Abstract
Background
Clostridium difficile infection (CDI) is associated with medical care and may cause readmission following hospitalization for any reason. The incidence of readmissions due to CDI is not well known.
Design
Retrospective cohort study of adult inpatients in one county from 2000–2007, using mandatory hospital discharge data.
Setting
All hospitals in Orange County, California
Patients
All adult inpatients readmitted with new-onset Clostridium difficile infection within 12 weeks of discharge.
Measurements
We assessed trends in hospital-associated CDI (HA-CDI) incidence, with and without inclusion of post-discharge CDI (PD-CDI) events resulting in re-hospitalization within 12 weeks of discharge. We measured the effect of including PD-CDI events on hospital-specific CDI incidence, a mandatory reporting measure in California, and on relative hospital ranks by CDI incidence.
Results
From 2000 to 2007, countywide hospital-onset CDI (HO-CDI) incidence increased from 15/10,000 to 22/10,000 admissions. When including PD-CDI events, HA-CDI incidence doubled (29/10,000 in 2000 and 52/10,000 in 2007). Overall, including PD-CDI events resulted in significantly higher hospital-specific CDI incidence, although hospitals had disproportionate amounts of HA-CDI occurring post-discharge. This resulted in substantial shifts in some hospitals’ rankings by CDI incidence. In multivariate models, both HO and PD-CDI were associated with increasing age, higher length of stay, and select comorbidities. Race and Hispanic ethnicity were predictive of PD-CDI but not HO-CDI.
Limitations
PD-CDI incidence may be underestimated since outpatient events were not evaluated. Inaccuracies in claims data may cause under or over-estimation of CDI cases. Whether C. difficile was acquired in the hospital or community post-discharge for PD-CDI is not known.
Conclusions
PD-CDI events associated with re-hospitalization are increasingly common. The majority of HA-CDI cases now may be occurring post-discharge, raising important questions about both accurate reporting and effective prevention strategies. Some risk factors for PD-CDI may be different than those for HO-CDI, allowing additional identification of high-risk groups before discharge.
INTRODUCTION
Hospital length of stay has steadily decreased over the past 30 years1 and increasingly complex medical care is provided after discharge through home health and skilled nursing facilities. In turn, adverse events related to hospitalization, including hospital-associated infection, may increasingly present after discharge and result in readmission.2–5 The costs and sequelae of hospital readmission have made it a target for hospital quality indicators and value based purchasing. 6,7 Currently, the Centers for Medicare and Medicaid Services reports hospitals’ rates of readmission following treatment for myocardial infarction, congestive heart failure and pneumonia, prompting a national focus on preventing readmission.8 However, readmission rates for other important conditions, such as hospital-associated infections (HAIs), are not well studied despite national and state requirements for reporting hospital-specific rates of HAIs.9–12
Clostridium difficile infection (CDI) is a common cause of diarrhea in healthcare settings and may be an important source of hospital readmissions.13–15 Hospital-associated C. difficile acquisition may not be evident until after hospital discharge, especially since the average hospital length of stay is 3–5 days and acquisition may initially be asymptomatic.1,16 In addition, risk factors related to medical care, such as predisposing antibiotics, may require time to deplete the normal intestinal flora and allow C. difficile to flourish and produce symptoms. The exact incubation period for CDI is unknown, but three studies found the incubation period to be less than 1 week.22,23 However, several studies have found patients may be at an increased risk for developing CDI up to 3 months after hospital discharge.18–21 One recent study found that among patients who developed CDI within 100 days post-discharge, 89% of patients developed CDI in the first 60 days and 85% occurred in the first month.17 To account for the range of incubation period, national guidance considers CDI occurring within 4 weeks of hospitalization as hospital-associated, and CDI occurring within 4–12 weeks of a hospitalization as potentially hospital-associated.22,23
Concerns about CDI have been increasing in the US. Hospitals’ incidence of CDI has been rising in the past decade. This has been associated with the emergence of a new epidemic strain, BI/NAP1/027, that produces 20-fold more toxin than other strains and is associated with high rates of colectomy and death.24–28 In fact, there is evidence in some hospitals that CDI prevalence may have surpassed that of methicillin-resistant Staphylococcus aureus (MRSA).29 With continued pressure to reduce hospital length-of-stay, the frequency of post-discharge CDI (PD-CDI) and the opportunity for prevention may be increasing as well.
In response to rising CDI incidence, the Centers for Disease Control and Prevention (CDC) and the Society for Healthcare Epidemiology of America (SHEA) have recommended surveillance of HA-CDI rates.9,10 In addition, reporting of CDI rates has been legislated or is under legislative consideration in several states.30,31 Despite national guidance that post-discharge CDI events occurring within 4 weeks should be considered hospital associated and events between 4–12 weeks of discharge could potentially be hospital-associated, hospitals performing CDI surveillance often do not track PD-CDI events. However, tracking post-discharge events may facilitate efforts to prevent readmissions and may be helpful for reporting hospital-specific CDI incidence. Moreover, patients requiring readmission for PD-CDI may not return to the original facility, suggesting that the incidence of HA-CDI may be significantly underestimated if PD-CDI events are not uniformly identified among hospitals.14
We sought to identify CDI cases occurring at all hospitals in a large California county (population 3 million). We assessed the frequency of admission for new-onset CDI after a recent hospitalization, and the impact of including PD-CDI events resulting in readmission on hospital-specific CDI incidence.
METHODS
Description of Dataset
We conducted a population-based retrospective cohort study to assess the frequency of post-discharge CDI events among adult inpatients in all 29 hospitals serving adults in Orange County, California, from January 1, 2000 to December 31, 2007. We used mandatory California hospital discharge data which provides line item demographic and insurer information, ICD-9 codes (up to 25), and a unique identifier (Record Linking Number) that allows patients to be tracked across hospital admissions.32 This data also includes a code to indicate whether a given condition was present when the patient was admitted, known as the Present on Admission (POA) code, which has been used in California since 1996.33
We identified CDI cases using the ICD-9 diagnostic code 008.45 for pseudomembranous colitis. We defined four types of CDI cases: 1) hospital-onset CDI (HO-CDI) cases defined by POA=N; 2) PD-CDI cases defined by POA=Y with a history of hospitalization for any reason in the prior 12 weeks; 3) HA-CDI cases defined as the sum of HO-CDI and PD-CDI; and 4) community-associated CDI (CA-CDI) cases defined by POA=Y with no prior history of hospitalization in the previous 12 weeks. To reduce the chance that a code represented a past history of CDI without active infection during hospitalization, we limited cases with POA=Y to the first three coding positions. For POA=N cases, all coding positions were accepted. We excluded 932 cases of recurrent CDI, defined as cases occurring within 8 weeks of a previous CDI episode.9,22 Finally, we assessed the fraction of post-discharge events that occurred within 4 weeks of discharge. This study was approved by the Institutional Review Boards of the University of California Regents and the California Committee for the Protection of Human Subjects.
