 96% formic acid for 1 h. The paraffin-embedded blocks selected for the study included coronal sections at the level of the olfactory bulb, the frontal, parietal, and occipital cortices, the pyriform lobus, hippocampus, striatum, thalamus, brainstem, and sagittal sections through the cerebellum. Brains were also obtained from three routinely slaughtered cattle free of neurological disorders. Tissues from patients with CJD were obtained as described (15).
Bovine PrP Gene Determination. Genomic DNA was isolated from frozen brain tissues by using a QIAamp DNA Mini Kit (Qiagen). PCR amplification of the PrP gene was performed in 50-μl reaction volumes containing 0.5-1 μg of genomic DNA, 25 mM Tris·HCl at pH 8.7, 200 μM each dNTP, 1.5 mM MgCl2, 1 unit of Taq DNA polymerase, and 1 μM each primer, modified p78 (+) (5′-TAAGTGGGCATATGATGCTC-3′) and p9 (-) (5′-CTGGGATTCTCTCTGGTACT-3′), according to previously described procedures (16). Amplification reactions were performed in a Gene Amp PCR system 9700 (Applied Biosystems) for 41 cycles of 1 min at 94°C, 1.5 min at 56°C, and 1 min at 72°C. PrP polymorphisms were detected by DNA sequencing on both strands of the PCR products in an ABI 310 capillary system (Applied Biosystems). To determine the number of copies of the octapeptide repeats, PCR was carried out by using as primers modified p78 (+) and p60 (-) (5′-GATAGTAACGGTCCTCATAG-3′). PCR amplification products were examined in ethidium bromide-stained 3% agarose gels.
Neuropathology and PrP Immunohistochemistry. Histological sections obtained from each sampled specimen were deparaffinized, rehydrated, and stained with hematoxylin and eosin for evaluation of pathological changes; additional sections were stained with thioflavin-S. For the immunohistochemical study, after rehydration, sections were treated with 96% formic acid for 20 min at room temperature, followed by autoclaving at 121°C for 30 min. After rinsing, sections were incubated overnight at 4°C with anti-PrP monoclonal antibody F99/97.6.1 (17) diluted to 1/1,000. Subsequent antibody detection was carried out by using a biotinylated goat anti-mouse secondary antibody diluted to 1/200 for 20 min (Vector Laboratories, Burlingame, CA) at room temperature, followed by the avidin-biotin-peroxidase complex (Vectastain ABC kit, Vector Laboratories) according to manufacturer's protocol. Immunoreactivity was visualized by using 3,3′-diaminobenzidine as chromogen.
For electron microscopic study, formalin-fixed specimens of brain tissues were extensively washed in PBS, fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, and postfixed with 2% osmium tetroxide for 2 h. After dehydration in graded acetone solutions, tissues were embedded in Spurr's resin. Subsequently, the sections were prepared for electron microscopy and observed with a Zeiss EM 109 electron microscope.
Immunoblot Analysis. From each central nervous system sample, 100 mg of tissue was homogenized in 9 vol of lysis buffer (100 mM sodium chloride/10 mM EDTA/0.5% Nonidet P-40/0.5% sodium deoxycholate/10 mM Tris·HCl, pH 7.4) and digested with 50 μg/ml proteinase K (Boehringer Mannheim) for 1 h at 37°C. Digestion was blocked by the addition of phenylmethylsulfonyl fluoride at 2 mM. For deglycosylation, proteinase K-digested samples were deglycosylated with recombinant peptide N-glycosidase F (PNGase F) according to the supplier's instructions (Boehringer Mannheim). Samples, equivalent to 400 μg of wet tissue, were resolved by electrophoresis on 13% polyacrylamide gels and then transferred onto PVDF membrane (Immobilon P; Millipore) for 2 h at 60 V. Membranes were blocked with 1% nonfat dry milk in TBST (10 mM Tris·HCl/150 mM sodium chloride/0.1% Tween 20, pH 7.5) for 1 h at 37°C and incubated overnight at 4°C with anti-PrP monoclonal antibody 6H4 (Prionics) diluted to 1/5,000. Blots were developed by using the Amersham Pharmacia enhanced chemiluminescence (ECL) system, as described by the supplier and visualized on an autoradiography film. Films were scanned by using a densitometer (GS-710; Bio-Rad). The relative amounts of PrPSc distribution were calculated as previously described (18).
