Abstract
Background
The incidence of Clostridium difficile infection (CDI) has been increasing. Previous studies report that the number of colectomies for CDI is also rising. Outside of a few notable outbreaks, there are few published data documenting increasing severity of CDI. The specific aims of this multiyear, multicenter study were to assess CDI-related colectomy rates and compare CDI-related colectomy rates by CDI surveillance definition.
Methods
Cases of CDI and patients who underwent colectomy were identified electronically from 5 US tertiary-care centers from July 2000 through June 2006. Chart review was performed to determine if a colectomy was for CDI. Monthly CDI-related colectomy rates were calculated as the number of CDI-related colectomies per 1,000 CDI cases. Data between observational groups were compared using χ2 and Mann-Whitney U tests. Logistic regression was performed to evaluate risk factors for CDI-related colectomy.
Results
8569 cases of CDI were identified and 75 patients had CDI-related colectomy. The overall colectomy rate was 8.7/1,000 CDI cases. The CDI-related colectomy rate ranged from 0 to 23 per 1,000 CDI episodes across hospitals. The colectomy rates for healthcare facility (HCF)-onset CDI was 4.3/1000 CDI cases and 16.5 /1000 CDI cases for community-onset CDI (p <.05). There were significantly more CDI-related colectomies at hospitals B and C (p<.05).
Conclusions
The overall CDI-related colectomy rate was low, and there was no significant change in the CDI-related colectomy rate over time. Onset of disease outside of the study hospital was an independent risk factor for colectomy.
Introduction
Clostridium difficile infection (CDI) is one of the most common health-care associated infections, causing significant morbidity and mortality, increased healthcare costs, and prolonged hospital lengths-of-stay [1–3]. Previously published reports indicate that the incidence and severity of CDI are increasing [4–6]. The emergence of a hypervirulent, epidemic strain and community-acquired disease in patients who were previously considered to be low risk will likely contribute an even greater burden on patients and the healthcare system [7–9].
Subtotal colectomy with end ileostomy is the treatment of choice for patients with fulminant CDI refractory to medical therapy [10–15]. Studies have identified a variety of factors associated with improved survival after CDI-related colectomy, and colectomy performed earlier in the course of fulminant colitis, before the patient becomes critically ill, is generally associated with improved survival [10–11, 13, 15–18]. Prior studies have reported colectomy rates for C. difficile infection from <1–3% of CDI cases [9, 11, 14–20], and the rate of colectomies for severe disease appears to be rising over time [21]. These studies have been limited to single-centers or outbreak settings, so little is known about overall temporal trends in colectomy rates in non-outbreak settings. Furthermore, the relationship between the location of CDI symptom onset and the risk for colectomy for CDI is unknown.
The objective of this study was to conduct a multicenter, multiyear analysis of the incidence of colectomies for severe CDI in a non-outbreak setting. Colectomy cases were further classified by the location of CDI onset, using published recommended surveillance definitions.
METHODS
Setting and Population
Five geographically diverse academic hospitals participating in the Prevention Epicenters Program of the Centers for Disease Control and Prevention collected inpatient data from July 1, 2000, through June 30, 2006. These hospitals were Barnes-Jewish Hospital (Saint Louis, Missouri), Brigham and Women’s Hospital (Boston, Massachusetts), the Ohio State University Medical Center (Columbus, Ohio), Stroger Hospital of Cook County (Chicago, Illinois), and University of Utah Hospital (Salt Lake City, Utah). Notably, a prior study found that the rates of CDI were significantly different between the hospitals, but there were no sustained outbreaks [4]. Inpatients > 18 years old were included in the study. Electronic medical records were queried to retrospectively collect dates of positive C. difficile toxin assay results, demographic information, comorbid conditions, International Classification of Diseases ninth revision clinical modification (ICD-9-CM) diagnosis codes, ICD-9-CM procedure codes for colectomy or hemicolectomy (45.73, 45.75, 45.76, 45.79, 45.8), and admission and discharge dates. In order to provide a description of the overall population at the five hospitals, four patients not diagnosed with CDI were randomly selected among the patients discharged from the same study hospital during the same year for every case of CDI identified by toxin assay results or ICD-9-CM codes.