Data Analysis
Patient Characteristics
We collected demographic information for all patients in our cohort, including gender, age, race and ethnicity, and insurance type. We also assessed the proportion of hospitalized patients with select comorbidities using the Romano score33 and the proportion that had undergone surgery in the previous month. These characteristics were collected for all admissions and for those with CDI (HO-CDI, PD-CDI, and CA-CDI).
Annual Incidence of CDI
Annual CDI incidence across Orange County was determined for 2000–2007 and analyzed by chi-square tests for trend. We identified all cases and subsets of CDI as defined above. Incidences of HO-CDI, PD-CDI and HA-CDI were expressed per 10,000 admissions. CA-CDI incidence was expressed per 100,000 residents.
Hospital Readmission for CDI
We defined a PD-CDI readmission as a case with symptoms present on admission (POA=Y) that occurred within 12 weeks after a prior hospitalization for any reason, as described above. We calculated the percentage of all-cause readmissions that are due to PD-CDI. We excluded readmissions for recurrent CDI, which we defined as community-onset (POA=Y) cases readmitted within 8 weeks of a previous admission for CDI.22 We also determined how often patients readmitted for PD-CDI went to a different facility for their readmission. Finally, we assessed the fraction of PD-CDI that occurred within 4 weeks of discharge.
Impact of Including Post-Discharge CDI Readmissions in Hospital-Specific CDI Incidence
For each hospital, we determined the annual incidence of HO-CDI and HA-CDI for the years 2000–2007. Differences between annual HO-CDI and HA-CDI incidence were compared using paired t-tests. We determined whether relative rankings by quartile of hospitals by CDI incidence were affected by inclusion of PD-CDI.
Identifying Individual and Hospital Predictors of CDI
We identified the primary admission diagnoses of admissions that were associated with HO-CDI and PD-CDI. For primary admission diagnoses associated with greater than 25 HO-CDI or PD-CDI events, we calculated the frequency of CDI compared to those without that primary admission diagnosis.
We performed bivariate analyses using chi-square tests to identify individual and hospital level variables associated with the individual outcomes of HO-CDI and PD-CDI. For the PD-CDI outcome, we used characteristics from the PD-CDI (vs. the index) admission and removed all hospitalizations that resulted in death, since these hospitalizations could not result in readmission. Individual variables included demographics, comorbidities, primary admission diagnosis, recent surgery, insurance type, year of hospital admission, and length-of-stay. Hospital variables included annual admissions, average length-of-stay, and hospital type (acute vs. long-term acute care facility). Variables with p<0.1 from bivariate testing were entered into a generalized linear mixed model which accounted for clustering by hospital (ProcGLIMMIX, SAS9.2, Cary, NC). Variables were retained at alpha=0.05.
RESULTS
Patient Characteristics
Patients admitted with CDI were older, had more comorbidities, and were less likely to have undergone surgery in the past month compared to all hospitalized patients (Table 1). Among those with CDI, patients with HO-CDI and PD-CDI had similar distributions of age, race and ethnicity, and comorbidities, but those with HO-CDI were more likely to be male and to have undergone surgery in the past month.