Results
Genetic Analysis. In four cattle a silent mutation at codon 70 (CAG → CAA) was found. As to the number of octapeptide repeats, a common cattle polymorphism, five animals were homozygous for PrP genotype with six copies and one for seven copies, whereas two Bruna Alpina cattle were heterozygous, having five/seven and six/seven repeats, respectively. Genetic, pathological and biochemical findings are summarized in Table 1.
Table 1. Epidemiological, neuropathological, and biochemical findings in examined cattle.
| Code | Breed | Age, yr | Alleles, octapeptide repeats | Genetic variation | PrP-amyloid plaques | Prevailing PrPSc glycoform on Western blot |
|---|---|---|---|---|---|---|
| 1088 | Piemontese | 15 | 6/6 | Wild type | + | Low molecular mass |
| 109655 | Bruna Alpina | 5 | 5/7 | Wild type | 0 | High molecular mass |
| 102417 | Friesian | 9 | 6/6 | Wild type | 0 | High molecular mass |
| 141387 | Bruna Alpina | 11 | 6/7 | Codon 70 CAG/CAA, encodes Q/Q | + | Low molecular mass |
| 78437 | Bruna Alpina | 5 | 7/7 | Codon 70 CAA/CAA, encodes Q/Q | 0 | High molecular mass |
| 16193 | Friesian | 5 | 6/6 | Codon 70 CAG/CAA, encodes Q/Q | 0 | High molecular mass |
| 128204 | Friesian | 7 | 6/6 | Wild type | 0 | High molecular mass |
| 72797 | Friesian | 8 | 6/6 | Codon 70 CAG/CAA, encodes Q/Q | 0 | High molecular mass |
The presence of amyloid plaques was assessed after thioflavin-S staining, PrP immunohistochemistry, and ultrastructural examination. Codon 70 in control cattle and other affected animals was CAG/CAG, encoding Q/Q.
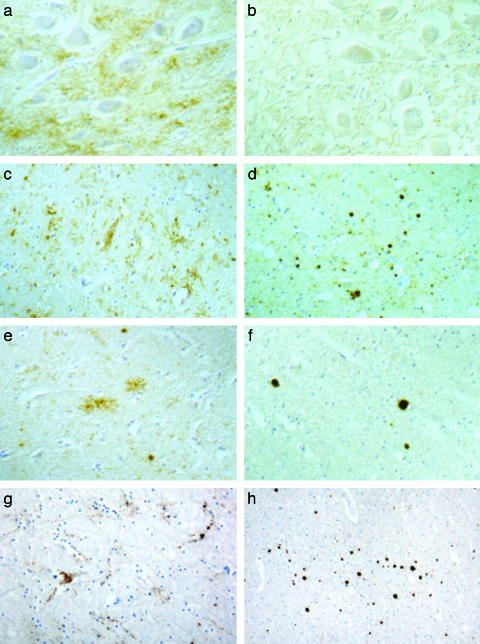
Neuropathology and Immunohistochemistry. Although the presence of early autolysis precluded an accurate pathological assessment in some brain areas, in all animals spongiosis was not consistently found in the brainstem, at the level of the obex or in more rostral areas. The frontal, parietal, and occipital cortices were apparently spared, and no vacuolation was detected in the olfactory bulb, piriform cortex, and hippocampus. Mild spongiform changes of the neuropil were observed only in two Friesian cattle at the level of the thalamus. However, after PrP immunohistochemistry, two groups of animals were readily distinguished, because of striking differences in patterns and topography of PrP deposition (Fig. 1). Group 1, comprising six Friesian and Bruna Alpina cattle, including the two cases with thalamic spongiosis, matched the typical phenotype of BSE, characterized by the occurrence of PrP deposits of granular type (in the neuronal cytoplasm or in gray matter neuropil), linear type (thick, thread-like profiles), and glial type, which confers a star-like appearance (Fig. 1 a, c, e, and g). By contrast, group 2, one each Piemontese and Bruna Alpina cattle 15 and 11 years old, respectively, was characterized by the presence of PrP-amyloid plaque-like deposits, kuru-like plaques, and granular extracellular and