Definitions
The charts of all patients with a positive assay for C. difficile or the ICD-9-CM diagnosis code for CDI and an ICD-9-CM procedure code of colectomy were reviewed to determine if the colectomy was for CDI (CDI-related colectomy). For patients without a colectomy, a case of CDI was defined as a positive C. difficile stool toxin assay. For patients with CDI-related colectomy, a case of CDI was defined as the presence of a positive C. difficile toxin assay or the presence of the ICD-9-CM code for CDI and a CDI-related colectomy. The reason to have different definitions for a case of CDI based on whether there was a colectomy for CDI is two-fold. All study hospitals are academic medical centers. Patients may be transferred for severe CDI and proceed to colectomy without repeating a toxin assay. In addition, patients with fulminant CDI may have an ileus or be taken to surgery prior to obtaining stool for C. difficile testing. Therefore it was determined that charts of patients assigned the ICD-9-CM code for CDI and a procedure code for colectomy would be reviewed. Conversely, most patients assigned the ICD-9-CM code for CDI without a positive toxin assay do not have CDI based on further chart review [22]. Because of this, it was determined to be unnecessary to review the charts of the approximately 3,000 patients assigned the ICD-9-CM code for CDI but who did not have a positive toxin assay or a colectomy.
Cases of CDI were categorized based on previously published surveillance definitions [4, 23–25]. Charts of all patients with CDI onset ≤ 48 hours from admission were reviewed to determine recent healthcare exposures. Cases were categorized as healthcare facility (HCF) onset; community-onset, study hospital associated; community-onset, non-study HCF associated; indeterminate; and recurrent (Table 1). Recurrent cases were cases of CDI with a history of a positive toxin assay within the previous 8 weeks.
Table 1.
Definitions of Clostridium difficile Infection (CDI) According to Exposures
| Type of CDI | Definition |
|---|---|
| Health care facility-onset, health care facility-associated | Patient's stool sample tested positive >48 h after admission to study hospital |
| Community-onset, study hospital associated | Patient’s stool sample tested positive ≤48 hours after admission, provided that symptom onset was less than 4 weeks after the last discharge from a HCF and the most recent discharge was from an Epicenter |
| Community-onset, non-study hospital associated | Patient’s stool sample tested positive ≤48 hours after admission, provided that symptom onset was less than 4 weeks after the last discharge from a HCF and the most recent discharge was from a HCF other than an Epicenter |
| Community-onset, community associated | Patient’s stool sample tested positive ≤48 hours after admission, provided that more than 12 weeks have elapsed since the last discharge from a HCF |
| Indeterminate | Patient’s exposure setting does not fit any of the other criteria (e.g., positive stool sample ≤48 hours after admission with HCF exposure 4–12 weeks prior to sample collection) |
| Recurrent | Patient’s stool sample tested positive ≤8 weeks after a prior positive stool sample |
| Unknown | Patient’s exposure setting cannot be determined because of lack of available data |
Data Analysis
Colectomy rates were calculated as the number of CDI-related colectomies per 1,000 toxin-positive cases. Data from hospital E were incomplete for the first 14 months; these months for hospital E were excluded from the analysis. Composite Charlson scores were calculated to assess comorbidity [26]. All tests were 2-tailed, and a P value of less than 0.05 was considered statistically significant, with Bonferroni adjustments for multiple comparisons when applicable. Data were compared with χ2, χ2 for trend, Fisher exact tests, and Mann-Whitney U tests. Univariate logistic regression was used to examine the contributions of age, sex, race, location of onset, Charlson score, and study center to the risk of colectomy. Variables associated with colectomy in unadjusted analysis (p<.05) were included in a multivariable regression logistic model to identify independent predictors of colectomy. Stepwise, backwards logistic regression was used for multivariate analysis. Variables with significance levels p <0.05 were retained in the final model. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated for each variable. The data were analyzed using Epi-Info, version 3.4 (Centers for Disease Control and Prevention, Atlanta, GA), and SPSS for Windows, version 17.0 (SPSS Inc., Chicago, IL). Approval was obtained from the institutional review boards of the Centers for Disease Control and Prevention and at each of the participating centers.
RESULTS
During the study period there were 8569 episodes of toxin positive CDI in 8033 patients, including 540 (6.3%) cases of recurrent CDI. There were 252 patients with a positive toxin assay or who were assigned the ICD-9-CM code for CDI and underwent a colectomy during the same hospitalization. Seventy-five (29.8%) of these patients had a CDI-related colectomy. Forty-nine patients (65%) with CDI-related colectomy had a documented positive toxin assay during the same admission. The colectomy incidence over the entire study period was 8.7 CDI-related colectomies per 1000 CDI episodes. There was no significant overall trend in the annual incidence of CDI-related colectomy during the study period.
Demographic data are provided in Table 2. Overall, patients with CDI were older (p<.05) and had higher Charlson scores than patients without CDI (p<.05). The median age of patients who had a CDI-related colectomy was higher than patients with CDI who did not have a colectomy (68.2 years versus 61.5 years, p=.03). White patients made up 71% of toxin-positive CDI cases, but 83% of patients who had a CDI-related colectomy (p=.03). The proportion of colectomy patients who were female was 43%, compared to 50% of patients with a CDI-related colectomy (p=.23). The population of patients who underwent a CDI-related colectomy had lower mean Charlson scores compared to patients with CDI who did not undergo colectomy (p=.04).