Table 1.
Characteristics of Hospital Inpatients, Orange County, CA 2000–2007
| Characteristics of Hospital Admissions, N (%) | |||||
|---|---|---|---|---|---|
| Characteristic | All | HO-CDI | PD-CDI | CA-CDI | Total CDI |
| No. Patients | N=1,768,686 | N=1,952 | N=3,077 | N=5,667 | N = 10,750 |
| Male Gender | 675,111(38%) | 919 (47%) | 1,213 (39%) | 2,416 (43%) | 4580 (43%) |
| Age | |||||
| 18-<40 | 497,982 (28%) | 146 (7%) | 194 (6%) | 364 (6%) | 703 (7%) |
| 40–49 | 220,320 (13%) | 164 (9%) | 218 (7%) | 443 (8%) | 819 (8%) |
| 50–59 | 220,859 (13%) | 243 (13%) | 255 (8%) | 596 (11%) | 1,105 (10%) |
| 60-<75 | 365,572 (21%) | 590 (31%) | 758 (25%) | 1,518 (28%) | 2,878 (28%) |
| 75+ | 434,378 (25%) | 762 (40%) | 1,569 (52%) | 2,572 (47%) | 4,929 (47%) |
| Race | |||||
| White | 1,419,979 (80%) | 1,582 (81%) | 2,698 (88%) | 4,775 (84%) | 9,103 (85%) |
| Black | 42,500 (3%) | 40 (2%) | 34 (1%) | 117 (2%) | 193 (2%) |
| Asian | 167,465 (9%) | 212 (11%) | 174 (6%) | 429 (8%) | 823 (7%) |
| Other | 138,761 (8%) | 118 (6%) | 171 (5%) | 346 (6%) | 631 (6%) |
| Hispanic Ethnicity | 281,701 (16%) | 208 (11%) | 290 (9%) | 643 (11%) | 1,164 (11%) |
| Romano Score | |||||
| 0 | 880,857 (50%) | 349 (18%) | 704 (23%) | 1,203 (21%) | 2,120 (20%) |
| 1–2 | 407,651 (23%) | 405 (21%) | 750 (24%) | 1,187 (21%) | 2,275 (21%) |
| 3–4 | 221,440 (12%) | 397 (20%) | 621 (20%) | 1,068 (19%) | 2,110 (20%) |
| 5+ | 258,757 (15%) | 801 (41%) | 1,002 (33%) | 2,209 (39%) | 4,245 (39%) |
| Recent Surgery* | 570,445 (32%) | 869 (44%) | 986 (32%) | 1,143 (20%) | 2,848 (26%) |
Recent Surgery includes surgery during the current admission or within the previous 30 days
Annual Incidence of HO-CDI and HA-CDI
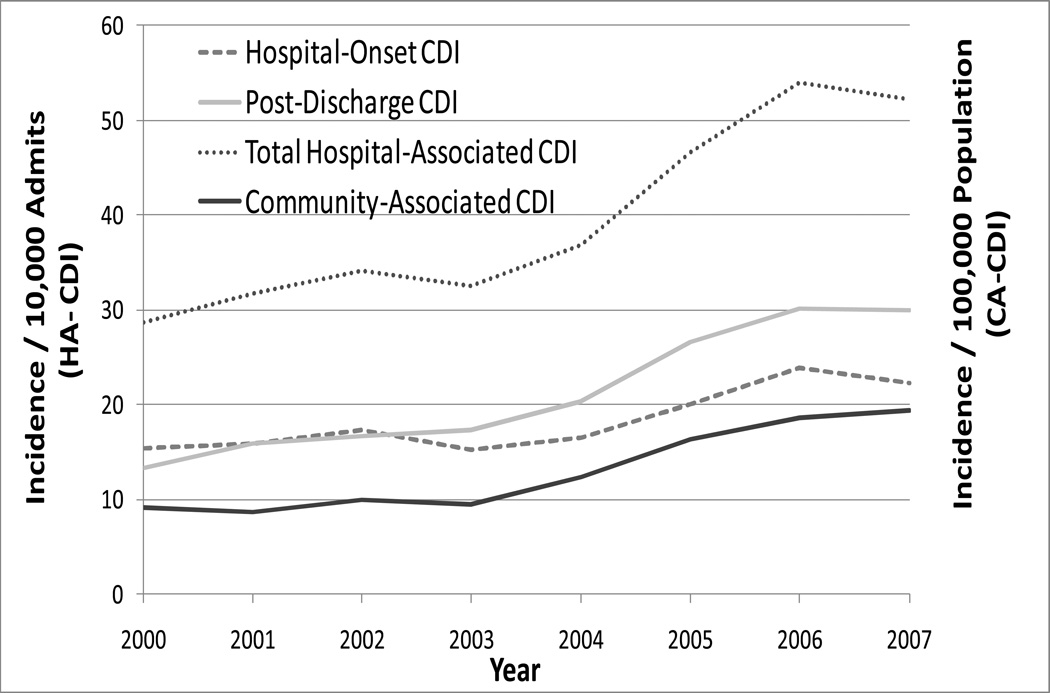
Annual incidence of HO-CDI in Orange County increased from 2000 to 2007, as shown in Figure 1 (p<0.001 for test of trend). After including PD-CDI events, the annual incidence of HA-CDI increased 1.9-fold during the same period, from 28 to 52 per 10,000 admissions (chi-square, p<0.001). By 2007, PD-CDI composed the majority of HA-CDI cases (increasing from 46% in 2000 to 57% in 2007, p<0.001 for test of trend).
Figure 1.
CDI Burden among Hospitalized Adult Patients in Orange County, California, from 2000 to 2007. Incidence of HO-CDI, PD-CDI and HA-CDI is expressed per 10,000 adult admissions; incidence of CA-CDI is expressed per 100,000 Orange County adult population. HA-CDI consists of HO-CDI and PD-CDI.
Frequency of New-Onset CDI as Reason for Hospital Readmission
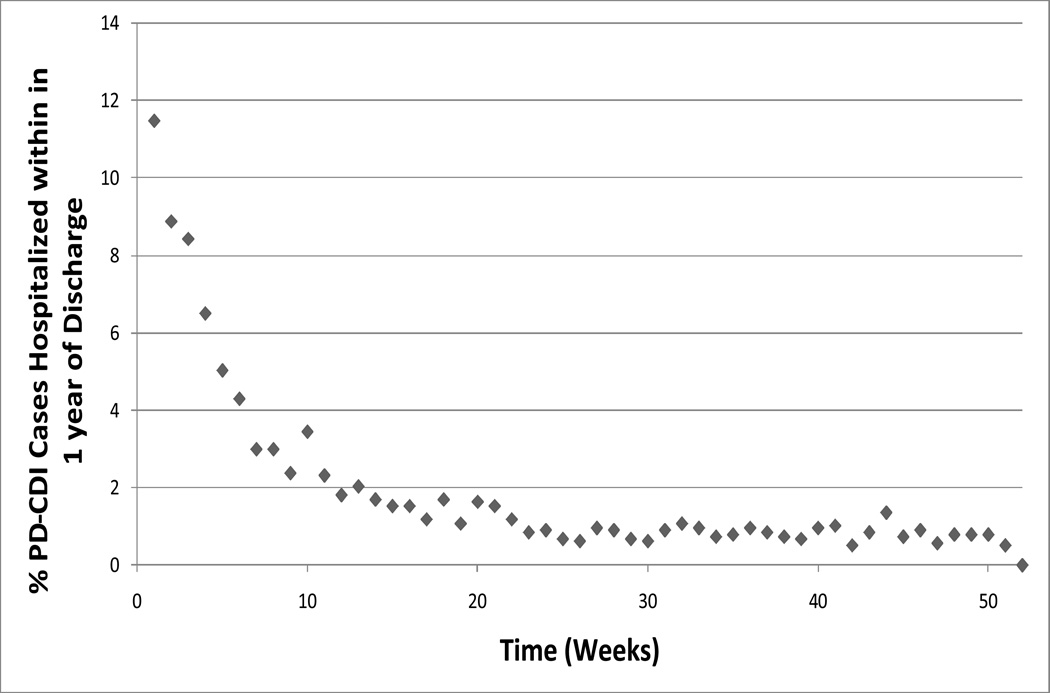
Over 2000–2007, PD-CDI events resulting in readmission represented 1.8% (2,998 of 170,995) of all-cause readmissions within 12 weeks after discharge. When evaluating all admissions related to CDI occurring within 365 days of discharge, we found that the risk of readmission for CDI was higher in the first 12 weeks post-discharge, and highest in the first 4 weeks post-discharge (Figure 2). Of PD-CDI event occurring within 12 weeks of discharge, 58% (624 of 1071) occurred within the 4 weeks after discharge. After 12 weeks, the risk of readmission for CDI dropped to a stable, low level. Among PD-CDI cases readmitted within 12 weeks, 25% (746 of 2,998) were readmitted to a different hospital than the initial hospitalization.