glial deposits (Fig. 1 b, d, f, and h). The kuru-like plaques appeared as dense unicentric (Fig. 2a), or less frequently multicentric, round structures up to 25 μm in diameter (Fig. 2b), with a pale core and a dark radial periphery. PrP-positive plaques were also fluorescent after thioflavin-S and were ultrastructurally composed of bundles of straight, unbranched fibrils with a diameter of ≈7 nm (Fig. 2c and d). The two groups of cattle also showed remarkable differences in brain regional distribution of PrP deposits. In group 1, large amounts of granular PrP deposits were observed in brainstem (Fig. 1a) and thalamus (Fig. 1c), whereas the lobus piriformis (Fig. 1e), the olfactory bulb (Fig. 1g), and cerebral cortexes were less involved. By contrast, the brainstem showed only a weak PrP positivity in group 2, and the dorsal motor nucleus of the vagus was unstained (Fig. 1b); PrP-amyloid plaques were seen in the thalamus (Fig. 1d), subcortical white matter and deeper layers of cerebral cortexes (Fig. 1f), and olfactory bulb (Fig. 1h). Finally, the molecular layer of the cerebellum exhibited PrP deposits of the glial type in group 1, whereas some amyloid plaques were observed in group 2.
Fig. 1.
PrP deposition in the brains of group 1 and group 2 cattle. Immunohistochemistry showing the glial and granular patterns of PrP deposition observed in the dorsal nucleus of vagus nerve (a, ×210), thalamus (c, ×210), pyriform cortex (e, ×220), and olfactory bulb (g, ×150) of an animal representative of group 1. In group 2 cattle, the dorsal nucleus of the vagus nerve is unstained (b, ×210), whereas PrP-positive plaques are observed in the thalamus (d, ×210), pyriform cortex (f, ×210), and olfactory bulb (h, ×80).
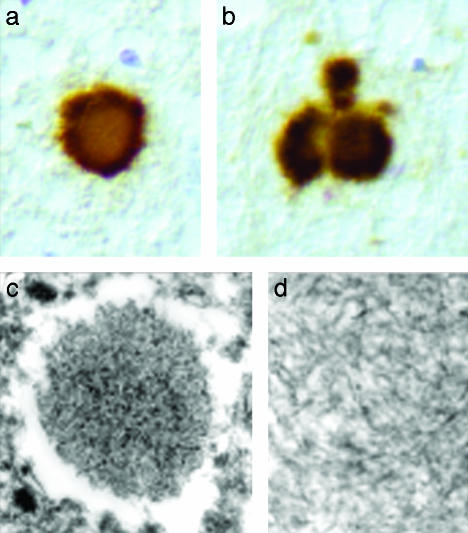
Fig. 2.
PrP-positive amyloid plaques in group 2 animals. PrP-immunostaining of the pyriform cortex from group 2 cattle, showing the presence of kuru-like amyloid plaques (a and b, ×450). At ultrastructural examination amyloid deposits are composed of aggregates and bundles of unbranched fibrils (c, ×12,550; d, ×60,000).
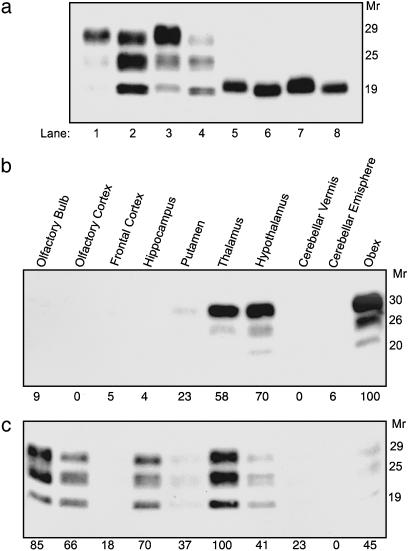
Biochemical Characterization and Regional Distribution of PrPSc. Western immunoblots of proteinase K-treated brain homogenates, obtained from different cortical and subcortical regions of group 1 and group 2 animals, showed the presence of two distinct PrPSc types, which were distinguishable on the basis of the molecular mass of their unglycosylated fragments and the ratio of differently glycosylated forms. The typical molecular ”BSE signature,” characterized by overrepresentation of the high molecular mass glycoform, was detected in group 1 animals (Fig. 3a, odd lanes). In contrast, the Piemontese and Bruna Alpina cattle (group 2) showed a predominance of the low molecular mass glycoform and a protease-resistant fragment with a faster electrophoretic mobility (Fig. 3a, even lanes).