Table 2.
Patient Characteristics
| Characteristic | Patients Without CDI |
Patients With CDI1 | Patients With Colectomy for CDI |
|
|---|---|---|---|---|
|
Hospital A (n=28215) |
Number of Patients | 24232 | 3955 | 28 |
| Median Age, y (range) | 55.1 (18–103) | 64.1 (18–105) | 68.1 (23–87) | |
| Female (%) | 13727 (57) | 2015 (51) | 15 (54) | |
| Non-White (%) | 9075 (37) | 1192 (30) | 7 (25) | |
| Mean Charlson Score (range) | 1.5 (0–16) | 2.2 (0–15) | 1.4 (0–7) | |
| Mortality (%) | 2.8 | 12.7 | 53.6 | |
|
Hospital B (n=8313) |
Number of Patients | 6977 | 1332 | 4 |
| Median Age, y (range) | 47.1 (18–101) | 56.2 (18–102) | 42.2 (36–66) | |
| Female (%) | 4016 (58) | 662 (50) | 0 (0) | |
| Non-White (%) | 1315 (19) | 168 (13) | 0 (0) | |
| Mean Charlson Score (range) | 1.1 (0–12) | 2.2 (0–14) | 1.3 (0–4) | |
| Mortality (%) | 2.2 | 6.5 | 25.0 | |
|
Hospital C (n=10692) |
Number of Patients | 9641 | 1026 | 25 |
| Median Age, y (range) | 54.3 (18–106) | 65.6 (18–97) | 69.6 (42–87) | |
| Female (%) | 5612 (58) | 535 (52) | 11 (44) | |
| Non-White (%) | 2643 (27) | 166 (16) | 4 (16) | |
| Mean Charlson Score (range) | 1.6 (0–15) | 2.5 (0–14) | 2.2 (0–11) | |
| Mortality (%) | 3.3 | 8.4 | 28.0 | |
|
Hospital D (n=10201) |
Number of Patients | 9147 | 1036 | 18 |
| Median Age, y (range) | 53.9 (18–104) | 63.4 (19–100) | 70.1 (44–88) | |
| Female (%) | 5266 (58) | 512 (49) | 6 (33) | |
| Non-White (%) | 3153 (34) | 231 (22) | 2 (11) | |
| Mean Charlson Score (range) | 1.3 (0–15) | 1.4 (0–11) | 0.6 (0–3) | |
| Mortality (%) | 2.4 | 8.6 | 33.3 | |
|
Hospital E (n=4165) |
Number of Patients | 3530 | 635 | 0 |
| Median Age, y (range) | 49.9 (18–100) | 50.6 (18–99) | - | |
| Female (%) | 1691 (48) | 237 (37) | - | |
| Non-White (%) | 3139 (89) | 552 (87) | - | |
| Mean Charlson Score (range) | 0.5 (0–10) | 0.7 (0–11) | - | |
| Mortality (%) | 2.4 | 7.7 | - | |
|
Overall (n=61586) |
Number of Patients | 53527 | 7984 | 75 |
| Median Age, y (range) | 53.4 (18–106) | 61.5 (18–105)2 | 68.2 (23–88)3 | |
| Female (%) | 30312 (57) | 3961 (50) | 32 (43) | |
| Non-White (%) | 19325 (36) | 2309 (29) | 13 (17) | |
| Mean Charlson Score (range) | 1.4 (0–16) | 2.0 (0–15)4 | 1.5 (0–11)5 | |
| Mortality (%) | 2.7 | 10.2 | 38.7 |
Cases of recurrent CDI are excluded