Figure 2.
Time to Readmission for Post-Discharge CDI Cases (PD-CDI), 2000–2007, for Cases Occurring within 1 Year after Discharge (N=1,766).
Impact of Including CDI Readmissions on Hospital-Specific Rates
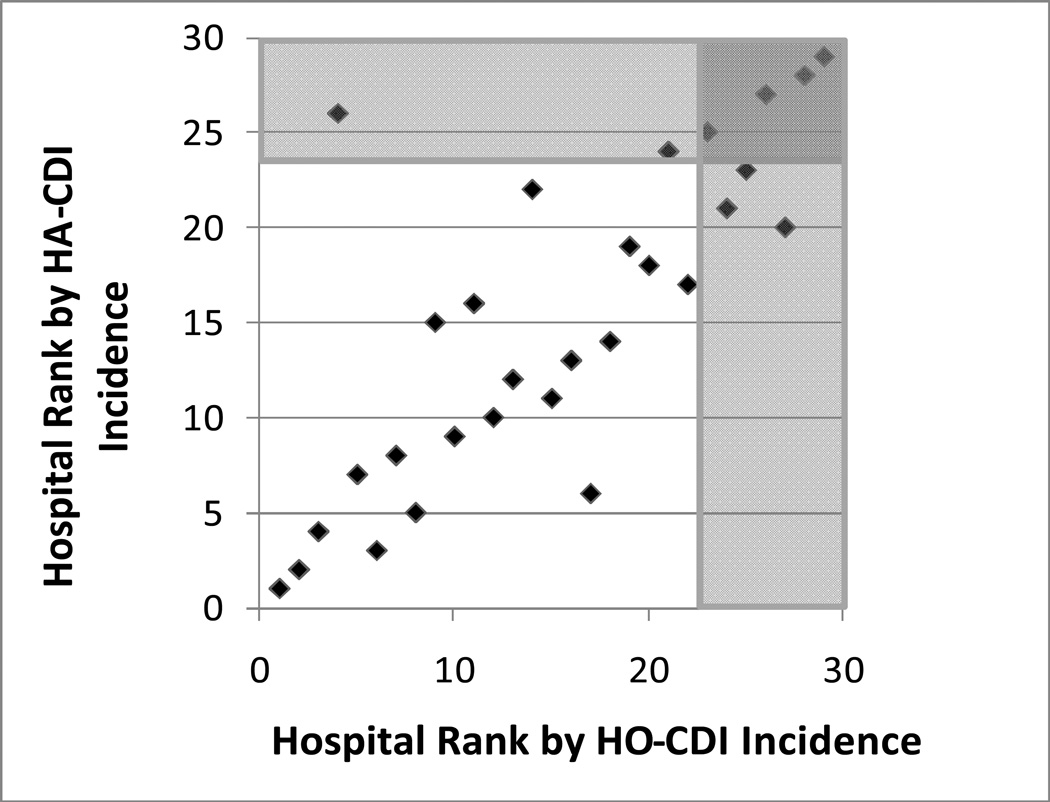
Figure 3 shows hospital-specific rankings according to CDI incidence for 2007, with and without including PD-CDI events (HO-CDI vs. HA-CDI, respectively). The proportion of hospitals’ HA-CDI comprised by PD-CDI varied greatly (median 60% PD-CDI, range 0–100%, for 2007). Hospital ranking by CDI incidence changed by a mean of 3 places after including PD-CDI events; only 5 of 29 hospitals did not change rank. Three hospitals became ranked in the worst quartile after including PD-CDI, including one hospital that had been ranked in the best quartile when PD-CDI events were excluded. Another 3 hospitals were no longer ranked in the worst quartile when PD-CDI events were included.
Figure 3.
Hospital-specific Rankings by HA-CDI vs. HO-CDI Incidence for 2007. Shaded areas indicate the quartile of hospitals with the highest CDI incidence based upon HA-CDI vs HO-CDI.
Identifying Individual and Hospital Predictors of CDI
Primary admission diagnoses that occurred most often during HO-CDI and PD-CDI admissions are listed in Table 3. Several primary admission diagnoses were significantly associated with both HO-CDI and PD-CDI admissions on bivariate analysis, including septicemia, pneumonia, post-operative infection and urinary tract infection.
Table 3.
Frequent Primary Admission Diagnoses for Hospital Patients at High Risk for CDI
| Primary Admission Diagnosis |
N(%) with HO-CDI | OR | p-value | N(%) with PD-CDI | OR | p-value |
|---|---|---|---|---|---|---|
| S.aureus Septicemia | 31 (0.6%) | 10.09 | <0.001 | |||
| S.aureus Pneumonia | 33 (0.7%) | 9.76 | <0.001 | |||
| Acute Respiratory Failure | 117 (2.4%) | 8.56 | <0.001 | 31 (0.4%) | 1.22 | 0.007 |
| Aspiration Pneumonitis | 99 (2.1%) | 6.55 | <0.001 | 69 (0.8%) | 2.47 | <0.001 |
| Infection of Vascular Device | 33 (0.7%) | 6.16 | <0.001 | 26 (0.3%) | 2.65 | |
| Chemotherapy | 30 (0.6%) | 5.43 | <0.001 | |||
| E.coli Septicemia | 26 (0.5%) | 5.39 | <0.001 | 26 (0.3%) | 2.94 | <0.001 |
| Septicemia | 68 (1.4%) | 3.72 | <0.001 | 184 (1.2%) | 3.20 | <0.001 |
| Post-operative Infection | 28 (0.6%) | 3.28 | <0.001 | 26 (0.3%) | 1.67 | <0.001 |
| Acute Renal Failure | 39 (0.8%) | 2.80 | <0.001 | 47 (0.5%) | 1.85 | <0.001 |
| Pneumonia | 98 (2.0%) | 1.69 | <0.001 | 184 (2.1%) | 1.74 | <0.001 |
| Acute Pancreatitis | 31 (0.6%) | 1.51 | 0.002 | 26 (0.3%) | 0.69 | 0.8 |
| Urinary Tract Infection | 37 (0.8%) | 1.43 | 0.19 | 96 (1.1%) | 2.04 | <0.001 |
| Hip Fracture | 31 (0.4%) | 1.76 | <0.001 | |||
| Cellulitis | 45 (0.5%) | 1.69 | <0.001 | |||
| Colon Diverticulitis | 45 (0.5%) | 1.68 | <0.001 |
Results from bivariate analysis (Table 2) were similar to those from multivariate analysis (Table 4). In multivariate analysis, HO-CDI and PD-CDI were both associated with increasing age, longer length of stay, Medicare insurance, recent surgery, comorbidities, select primary admission diagnoses (septicemia, post-operative infection and pneumonia), and hospitals with a high percent of patients with a high comorbidity index. Non-white race, Hispanic ethnicity and male gender were protective against PD-CDI, but not HO-CDI.