Fig. 3.
Biochemical analysis and regional distribution of PrPSc in group 1 and group 2 cattle. (a) Immunoblot with 6H4 monoclonal antibody of proteinase K-treated brain homogenates from the thalamus of group 1 (odd lanes) and group 2 animals (even lanes), before (lanes 1-4) and after (lanes 5-8) enzymatic deglycosylation. (b and c) Regional distribution of brain PrPSc in group 1 (b) and group 2 (c) cattle; values of PrPSc are reported below each gel as the percentage of the highest value obtained. Molecular size markers are shown on the right as Mr × 10-3.
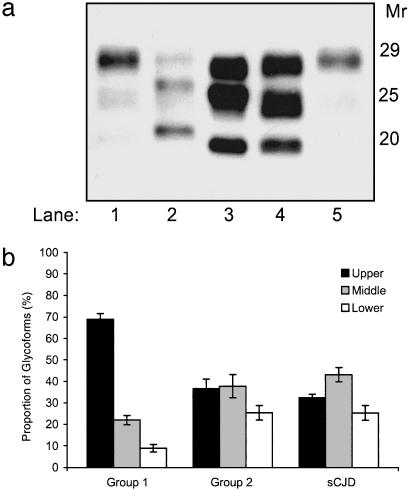
In prion diseases, distinct PrPSc types usually result in different patterns of deposition and brain regional distribution of the abnormal protein. In group 1 animals, the highest amounts of PrPSc were recovered, as expected, in the brainstem, hypothalamus, and thalamus, and very low PrPSc levels were found in the olfactory bulb and pyriform cortex (Fig. 3b). Conversely, the distribution of PrPSc in group 2 cattle was more widespread than in typical BSE cases, and the largest amounts of PrPSc were detected in the thalamus, olfactory bulb, hippocampus, and olfactory cortex, whereas lower PrPSc levels were recovered in the brainstem (Fig. 3c). On the basis of the neuropathological phenotype and the PrPSc distribution and glycotype, group 2 cattle were reminiscent of the sCJD phenotype seen in subjects with M/V at PRNP codon 129 and type 2 PrPSc (M/V2) (9, 19). Therefore, we compared proteinase K-treated brain homogenates from group 1 and group 2 cattle with sCJD with different molecular types of PrPSc, either homozygous or heterozygous at PRNP codon 129. Remarkably, the PrPSc type detected in TSE-affected cattle from group 2 had fragment size (Fig. 4a) and glycoform ratios (Fig. 4b) similar to a PrPSc type encountered in sCJD M/V2 (9, 19).
Fig. 4.
Electrophoretic analysis of PrPSc in cattle TSE and sCJD. (a) Western blot detection of PrPSc in brains of group 1 animals (lanes 1 and 5); subject with sCJD and type 1 PrPSc, methionine/methionine at codon 129 (lane 2); subject with sCJD and type 2 PrPSc, methionine/valine at codon 129 (lane 3); and group 2 cattle (lane 4). (b) Relative proportions of the three PrPSc glycoforms in group 1 and group 2 cattle compared with glycoform profiles obtained in nine sCJD patients, methionine/valine at codon 129 and with type 2 PrPSc. Mean ± standard deviation is shown. Upper band, diglycosylated form; middle band, monoglycosylated form; and lower band, unglycosylated form.
Discussion
In natural and experimental TSEs, PrPSc deposition represents an early event that occurs weeks to months before the development of spongiform changes (20, 21). As a consequence, the detection of PrPSc by Western immunoblot provides a unique opportunity in the diagnosis of BSE early in the incubation period and, therefore, in presymptomatic animals. The identification of the present cattle by postmortem biochemical tests, in the absence of clear neurological involvement, suggests that the disorder was detected at early stages, and this may also explain the lack of widespread vacuolar changes.