p<.05 comparing age of patients with and without CDI
p=.03 comparing age of patients with CDI with and without colectomy
p<.05 comparing Charlson score of patients with and without CDI
p=0.04 comparing Charlson score of patients with CDI with and without colectomy
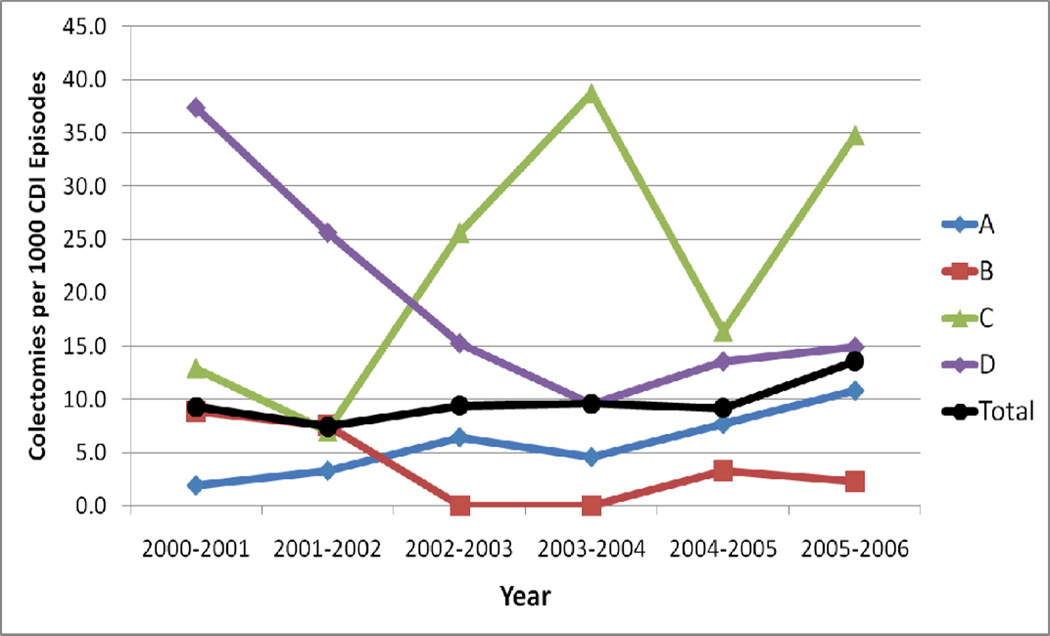
Colectomy incidence at each hospital during the study period ranged from 0 to 23 per 1,000 CDI episodes. The only hospital with a significant linear trend in the annual colectomy rate over time was hospital A, with an increase in CDI-related colectomy during the study period (p=.01) (Figure 1). Though annual variations were evident, particularly at hospital C and D, there were likely too few colectomies to find a significant linear trend. No colectomies for CDI were reported at hospital E during the study period. Comparisons by study center are presented in Table 2. Among patients without CDI, those at hospital A were the oldest and those at hospital B were the youngest (55 years versus 47 years, p<.001). Patients at hospital C had the highest Charlson comorbidity scores; patients at hospital E had the lowest (1.6 versus 0.5, p<.001). Among patients with CDI who did not undergo colectomy, those at hospital C were the oldest (66 years) and had the highest mean Charlson scores (2.5), while those at hospital E were the youngest (51 years) and had the lowest Charlson scores (0.7) (both p<.001). Patients with CDI-related colectomy were the oldest (70 years) at hospital D and the youngest at hospital B (42 years) (p=.02, not significant after Bonferroni correction). Those who had CDI-related colectomy at hospital D had the lowest Charlson scores, and those at hospital C had the highest scores (0.6 versus 2.2, p=.01).
Figure 1.
Colectomy rates by hospital over time. There was a significant increase in colectomies over time at hospital A (p=.01)
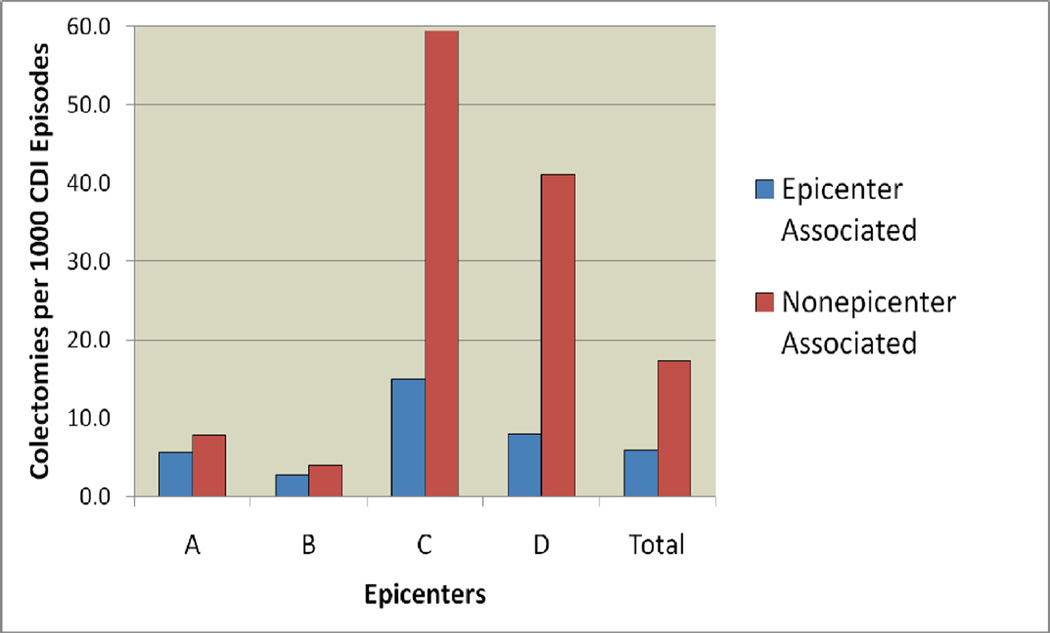
HCF-onset CDI represented 4897 episodes of CDI (58% of all toxin-positive episodes) (Table 3). There were 21 CDI-related colectomies for HCF-onset CDI, accounting for 29% of all CDI-related colectomies. There were 794 episodes of community-onset, study hospital associated CDI (9% of all episodes) and 10 CDI-related colectomies (13% of CDI-related colectomy). The community-onset, non-study