Table 2.
Bivariate Analysis of Predictors of Hospital-Onset CDI (HO-CDI) and Post-Discharge CDI (PD-CDI) among all Adult Inpatients
| Individual Variables (%) | HO- CDI |
Non HO- CDI |
p- value |
PD- CDI |
Non PD- CDI |
p- value |
|---|---|---|---|---|---|---|
| N | 2,403 | 1,766,753 | 3,077 | 1,725,165 | ||
| Age | <0.001 | <0.001 | ||||
| 18 – < 40 | 6.7% | 28.7% | 6.5% | 29.3% | ||
| 40 – 49 | 7.8% | 12.7% | 7.3% | 12.8% | ||
| 50 – 59 | 11.9% | 12.7% | 8.5% | 12.8% | ||
| 60 – < 75 | 31.7% | 21.0% | 25.3% | 20.9% | ||
| 75+ | 41.9% | 24.9% | 52.4% | 24.2% | ||
| Male Gender | 48.2% | 38.2% | <0.001 | 39.4% | 37.9% | 0.08 |
| Race | 0.01 | <0.001 | ||||
| White | 80.9% | 80.3% | 87.7% | 80.2% | ||
| Black | 2.2% | 2.4% | 1.1% | 2.4% | ||
| Asian | 10.6% | 9.5% | 5.6% | 9.5% | ||
| Other | 6.3% | 7.8% | 5.6% | 7.9% | ||
| Hispanic Ethnicity | 11.1% | 16.2% | <0.001 | 9.5% | 16.4% | <0.001 |
| Medicare Insurance | 36.8% | 59.8% | <0.001 | 29.9% | 60.6% | <0.001 |
| Medicaid Insurance | 92.0% | 89.5% | <0.001 | 95.2% | 89.4% | <0.001 |
| Admit to Acute Hospital (vs. LTAC)a | 87.2% | 96.8% | <0.001 | 96.3% | 96.8% | 0.08 |
| Admission Year | <0.001 | <0.001 | ||||
| 2000 | 10.2% | 11.7% | 7.9% | 11.6% | ||
| 2001 | 10.6% | 12.2% | 9.1% | 12.2% | ||
| 2002 | 11.7% | 12.4% | 9.6% | 12.4% | ||
| 2003 | 10.8% | 12.9% | 10.9% | 12.9% | ||
| 2004 | 11.8% | 12.7% | 12.2% | 12.7% | ||
| 2005 | 13.5% | 12.7% | 16.3% | 12.7% | ||
| 2006 | 16.3% | 12.5% | 17.6% | 12.6% | ||
| 2007 | 15.1% | 12.9% | 16.4% | 12.9% | ||
| Length of Stay >5 days | 96.7% | 27.4% | <0.001 | 56.2% | 26.8% | <0.001 |
| Surgeryb | 42.3% | 32.2% | <0.001 | 25.7% | 30.5% | <0.001 |
| Comorbidities | ||||||
| Diabetes | 29.6% | 16.9% | <0.001 | 24.7% | 16.6% | <0.001 |
| Cancer | 15.1% | 7.8% | <0.001 | 13.5% | 7.5% | <0.001 |
| Dementia | 5.9% | 2.9% | <0.001 | 5.8% | 2.8% | <0.001 |
| Ulcer | 4.6% | 1.8% | <0.001 | 2.7% | 1.7% | <0.001 |
| AIDS | 0.6% | 0.2% | <0.001 | 0.4% | 0.2% | 0.02 |
| High Comorbidity Indexc | 64.5% | 27.1% | <0.001 | 52.8% | 26.0% | <0.001 |
| Admitted to High Volume Hospitald | 61.9% | 61.1% | <0.001 | 72.2% | 67.2% | <0.001 |
| Admitted to Hospital with High Length of Staye |
85.4% | 77.2% | <0.001 | 71.5% | 77.1% | <0.001 |
LTAC = long-term acute care facility
Surgery indicates surgery during the current admission or within the previous 30 days
Comorbidity Index measured by Romano score
High volume = >10,000 annual admissions
High length of stay = >5 days
Table 4.