Previous pathological studies in clinically suspect cases of BSE in Great Britain have provided evidence for a uniform pattern in the severity and distribution of vacuolar lesions in affected animals, with medulla oblongata nuclei being the most involved (22). While confirming that the BSE epidemic has been sustained by a single agent, these studies have assessed the validity of statutory criteria for the diagnosis of BSE, which is currently based on both histopathological and immunobiochemical examination of the medulla. However, the prevailing involvement of cortical regions in the cattle with amyloid deposition suggests that postmortem brain sampling should not be limited to the obex. In addition, a careful analysis of PrPSc glycoform profiles at the confirmatory Western immunoblot may provide a molecular means of identifying atypical cases of bovine TSE.
Bovine Amyloidotic Spongiform Encephalopathy (BASE): A Second Bovine TSE. The present findings show that a previously undescribed pathological and immunohistochemical phenotype, associated with cattle TSE, is related to the presence of a PrPSc type with biochemical properties, including the gel mobility of the protease-resistant fragment and glycoform ratios, different from those encountered in cattle BSE. Brain deposition of this pathological isoform of cattle PrP correlates with the formation of PrP-amyloid plaques, as opposed to typical BSE cases. Although in several natural and experimental recipients of the BSE agent, including humans (13), neuropathological changes are characterized by the presence of PrP-positive amyloid deposits with surrounding vacuolation, cattle BSE is not associated with PrP-amyloid plaque formation. On the basis of the above features, we propose to name the disease described here BASE. Although observed in only two cattle, the BASE phenotype could be more common than expected. In previous studies, amyloid congophilic plaques were found in 1 of 20 BSE cases examined systematically for amyloid (23), and it was reported that focal cerebral amyloidosis is present in a small proportion of BSE cases (24). Although no biochemical analysis of PrPSc glycotype is available for these animals with ”atypical BSE phenotype,” our present results underscore the importance of performing a strain-typing in bovine TSE with amyloid deposition.
In sCJD, the neuropathological phenotype largely correlates with the molecular type of PrPSc and distinct polymorphic sites of PRNP (9, 19). This is in contrast with the situation in cattle, where different genotypes have been reported based on the variable numbers of octapeptide repeats in each allele, but no evidence for single-codon polymorphisms in the PrP gene has been established (25, 26). Because the present animals shared a similar genetic background and breed, differences in disease phenotypes between cattle with BSE and BASE can be tentatively related only to distinct PrPSc types or alternative routes of infection and spread of prion pathology. Accordingly, the lack of involvement of the motor dorsal nucleus of the vagus and the slight involvement of the brainstem in BASE, suggests a route for spreading of the agent other than the alimentary tract. Therefore, unless the BASE agent propagates throughout the olfactory pathway or other peripheral routes, it is possible that this disorder represents a sporadic form of cattle TSE, which would also explain the difference in ages between the two groups of affected animals.
Phenotypic Similarities Between BASE and sCJD. The transmissibility of CJD brains was initially demonstrated in primates (27), and classification of atypical cases as CJD was based on this property (28). To date, no systematic studies of strain typing in sCJD have been provided, and classification of different subtypes is based on clinical, neuropathological, and molecular features (the polymorphic PRNP codon 129 and the PrPSc glycotype) (8, 9, 15, 19). The importance of molecular PrPSc characterization in assessing the identity of TSE strains is underscored by several studies, showing that the stability of given disease-specific PrPSc types is maintained upon experimental propagation of sCJD, familial CJD, and vCJD isolates in transgenic PrP-humanized mice (8, 29). Similarly, biochemical properties of BSE- and vCJD-associated PrPSc molecules remain stable after passage to mice expressing bovine PrP (30). Recently, however, it has been reported that PrP-humanized mice inoculated with BSE tissues may also propagate a distinctive PrPSc type, with a ”monoglycosylated-dominant” pattern and electrophoretic mobility of the unglycosylated fragment slower than that of vCJD and BSE (31). Strikingly, this PrPSc type shares its molecular properties with the a PrPSc molecule found in classical sCJD. This observation is at variance with the PrPSc type found in M/V2 sCJD cases and in cattle BASE, showing a monoglycosylated-dominant pattern but faster electrophoretic mobility of the protease-resistant fragment as compared with BSE. In addition to molecular properties of PrPSc, BASE and M/V2 sCJD share a distinctive pattern of intracerebral PrP deposition, which occurs as plaque-like and amyloid-kuru plaques. Differences were, however, observed in the regional distribution of PrPSc. While in M/V2 sCJD cases the largest amounts of PrPSc were detected in the cerebellum, brainstem, and striatum, in cattle BASE these areas were less involved and the highest levels of PrPSc were recovered from the thalamus and olfactory regions.