hospital HCF associated category includes 1063 episodes of CDI (12% of all episodes) and 23 CDI-related colectomies (31% of CDI-related colectomies). There were 520 episodes of community-associated CDI (6% of all episodes) and 5 CDI-related colectomies (7% of CDI-related colectomies). Community-onset cases had higher colectomy rates than hospital-onset cases at all four hospitals that reported colectomies during the study period (Figure 2). Six percent of cases and four percent of colectomies were patients with recurrent CDI.
Table 3.
Comparison of CDI Incidence and Deaths, by Surveillance Definitions
| Patients With CDI, No Colectomy (%) |
Patients With Colectomy for CDI (%) |
Colectomies for CDI per 1000 Cases |
|||
|---|---|---|---|---|---|
| Number of patients (%) |
Deaths (%) |
Number of patients (%) |
Deaths (%) |
||
| Hospital-Onset (%) | 4,897 (57.5) | 554 (11.3) | 21 (28.8) | 10 (47.6) | 4.3 |
| Community-Onset, Study Hospital Associated (%) | 794 (9.3) | 70 (8.8) | 10 (10.7) | 4 (80) | 12.4 |
| Community-Onset, Other Healthcare Facility Associated (%) | 1,063 (12.5) | 113 (10.6) | 23 (30.7) | 8 (80) | 21.2 |
| Indeterminate (%) | 365 (4.3) | 20 (5.5) | 8 (10.7) | 3 (37.5) | 21.4 |
| Community Associated (%) | 520 (6.1) | 26 (5.0) | 5 (6.7) | 0 | 9.5 |
| Recurrent (%) | 536 (6.3) | 71 (13.2) | 3 (4) | 3 (100) | 5.6 |
| Unknown (%) | 345 (4.0) | 19 (5.5) | 5 (6.7) | 1 (38.7) | 14.3 |
Figure 2.
CDI-related colectomy rates by location of symptom onset.
Risk factors for CDI-related colectomy are presented in Table 4. Advanced age (65 years and older) was associated with an odds ratio of 1.9 for colectomy (p<.05). White patients had a significantly higher odds ratio for colectomy compared to non-white patients (OR 2.0, p=.02). Admission to hospitals C and D was also associated with more CDI-related colectomies (p<.05). There were no differences in the risk of colectomy by patient gender, discharge year, or Charlson score. After multivariable adjustment, age 65 years or older (OR 1.6, 95% CI 1.0–2.6), admission to hospital C (OR 4.0 95% CI 2.3–7.0) or D (OR 2.4 95% CI 1.3–4.5), and community-onset CDI (OR 3.4, 95% CI 2.0–5.5) remained significantly associated with CDI-related colectomy.
Table 4.
Factors associated with CDI-related colectomy.