Multivariate Analysis of Predictors of Hospital-Onset CDI (HO-CDI) and Post-Discharge CDI (PD-CDI) Among all Adult Inpatients
| HO-CDI vs. All non HO-CDI | PD-CDI vs. All non HO-CDI | |||
|---|---|---|---|---|
| Individual Variables | OR (95% CI) | p-value | OR (95% CI) | p-value |
| Age | <0.001 | <0.001 | ||
| 18 – < 40 | Reference | Reference | ||
| 40 – 49 | 1.93 (1.29–2.89) | 2.20 (1.53–3.17) | ||
| 50 – 59 | 1.82 (1.23–2.71) | 2.21 (1.54–3.17) | ||
| 60 – < 75 | 1.83 (1.28–2.61) | 2.76 (1.99–3.82) | ||
| 75+ | 2.16 (1.51–3.09) | 3.93 (2.85–5.44) | ||
| Male Gender | 1.04 (0.93–1.16) | 0.49 | 0.87 (0.80–0.95) | 0.001 |
| Race | 0.01 | <0.001 | ||
| White | Reference | Reference | ||
| Black | 0.94 (0.61–1.45) | 0.49 (0.31–0.78) | ||
| Asian | 1.27 (1.06–1.52) | 0.64 (0.53–0.77) | ||
| Other | 0.79 (0.59–1.05) | 1.04 (0.85–1.28) | ||
| Hispanic Ethnicity | 0.94 (0.77–1.15) | 0.56 | 0.73 (0.62–0.86) | <0.001 |
| Medicare Insurance | 0.61 (0.48–0.77) | <0.001 | 0.70 (0.56–0.87) | 0.001 |
| Admission Year | 1.07 (1.05–1.09) | <0.001 | 1.11 (1.09–1.13) | <0.001 |
| 2000 (reference) | ||||
| 2001 | 1.03 (0.81–1.33) | 0.92 (0.75–0.86) | ||
| 2002 | 1.12 (0.88–1.43) | 1.04 (0.86–1.27) | ||
| 2003 | 0.94 (0.73–1.21) | 1.14 (0.94–1.38) | ||
| 2004 | 1.22 (0.96–1.55) | 1.35 (1.12–1.62) | ||
| 2005 | 1.25 (0.99–1.58) | 1.67 (1.40–1.99) | ||
| 2006 | 1.55 (1.24–1.94) | 1.83 (1.53–2.18) | ||
| 2007 | 1.39 (1.10–1.74) | 1.71 (1.43–2.04) | ||
| Length of Stay | 1.02 (1.02–1.02) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| Surgerya | 2.16 (1.91–2.43) | <0.001 | 1.23 (1.12–1.35) | <0.001 |
| Comorbidities | ||||
| Diabetes | 1.36 (1.20–1.53) | <0.001 | 1.14 (1.03–1.25) | 0.009 |
| Cancer | 1.32 (1.13–1.55) | <0.001 | 1.24 (1.09–1.42) | 0.001 |
| Dementia | 1.57 (1.28–1.93) | <0.001 | 1.03 (0.87–1.21) | 0.74 |
| Ulcer | 1.92 (1.49–2.47) | <0.001 | 1.31 (1.04–1.67) | 0.02 |
| AIDS | 4.15 (2.02–8.50) | <0.001 | 3.27 (1.60–6.65) | 0.001 |
| High Comorbidity Indexb | 1.06 (1.02–1.11) | <0.001 | 1.02 (1.01–1.03) | 0.001 |
| Primary Admission Diagnosis: | ||||
| Chemotherapy | 7.08 (4.04–12.42) | <0.001 | ---------------------- | |
| S.aureus Pneumonia | 5.13 (3.23–8.16) | <0.001 | ---------------------- | |
| Infection due to Vascular Device | 3.05 (1.87–4.97) | <0.001 | ---------------------- | |
| Septicemia | 2.70 (3.71–1.96) | <0.001 | 3.55 (2.87–4.40) | <0.001 |
| Post-operative Infection | 2.36 (3.89–1.43) | <0.001 | 2.39 (1.51–3.76) | <0.001 |
| Acute Respiratory Failure | 2.25 (1.63 – 3.10) | <0.001 | ---------------------- | |
| Pneumonia | 1.47 (1.93–1.11) | 0.006 | 1.82 (1.54–2.16) | <0.001 |
| Cellulitis | ---------------------- | 2.41 (1.72–3.38) | <0.001 | |
| Colon Diverticulitis | ---------------------- | 2.37 (1.64–3.43) | <0.001 | |
| Urinary Tract Infection | ---------------------- | 1.86 (1.48–2.33) | <0.001 | |
| Acute Renal Failure | ---------------------- | 1.73 (1.25–2.39) | <0.001 | |
Surgery indicates surgery during the current admission or within the previous 30 days
Comorbidity Index measured by Romano score
Annual Incidence of Community-Associated CDI
Orange County’s incidence of CA-CDI also rose during 2000 to 2007. In this period, CA-CDI incidence increased 2.1-fold from 9 to 19 cases per 100,000 residents (chi-square, p<0.001), exclusive of PD-CDI cases.
DISCUSSION
C. difficile disease is a major cause of healthcare-associated infection and morbidity. Due to the known delay in presentation following antibiotic exposure, national guidelines consider cases up to 12 weeks following hospital discharge as potentially healthcare-associated and possibly preventable. Nevertheless, the majority of hospitals do not track post-discharge cases, and the impact of post-discharge cases has remained largely unknown. Remarkably, we found that PD-CDI cases within 12 weeks after hospital discharge accounted for the majority of HA-CDI and led to a 2-fold increase in HA-CDI incidence across hospitals in a large metropolitan county. This finding illustrates the need to expand prevention and education strategies to include the post-discharge period and thereby reduce the frequency of PD-CDI events.
Inclusion of post-discharge CDI events substantially altered hospital-specific CDI incidence, but the impact varied widely by hospital. For example, PD-CDI cases accounted for all HA-CDI cases in one hospital and none of the cases in another. This suggests that tracking PD-CDI events may impact the validity of inter-facility comparisons, since hospitals are affected differentially by including or excluding PD-CDI. These discrepancies could be magnified if some, but not all, hospitals track PD-CDI. When we ranked hospitals by HO-CDI incidence, half the hospitals captured in the quartile with the highest HO-CDI incidence changed when PD-CDI was included. In fact, one hospital changed from the best quartile to the worst quartile when PD-CDI cases were captured. In addition, since 75% of patients with PD-CDI returned to the same hospital for readmission, hospitals may be able to track most PD-CDI cases by performing post-discharge surveillance for PD-CDI cases that readmit to their own facility. Additional notification of PD-CDI cases back to transferring or recently discharging hospitals may also improve accuracy of CDI rates.
For prevention, patient characteristics may be utilized to identify populations at elevated risk for post-discharge CDI. We found that risk factors for HO-CDI and PD-CDI were often the same, including increasing age, higher length of stay, and overall poor health, including diabetes, cancer, and AIDS. In addition, prevention may be targeted at patients with specific primary admission diagnoses such as septicemia, post-operative infection, and pneumonia. These primary admission diagnoses all represent conditions likely to be treated with antibiotics, the main risk factor for CDI. The immediate post-discharge period should be considered an extension of the risk of CDI that begins during a hospital stay. This heightens the importance of educating high risk patients prior to hospital discharge about the potential for post-discharge diarrhea and of identifying prophylactic solutions to prevent disease in the high risk patient population.