In conclusion, decoding the biochemical PrPSc signature of individual human and animal TSE strains may allow the identification of potential risk factors for human disorders with unknown etiology, such as sCJD. However, although BASE and sCJD share several characteristics, caution is dictated in assessing a link between conditions affecting two different mammalian species, based on convergent biochemical properties of disease-associated PrPSc types. Strains of TSE agents may be better characterized upon passage to transgenic mice. In the interim until this is accomplished, our present findings suggest a strict epidemiological surveillance of cattle TSE and sCJD based on molecular criteria.
Acknowledgments
We are grateful to Giuseppe Ru (Centro di Referenza Nazionale per le Encefalopatie Animali, Istituto Zooprofilattico Sperimentale di Torino) for the provision of surveillance data. We also thank Diana Bazan for preparing material for transmission electron microscopy, and Ines Crescio, Cristiano Corona, Cristiano Longo, Michele Fiorini, Alessia Farinazzo, and Matteo Gelati for technical assistance. This work was supported by a grant from the Italian Ministry of Health (IZS PLV 004/01 to M.C. and S.M.), a grant from Fondazione Cariverona (2002-Malattie neurodegenerative to S.M.), and in part by the Italian Ministry of Health (RF 2001.96 to F.T.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TSE, transmissible spongiform encephalopathy, BSE, bovine spongiform encephalopathy; CJD, Creutzfeldt-Jakob disease; vCJD, variant CJD; sCJD, sporadic CJD; PrP, prion protein, PrPSc pathological PrP; BASE, bovine amyloidotic spongiform encephalopathy.
References
- 1.Prusiner, S. B. (1998) Proc. Natl. Acad. Sci. USA 95, 13363-13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collinge, J. (2001) Annu. Rev. Neurosci 24, 519-550. [DOI] [PubMed] [Google Scholar]
- 3.Parchi, P. & Gambetti, P. (1995) Curr. Opin. Neurol. 8, 286-293. [DOI] [PubMed] [Google Scholar]
- 4.Brown, P., Goldfarb, L. G. & Gajdusek, D. C. (1991) Lancet 337, 1019-1022. [DOI] [PubMed] [Google Scholar]
- 5.Prusiner, S. B. (1992) Biochemistry 31, 12277-12288. [DOI] [PubMed] [Google Scholar]
- 6.Bessen, R. A. & Marsh, R. F. (1992) J. Virol. 66, 2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bessen, R. A. & Marsh, R. F. (1994) J. Virol. 68, 7859-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collinge, J., Sidle, K. C. L., Meads, J., Ironside, J. & Hill, A. F. (1996) Nature 383, 685-690. [DOI] [PubMed] [Google Scholar]
- 9.Parchi, P., Castellani, R., Capellari, S., Ghetti, B., Young, K., Chen, S. G., Farlow, M., Dickson, D. W., Sima, A., Trojanowski, J. Q., et al. (1996) Ann. Neurol. 39, 767-778. [DOI] [PubMed] [Google Scholar]
- 10.Hill, A. F, Sidle, K. C. L., Joiner, S., Keyes, P., Martin, T. C., Dawson, M. & Collinge, J. (1998) Neurosci. Lett. 255, 159-162. [DOI] [PubMed] [Google Scholar]
- 11.Gajdusek, D. C. (1977) Science 197, 943-960. [DOI] [PubMed] [Google Scholar]
- 12.Brown, P., Preece, M. A. & Will, R. G. (1992) Lancet 340, 24-27. [DOI] [PubMed] [Google Scholar]
- 13.Will, R. G., Ironside, J. W., Zeidler, M., Cousens, S. N., Estibeiro, K., Alperovitch, A., Poser, S., Pocchiari, M., Hofman, A. & Smith, P. G. (1996) Lancet 347, 921-925. [DOI] [PubMed] [Google Scholar]