| Characteristic | Colectomies for CDI per 1000 Patients |
Odds Radio (95% CI) |
p | Multivariable OR (95% CI) |
Multivariable p- value |
|
|---|---|---|---|---|---|---|
| Age | ||||||
| 18–64 | 6.2 | 1 | - | |||
| ≥65 | 12.1 | 1.9 (1.2, 3.2) | <0.05 | 1.6 (1.0, 2.6) | 0.05 | |
| Sex | ||||||
| Female | 7.6 | 1 | - | |||
| Male | 10.0 | 1.3 (0.8, 2.1) | 0.2 | 1.4 (0.9, 2.3) | 0.15 | |
| Race | ||||||
| Non-White | 5.3 | 1 | ||||
| White | 10.3 | 2.0 (1.1, 3.8) | 0.02 | 1.6 (0.8, 2.9) | 0.16 | |
| Charlson Score | ||||||
| 0–1 | 10.4 | 1 | - | - | - | |
| 2–3 | 8.8 | 0.9 (0.5, 1.4) | 0.5 | - | - | |
| 4–5 | 2.2 | 0.4 (0, 2.9) | 0.7 | - | - | |
| ≥6 | 4.3 | 0.2 (0.2, 1.2) | 0.1 | - | - | |
| Surveillance Definition | ||||||
| HCF-Onset | 4.3 | 1 | - | - | ||
| Community-Onset | 16.5 | 3.9 (2.3, 6.8) | <0.05 | 3.4 (2.0, 5.5) | <0.05 | |
| Discharge Year | ||||||
| 2000–2001 | 9.4 | 1 | - | - | - | |
| 2001–2002 | 6.0 | 0.6 (0.2, 1.9) | 0.4 | - | - | |
| 2002–2003 | 7.8 | 0.8 (0.3, 2.2) | 0.7 | - | - | |
| 2003–2004 | 7.7 | 0.8 (0.3, 2.2) | 0.7 | - | - | |
| 2004–2005 | 7.4 | 0.8 (0.3, 2.0) | 0.6 | - | - | |
| 2005–2006 | 12.8 | 1.4 (0.6, 3.2) | 0.4 | - | - | |
| Study Hospital1 | ||||||
| A | 6.4 | 1 | - | 1 | - | |
| B | 3.0 | 0.5 (0.1, 1.4) | 0.1 | 0.6 (0.2, 1.6) | 0.27 | |
| C | 23.1 | 3.6 (2.0, 6.4) | <0.05 | 4.0 (2.3, 7.0) | <0.05 | |
| D | 16.2 | 2.5 (1.3, 4.7) | <0.05 | 2.4 (1.3, 4.5) | <0.05 | |
Hospital E was not included in this analysis because there were no colectomies for CDI reported in the study period.
DISCUSSION
This study examined CDI-related colectomy rates at multiple healthcare facilities over a six year time period. We also assessed risk factors associated with CDI-related colectomy. Prior studies of colectomy for CDI were limited by being conducted at single-centers, over relatively brief periods of time, or in response to CDI outbreaks. These studies have potentially biased the literature to more severe outbreaks of CDI. Our study minimizes these biases by including multiple healthcare facilities over several years in the absence of any sudden increases in CDI incidence or clinically apparent increases in deaths due to CDI [4].
CDI-related colectomy rates in the literature differ based on the clinical setting, drawing from a wide variety of patient populations and CDI rates. The overall CDI-related colectomy rate in this study was 8.7/1,000 CDI cases, which is consistent with previously published rates, ranging from 2.7 to 32 colectomies per 1,000 CDI cases [9, 11, 14–20]. Kutty et al (2010) recently published a report of a community-based CDI outbreak in 6 hospitals in North Carolina involving 109 patients with CDI; none required ICU admission or a colectomy [9]. It was hypothesized that the paucity of severe cases was due to the young age of the study population (median age of 62 years). In contrast, Lamontagne et al (2007) found that 23% of the 165 patients in 2 hospitals’ ICUs with fulminant colitis required a colectomy [13]. The hospitalized population in our study includes low and high acuity patients, and the colectomy rate fell between those two extremes.
There are few published data describing colectomy rates according to CDI surveillance definitions. One single-center study during an outbreak at a community hospital reported a colectomy incidence for healthcare facility onset CDI of 2% [19]. The HCF-onset colectomy rate in our study was lower, at 0.43%. We found that patients who had community-onset CDI with recent HCF exposure had the highest colectomy rate (Table 3). To our knowledge, there are no prior studies on colectomy incidence in community-onset cases that can be used for comparison. The colectomy rate in patients with community-onset CDI in this study may be biased high. Four of the five study hospitals are academic referral centers. It is not uncommon for patients with severe CDI to be transferred for management of severe CDI and possible colectomy.
There are no standard guidelines for selecting patients with CDI for colectomy, and this may account for the differences in CDI-related colectomy rates across the study hospitals. Some patterns did emerge. Overall, patients who underwent CDI-related colectomy were significantly older compared to patients with CDI who did not have a colectomy. This age difference was present at all centers, and likely reflects the higher likelihood that older patients develop severe CDI [6, 20, 27]. Patients who had a CDI-related colectomy had lower Charlson scores compared to patients with CDI who did not have a colectomy. This may reflect a greater willingness to proceed to colectomy among patients with fewer comorbidities who are more likely to survive an emergent, high-risk procedure. Patients were more likely to have a colectomy for CDI at hospitals C and D. There were no obvious associations between mortality of patients without CDI and patients with CDI, and CDI-related colectomy rates. Data on treatment for CDI was not collected. Therefore it is not possible to determine if differences in CDI-related colectomy rates translated to improved patient outcomes.