In addition, we found that white and non-Hispanic patients had a higher risk of PD-CDI. While we did not evaluate reasons for this difference, racial and ethnic disparities, including access to healthcare, have been well-documented and may be magnified in the outpatient arena.35–36 Differences in access to outpatient care are likely to impact antibiotic use in these groups, and their subsequent risk of PD-CDI. In addition, male gender was associated with lower risk of PD-CDI but not with HO-CDI. This difference may not be due to an increased post-discharge risk for women but may instead reflect men’s reduced tendency to seek care and therefore be hospitalized for PD-CDI.37–39 More research is needed to understand the reasons for differential risk in these groups in order to develop effective prevention strategies for the post-discharge setting.
Our study has several limitations. We did not capture PD-CDI cases treated in the outpatient setting, which may have led to an underestimate of PD-CDI incidence. Nevertheless, the focus on PD-CDI associated with re-hospitalization ensured capture of the most serious cases. While errors present in administrative data may lead to an under or over estimation of CDI incidence, this California dataset is notable for containing present on admission codes that indicate hospital vs. community disease onset. These codes have been well established in California hospitals for over a decade and have been validated for select diseases such as community-acquired pneumonia and acute myocardial infarction.40 We also minimized the chances that a code represented a history of CDI only versus active disease by limiting diagnoses to the first 3 coded positions. Another limitation is that we did not account for certain known risk factors (such as antibiotic use) that were unavailable in administrative data. However, we included primary admission diagnoses that are frequently treated with antibiotics. Finally, we attributed a PD-CDI event to the most recent hospitalization within 12 weeks. This definition does not account for multiple hospital exposures during that period or for intervening nursing home admissions, which may also contribute to CDI acquisition.41 Further, since the incubation period for CDI is unknown, some cases occurring within our 12 week window may be due to exposures in the community, including outpatient antibiotic use, household pets, and contamination of food.42–43 Nonetheless, over half of PD-CDI cases detected in this study occurred within the first 4 weeks after discharge, a time window that is accepted by national guidance to most likely reflect healthcare associated CDI events.
In summary, tracking PD-CDI cases doubled the incidence of HA-CDI in a large county. Since the majority of hospitals do not track PD-CDI cases, the frequency and impact of PD-CDI may be widely underestimated, resulting in missed opportunities to prevent readmissions. Importantly for public reporting purposes, including PD-CDI affected individual hospitals differently, leading to substantial changes in hospital rankings by CDI incidence. Uniform tracking of PD-CDI events would allow more accurate estimates of overall CDI incidence and more equitable hospital-to-hospital comparisons. We found that the vast majority of cases can be captured if hospitals track PD-CDI cases that return to the same facility. We also identified several patient characteristics that were associated with PD-CDI, suggesting that preventative strategies may effectively focus upon specific patient groups. Targeted education and prevention for CDI may become increasingly important to help hospitals lower their readmission rates.
ACKNOWLEDGEMENTS
We thank Leah Terpstra and Kristen Elkins for their contributions. This study was funded by the CDC Prevention Epicenters Program (1U01 CI000344, Platt).
E.R.D. reports that he is a consultant for Optimer, Pfizer and and has received research support from Optimer and Merck. Support for ERD came from NIAID (1 K23AI065806).
Footnotes
All other authors report no conflict of interest.
REFERENCES
- 1.Meltzer DO, Chung JWUS. Trends in Hospitalization and Generalist Physician Workforce and the Emergence of Hospitalists. J Gen Intern Med. 2010;25(5):453–459. doi: 10.1007/s11606-010-1276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yokoe DS, Avery TR, Huang SS. Surgical Site infection Surveillance Following Total hip and Knee Arthroplasty Using California Administrative Data. Dallas, TX: Oral Presentation, Society for Healthcare Epidemiology of America; Apr 1–4, 2011. [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, et al. Invasive Methicillin-Resistant Staphylococcus aureus Infections in the United States. JAMA. 2007;298(15):1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Datta R, Huang SS. Risk of Infection and Death Due to Methicillin-Resistant Staphylococcus aureus in Long-Term Carriers. Clin Infect Dis. 2008;47(2):176–181. doi: 10.1086/589241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang SS, Platt R. Risk of Methicillin-Resistant Staphylococcus aureus Infection After Previous Infection or Colonization. Clin Infect Dis. 2003;36(3):281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger M, Oddone EZ, Henderson WG. Does Increased Access to Primary Care Reduce Hospital Readmissions? N Engl J Med. 1996;334:1441–1447. doi: 10.1056/NEJM199605303342206. [DOI] [PubMed] [Google Scholar]
- 7.Jweinat JJ. Hospital Readmission Under the Spotlight. J Healthc Manag. 2010;55(4):252–264. [PubMed] [Google Scholar]
- 8.CMS/ Joint Commission. http://www.jointcommission.org/QualityCheck/mortality_help.htm.
- 9.Cohen AL, Calfee D, Fridkin SK, et al. Recommendations for Metrics for Multidrug-Resistant Organisms in Healthcare Settings: SHEA/HICPAC Position Paper. Infect Control Hosp Epidemiol. 2008;29:901–913. doi: 10.1086/591741. [DOI] [PubMed] [Google Scholar]
- 10.Multidrug-Resistant Organism (MDRO) and Clostridium difficile-Associated Disease (CDAD) Module. National Health Safety Network, Centers for Disease Control and Prevention. http://www.cdc.gov/nhsn/mdro_cdad.html.
- 11.Essentials of Public Reporting of Healthcare-Associated Infections: A Tool Kit. Healthcare-Associated Infection Working Group of the Joint Public Policy Committee (SHEA/APIC/CSTE/CDC) Infect Control Hosp Epidemiol. 2006 [Google Scholar]
- 12.Association for Professionals in Infection Control and Epidemiology. Healthcare-Associated Infection Legislation in Progress. 2010 http://www.apic.org/downloads/legislation/HAI_map.gif.