- 14.Bruce, M. E., Will, R. G., Ironside, J. W., McConnel, I., Drummond, D., Suttle, A., McCardle, L., Chree, A., Hope, J., Birkett, C., et al. (1997) Nature 389, 498-501. [DOI] [PubMed] [Google Scholar]
- 15.Zanusso, G., Farinazzo, A., Fiorini, M., Gelati, M., Castagna, A., Rigetti, P. G., Rizzuto, N. & Monaco, S. (2001) J. Biol. Chem. 276, 40377-40380. [DOI] [PubMed] [Google Scholar]
- 16.Belt, B. G. M., Muileman, I. H., Schreuder, B. E. C., Bos-de Ruijter, J., Gielkens, A. L. J., Smits, M. A. (1995) J. Gen. Virol. 76, 509-517. [DOI] [PubMed] [Google Scholar]
- 17.O'Rourke, K. I., Baszler, T. V., Besser, T. E., Miller, J. M., Cutlip, R. C., Wells, G. A., Ryder, S. J., Parish, S. M., Hamir, A. N., Cockett, N. E., et al. (2000) J. Clin. Microbiol. 38, 3254-3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zanusso, G., Ferrari, S., Cardone, F., Zampieri, P., Gelati, M., Fiorini, M., Farinazzo, A., Gardiman, M., Cavallaro, T., Bentivoglio, M., et al. (2003) N. Engl. J. Med. 348, 711-719. [DOI] [PubMed] [Google Scholar]
- 19.Parchi, P., Giese, A., Capellari, S., Brown, P., Schulz-Schaeffer, W., Windl, O., Zerr, I., Budka, H., Kopp, N., Piccardo, P., et al. (1999) Ann. Neurol. 46, 224-233. [PubMed] [Google Scholar]
- 20.Castellani, R., Parchi, P., Stahl, J., Capellari, S., Cohen, M. & Gambetti, P. (1996) Neurology 46, 1690-1693. [DOI] [PubMed] [Google Scholar]
- 21.DeArmond, S. J. & Prusiner, S. B. (1995) Am. J. Pathol. 146, 785-811. [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons, M. M., Harris, P., Jeffrey, M., Meek, S. C., Blamire, I. W. H. & Wells, G. A. H. (1996) Vet. Rec. 138, 175-177. [DOI] [PubMed] [Google Scholar]
- 23.Wells, G. A. H. & Wilesmith, J. W. (1995) Brain Pathol. 5, 91-103. [DOI] [PubMed] [Google Scholar]
- 24.Wells, G. A. H., Wilesmith, J. W., McGill, I. S. (1991) Brain Pathol. 1, 69-78. [DOI] [PubMed] [Google Scholar]
- 25.Goldmann, W., Hunter, N., Martin, T., Dawson, M. & Hope, J. (1991) J. Gen. Virol. 72, 201-204. [DOI] [PubMed] [Google Scholar]
- 26.Hunter, N., Goldmann, W., Smith, G. & Hope, J. (1994) Vet. Rec. 135, 400-403. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs, C. J., Jr., Gajdusek, D. C., Asher, D. M., Alpers, M. P., Beck, E., Daniel, P. M. & Matthews, W. B. (1968) Science 161, 388-389. [DOI] [PubMed] [Google Scholar]
- 28.Brown, P., Rodgers-Johnson, P., Cathala, F., Gibbs, C. J., Jr. & Gajdusek, D. C. (1984) Ann. Neurol. 16, 295-304. [DOI] [PubMed] [Google Scholar]
- 29.Korth, C., Kaneko, K., Groth, D., Heye, N., Telling, G., Mastrianni, J., Parchi, P., Gambetti, P., Will, R., Ironside, J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 4784-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott, M. R., Will, R., Ironside, J., Nguyen, H.-O. B., Tremblay, P., DeArmond, S. J. & Prusiner, S. B. (1999) Proc. Natl. Acad. Sci. USA 96, 15137-15142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asante, E. A., Linehan, J. M., Debruslais, M., Joiner, S., Gowland, I., Wood, A. L., Welch, J., Hill, A. F., Lloyd, S. E., Wadsworth, J. D. F. & Collinge, J. (2002) EMBO J. 21, 6358-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]