This study has some limitations. Different case definitions were used for the numerator and denominator to determine the rate of CDI-related colectomy. When conducting surveillance for a rare event, missing a few cases may result in large differences in identified disease incidence. On the other hand, missing a few non-cases has less of an impact on the identified disease incidence. These factors must be considered when balancing the accuracy of the method of surveillance with the resources necessary to conduct the surveillance. Using ICD-9-CM codes to identify patients with CDI has been shown to overestimate the number of cases of CDI, and most patients with the ICD-9-CM code for CDI without a corresponding positive toxin assay do not have CDI. Although ICD-9-CM codes are specific for CDI, the positive predictive value is only ~75% due to the relatively low prevalence of CDI [22]. Therefore, it was decided to limit the CDI without colectomy group to CDI cases with positive toxin assays only. Conversely, there was concern that some patients with CDI diagnosed at a referring healthcare facility would be transferred for severe CDI and proceed to colectomy without repeating a toxin assay at the study hospital, or patients with fulminant CDI may be taken to surgery prior to procurement of a stool specimen for toxin testing. Although these cases represent a minority of all CDI cases, they may represent a large proportion of patients who have a CDI-related colectomy. These patients were included in the definition of CDI-related colectomy to avoid missing these cases. Consequently, colectomy rates may be overestimated, particularly for community-onset, non-study hospital associated cases. This is notable considering the overall low incidence of colectomy identified in this study.
As reported previously [4], hospital B experienced a CDI pseudo-outbreak from July 1, 2004 to June 30, 2006 related to improper stool sample collection and transport. We chose to include data from hospital B during the pseudo-outbreak for this particular study, since two of the four colectomies performed during the study period at hospital B were performed during the pseudo-outbreak. The true colectomy incidence during these periods is therefore likely biased low. Another limitation of the data is that different toxin assays were used among the hospitals over the study period. It is unclear how or to what extent these different assays may have influenced CDI incidence. Additionally, data on infecting C. difficile strains are not available. It is possible the hospitals involved in this study have a low proportion of CDI cases due to highly-virulent, epidemic strains, thus accounting for the low colectomy incidence. The epidemic BI / NAP1 / 027 strain has been identified at hospital A.
This study reports that no significant change in overall colectomy rates occurred from 2000 to 2006, but that CDI-related colectomy is associated with community-onset CDI, there are differences in colectomy incidence across healthcare facilities without obvious impact on outcomes, and CDI-related colectomy is associated with high mortality. Because of the difficulties in establishing optimal criteria on when to take a patient with CDI to surgery, our study supports the need for prospective surveillance studies to track CDI-related colectomy trends in stable sentinel populations using standardized case definitions. Studies such as these will allow for comparisons between healthcare facilities to identify trends in CDI-related colectomies and factors associated with improved patient outcomes which can be used to improve patient selection for CDI-related colectomy.
Acknowledgements
This work was supported by grants from the Centers for Disease Control and Prevention (UR8/CCU715087-06/1 and 5U01C1000333 to Washington University, 5U01CI000344 to Eastern Massachusetts, 5U01CI000328 to The Ohio State University, and 5U01CI000334 to University of Utah) and the National Institutes of Health (K23AI065806, K24AI06779401, K01AI065808 to Washington University). Findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Preliminary data were presented in part at the Fifth Decennial Conference of The Society for Healthcare Epidemiology of America held in Atlanta, Georgia, March 18-22, 2010, abstract number 730.
Footnotes
These data were presented in part at the Fifth Decennial Conference of The Society for Healthcare Epidemiology of America held in Atlanta, Georgia, March 18–22, 2010, abstract number 730.
DISCLOSURES
ERD: research: Optimer, Merck, Viropharma; consulting: Optimer, Merck, Sanofi-Pasteur, and Pfizer.