- 13.Dubberke ER, Gerding DN, Classen D, et al. Strategies to prevent clostridium difficile infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(Suppl 1):S81–S92. doi: 10.1086/591065. [DOI] [PubMed] [Google Scholar]
- 14.Huang SS, Avery TR, Song Y, et al. Quantifying inter-hospital patient sharing as a mechanism for infectious disease spread. Infect Control Hosp Epidemiol. doi: 10.1086/656747. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubberke ER, Butler AM, Reske KA, et al. Attributable Outcomes of Endemic Clostridium difficile-Associated Disease in Non-Surgical Patients. Emerg Infect Dis. 2008;14(7):1031–1038. doi: 10.3201/eid1407.070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Issa M, Ananthakrishnan AN, Binion DG. Clostridium difficile and inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(10):1432–1442. doi: 10.1002/ibd.20500. [DOI] [PubMed] [Google Scholar]
- 17.Chang HT, Krezolek D, Johnson S, Parada JP, Evans CT, Gerding DN. Onset of Symptoms and Time to Diagnosis of Clostridium difficile-Associated Disease Following Discharge From an Acute Care Hospital. Infect Control Hosp Epidemiol. 2007;28(8):926–931. doi: 10.1086/519178. [DOI] [PubMed] [Google Scholar]
- 18.Dubberke ER, McMullen KM, Mayfield JL, et al. Hospital-Associated Clostridium difficile Infection: Is It Necessary to Track Community-Onset Disease? Infect Control Hosp Epidemiol. 2009;30(4):332–337. doi: 10.1086/596604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutty PK, Benoit SR, Woods CW, et al. Assessment of Clostridium difficile – Associated Disease Surveillance Definitions, North Carolina, 2005. Infect Control Hosp Epidemiol. 2008;29(3) doi: 10.1086/528813. [DOI] [PubMed] [Google Scholar]
- 20.Palmore TN, Sohn S, Malak SF, Eagan J, Sepkowitz KA. Risk Factors for Acquisition of Clostridium difficile-associated Diarrhea among Outpatients at a Cancer Hospital. Infect Control Hosp Epidemol. 2005;26:680–684. doi: 10.1086/502602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly CP, Pothoulakis C, Lamont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 22.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for Surveillance of Clostridium difficile-Associated Disease. Infect Control Hosp Epidemiol. 2007;28(2):140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SH, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 24.Campbell RJ, Giljahn L, Machesky K, et al. Clostridium difficile Infection in Ohio Hospitals and Nursing Homes During 2006. Infection Control Hosp Epidemiol. 2009;30:526–533. doi: 10.1086/597507. [DOI] [PubMed] [Google Scholar]
- 25.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009;15(13):1554–1580. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warny M, Pepin J, Fang A, et al. Toxin Production by an Emerging Strain of Clostridium difficile Associated with Outbreaks of Severe Disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 27.Miller M, Gravel D, Mulvey M, et al. Healthcare-associated Clostridium difficile Infection in Canada: Patient Age and Infecting Strain Type are Highly Predictive of Severe Outcome and Mortality. Clin Infect Dis. 2010;50(2):194–201. doi: 10.1086/649213. [DOI] [PubMed] [Google Scholar]
- 28.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile . N Engl J Med. 2005;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 29.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Top Four Abstracts at the Fifth Decennial International Conference on Healthcare-Associated Infections. Atlanta, GA, USA: Mar 18–22, 2010. The Impact of Hospital-Onset Healthcare Facility Associated (HO-HCFA) Clostridium difficile Infection (CDI) in Community Hospitals: Surpassing Methicillin-Resistant Staphylococcus aureus (MRSA) as the New Superbug. Oral Presentation. [Google Scholar]
- 30.Association for Professionals in Infection Control and Epidemiology. Clostridium difficile Legislation in Progress. 2010 http://www.cqstatetrack.com/texis/viewrpt/+yeOlOjemuAB?report=4cab75bbeb1.
- 31.Committee to Reduce Infection Deaths. State Legislation and Initiatives on Healthcare-Associated Infections. 2010 Updated March http://www.hospitalinfection.org/legislation.shtml.
- 32.Office of Statewide Health Planning and Development. http://www.oshpd.ca.gov/
- 33.Leibson CL, Needleman J, Buerhaus P, et al. Identifying in-hospital venous thromboembolism (VTE): a comparison of claims-based approaches with the Rochester Epidemiology Project VTE cohort. Med Care. 2008;46(2):127–132. doi: 10.1097/MLR.0b013e3181589b92. [DOI] [PubMed] [Google Scholar]
- 34.Musher DM, Aslam S, Logan N. Relatively Poor Outcome after Treatment of Clostridium difficile Colitis with Metronidazole. Clin Infect Diseases. 2005;40:1586–1590. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 35.Nelson A. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. J Natl Med Assoc. 2002;94:666–668. [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DR. Racial Variations in Adult Health Status: Patterns, Paradoxes and Prospects. In: Smelser N, Wilson WJ, Mitchell F, editors. America becoming: racial trends and their consequences. Vol. 2. Washington DC: National Academy of Sciences; 2001. pp. 371–410. [Google Scholar]
- 37.Courtenay W. Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Social Science and Medicine. 2000b;50(10):1385–1401. doi: 10.1016/s0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- 38.Lee C, Owens RG. Issues for a psychology of men’s health. Journal of Health Psychology. 2002a;7(3):209–217. doi: 10.1177/1359105302007003215. [DOI] [PubMed] [Google Scholar]
- 39.Lee C, Owens RG. The Psychology of Men’s Health. Buckingham: Open University Press; 2002b. [Google Scholar]
- 40.Goldman LE, Chu PW, Prothro C, Osmond D, Bindman AB. Accuracy of Condition Present on Admission, Do Not Resuscitate, and E-codes in California Patient Discharge Data. Prepared for the Office of Statewide Health Planning and Development. 2011 Spring [Google Scholar]
- 41.Crogan NL, Evans BC. Clostridium difficile: An Emerging Epidemic in Nursing Homes. Geriatr Nurs. 2007;28(3):161–164. doi: 10.1016/j.gerinurse.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Jhung MA, Thompson AD, Killgore GE, et al. Toxinotype V Clostridium difficile in Humans and Food Animals. Emerg Infect Dis. 2008;14(7):1039–1045. doi: 10.3201/eid1407.071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould LH, Limbago B. Clostridium difficile in Food and Domestic Animals: A New Foodborne Pathogen? Clin Infect Dis. 2010;51(5):577–582. doi: 10.1086/655692. [DOI] [PubMed] [Google Scholar]