References
- 1.Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235(3):363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubberke ER. Attributable Outcomes of Endemic Clostridium difficile associated Disease in Nonsurgical Patients. Emerg Infect Dis. 2008;14(7):1031–1038. doi: 10.3201/eid1407.070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubberke ER, Wertheimer AI. Review of current literature on the economic burden of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2009;30(1):57–66. doi: 10.1086/592981. [DOI] [PubMed] [Google Scholar]
- 4.Dubberke ER, Butler AM, Yokoe DS, Mayer J, Hota B, Mangino JE, et al. Multicenter Study of Clostridium difficile Infection Rates from 2000 to 2006. Infect Control Hosp Epidemiol. 2010;31(10):1030–1037. doi: 10.1086/656245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Eng J Med. 2005;353(23):2433. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 6.Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005;173(9):1037–1042. doi: 10.1503/cmaj.050978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, et al. Toxin production by an emerging strain of associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366(9491):1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 8.Severe Clostridium difficile-associated disease in populations previously at low risk--four states, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(47):1201–1205. [PubMed] [Google Scholar]
- 9.Kutty PK, Woods CW, Sena AC, Benoit SR, Naggie S, Frederick J, et al. Risk factors for and estimated incidence of community-associated Clostridium difficile infection, North Carolina, USA. Emerg Infect Dis. 2010;16(2):197–204. doi: 10.3201/eid1602.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali SO, Welch JP, Dring RJ. Early surgical intervention for fulminant pseudomembranous colitis. Am Surg. 2008;74(1):20–26. doi: 10.1177/000313480807400105. [DOI] [PubMed] [Google Scholar]
- 11.Hall JF, Berger D. Outcome of colectomy for Clostridium difficile colitis: a plea for early surgical management. Am J Surg. 2008;196(3):384–388. doi: 10.1016/j.amjsurg.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 12.Koss K, Clark MA, Sanders DS, Morton D, Keighley MR, Goh J. The outcome of surgery in fulminant Clostridium difficile colitis. Colorectal Dis. 2006;8(2):149–154. doi: 10.1111/j.1463-1318.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- 13.Lamontagne F, Labbé A-C, Haeck O, Lesur O, Lalancette M, Patino C, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg. 2007;245(2):267–272. doi: 10.1097/01.sla.0000236628.79550.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipsett PA, Samantaray DK, Tam ML, Bartlett JG, Lillemoe KD. Pseudomembranous colitis: a surgical disease? Surgery. 1994;116(3):491–496. [PubMed] [Google Scholar]
- 15.Seder CW, Villalba MR, Robbins J, Ivascu FA, Carpenter CF, Dietrich M. Early colectomy may be associated with improved survival in fulminant Clostridium difficile colitis: an 8-year experience. Am J Surg. 2009;197(3):302–307. doi: 10.1016/j.amjsurg.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Byrn JC, Maun DC, Gingold DS, Baril DT, Ozao JJ, Divino CM. Predictors of mortality after colectomy for fulminant Clostridium difficile colitis. Arch Surg. 2008;143(2):150–154. doi: 10.1001/archsurg.2007.46. [DOI] [PubMed] [Google Scholar]
- 17.Synnott K, Mealy K, Merry C, Kyne L, Keane C, Quill R. Timing of surgery for fulminating pseudomembranous colitis. Br J Surg. 1998;85(2):229–231. doi: 10.1046/j.1365-2168.1998.00519.x. [DOI] [PubMed] [Google Scholar]
- 18.Sailhamer EA, Carson K, Chang Y, Zacharias N, Spaniolas K, Tabbara M, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg. 2009;144(5):433–439. doi: 10.1001/archsurg.2009.51. [DOI] [PubMed] [Google Scholar]
- 19.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Eng J Med. 2005;353(23):2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 20.Miller M, Gravel D, Mulvey M, et al. Health care-associated Clostiridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis. 2010;50(2):194–201. doi: 10.1086/649213. [DOI] [PubMed] [Google Scholar]
- 21.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142(7):624–631. doi: 10.1001/archsurg.142.7.624. [DOI] [PubMed] [Google Scholar]
- 22.Dubberke ER, Butler AM, Yokoe DS, Mayer J, Hota B, Mangino JE, et al. Multicenter study of surveillance for hospital-onset Clostridium difficile infection by the use of ICD-9-CM diagnosis codes. Infect Control Hosp Epidemiol. 2010;31(3):262–268. doi: 10.1086/650447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dubberke ER, Butler AM, Hota B, Khan YM, Mangino JE, Mayer J, et al. Multicenter study of the impact of community-onset Clostridium difficile infection on surveillance for C. difficile infection. Infect Control Hosp Epidemiol. 2009;30(6):518–525. doi: 10.1086/597380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutty PK, Benoit SR, Woods CW, Sena AC, Naggie S, Frederick J, et al. Assessment of Clostridium difficile-associated disease surveillance definitions, North Carolina, 2005. Infect Control Hosp Epidemiol. 2008;29(3):197–202. doi: 10.1086/528813. [DOI] [PubMed] [Google Scholar]
- 25.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 26.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 27.Zilberberg MD, Shorr AF, Micek ST, Doherty JA, Kollef MH. Clostridium difficile-associated disease and mortality among the elderly critically ill. Crit Care Med. 2009;37(9):2583–2589. doi: 10.1097/CCM.0b013e3181ab8388. [DOI] [PubMed] [Google Scholar]