Abstract
The suppression of spontaneous motor impulses is an essential facet of cognitive control that is linked to frontal-basal ganglia circuitry. Basal ganglia dysfunction caused by Parkinson’s disease (PD) disrupts the proficiency of action suppression, but how pharmacotherapy for PD impacts impulsive motor control is poorly understood. Dopamine agonists improve motor symptoms of PD, but can also provoke impulsive-compulsive behaviors (ICB). We investigated whether dopamine agonist medication has a beneficial or detrimental effect on impulsive action control in thirty-eight PD patients, half of whom had current ICB. Participants performed the Simon conflict task, which measures susceptibility to acting on spontaneous action impulses as well as the proficiency of suppressing these impulses. Compared to an off agonist state, patients on their agonist were no more susceptible to reacting impulsively, but were less proficient at suppressing the interference from the activation of impulsive actions. Importantly, agonist effects depended on baseline performance in the off agonist state; more proficient suppressors off agonist experienced a reduction in suppression on agonist, whereas less proficient suppressors off agonist showed improved suppression on agonist. Patients with active ICB were actually less susceptible to making fast, impulsive response errors than patients without ICB, suggesting that behavioral problems in this subset of patients may be less related to impulsivity in motor control. Our findings provide further evidence that dopamine agonist medication impacts specific cognitive control processes and that the direction of its effects depends on individual differences in performance off medication.
Keywords: Parkinson’s disease, simon task, inhibition, dopamine agonist, impulse control
Introduction
Reacting spontaneously to external events is an ineludible challenge in a constantly changing environment. Spontaneous reactions to stimulus events can be impulsive (e.g., a driver slamming on her brakes to avoid being hit by a car that has run a red light) or highly overlearned (e.g., a driver slamming on her brakes to avoid running a red light). In many situations, spontaneous actions are advantageous, particularly if a swift action leads to reward or averts a negative consequence. However, action impulses sometimes conflict with optimal or goal-directed behavior. The cognitive neuroscience of action control has attracted considerable attention in recent years. This work has highlighted the role of frontal-basal ganglia circuitry as an important interface for selecting and inhibiting impulsive actions (for a review, see Ridderinkhof et al., 2011). Consistent with this view, many neuropsychiatric (e.g., Schizophrenia, Obsessive-Compulsive Disorder) and neurological disorders (Parkinson’s disease, Huntington’s disease, Tourette’s syndrome) associated with frontal-basal ganglia dysfunction have been linked with various forms of suboptimal impulsive behavior (Frank, Piedad, Rickards, & Cavanna, 2011; Grant et al., 2006; Kaladjian et al., 2011; van den Heuvel et al., 2010; Voon & Fox, 2007). An important aspect of this research is determining how treatments for these conditions affect the expression and control of impulsive actions. The current study extends this work in Parkinson’s disease by examining the effects of dopamine agonist treatment on impulsive action control and how these effects are driven by individual differences between patients.
Parkinson’s Disease Pharmacotherapy and Action Control
The cardinal clinical features of Parkinson’s disease (PD), bradykinesia, rigidity, and tremor, are attributed largely to neurodegeneration of the dopamine-producing substantia nigra pars compacta neurons of the basal ganglia (Bjorklund & Dunnett, 2007; McAuley, 2003). In addition to playing a key role in rudimentary motor control functions, the basal ganglia, via elaborate interconnections with prefrontal cortices, are increasingly recognized as vital nodes in complex cognitive and motor control networks involved in action selection and suppression (Alexander et al., 1986; Aron et al., 2007; Hikosaka, 1998; Mink, 1996; Mink & Thach, 1993; Redgrave et al., 1999; Robbins and Brown, 1990). Empirical support for this role is bolstered by functional imaging studies that reveal basal ganglia activity associated with action control functions (e.g., action suppression), and by studies in which PD patients have been found to be less proficient in action control than healthy age peers (Aron, 2007; Gauggel et al., 2007; Praamstra et al., 1998). While the dopamine precursor, levodopa, remains the gold standard of dopaminergic treatment in PD, dopamine agonists are frequently used to reduce clinical motor symptoms in patients who do not tolerate levodopa, develop a suboptimal response to it, or decide to delay its use in treatment to avert unwanted side effects (e.g., levodopa-induced dyskinesia) (Rascol et al., 2000; Parkinson Study Group, 2000). Despite their widespread clinical use, the impact of dopamine agonists on action control processes in PD patients has been relatively unexamined. The first aim of the current investigation was to study the effect of dopamine agonist administration on the ability of PD patients to suppress or inhibit the expression of action impulses that interfere with the execution of goal-directed behavior.
The importance of understanding the effects of dopamine agonists on inhibitory action control processes is underscored by at least two key considerations. First, previous studies have shown that the capacity of PD patients to suppress incorrect, prepotent action impulses is diminished (Praamstra et al., 1999; Wylie et al., 2009a, b). This reduction in action suppression has been demonstrated in separate studies of patients who were either under the influence of their dopamine replacement medications or temporarily withdrawn from their dopamine medications. Thus, while a deficit in inhibitory action control appears to be an important feature of PD, it remains unclear whether dopamine medications, including dopamine agonists, improve, worsen, or have no effect on inhibitory control processes within or across individuals.
The possibility that dopamine agonists may actually worsen the proficiency of inhibitory action control among PD patients is suggested by the second consideration that has emerged from the clinical literature. A subset of patients who are being treated with these agents develops uncharacteristic and often destructive behavioral changes that are expressed in impulsive decision-making (e.g., making an impromptu automobile purchase that depletes a retirement savings account) and/or difficulties controlling compulsive behaviors (e.g., spending entire days performing a hobby while neglecting to pay bills) (Dodd et al., 2005; Voon and Fox, 2007). These so-called impulsive-compulsive behaviors (ICB) are evoked by agonists in approximately 15-20% of PD patients and are thought to involve changes in a variety of underlying processes such as behavioral inhibition, reward anticipation, and biases in decision-making under risk (Voon et al., 2006; Weintraub et al., 2010; Ye et al., 2011; Claassen et al., 2011; Housden et al., 2010). Hypotheses concerning these changes are driven largely by the known affinity of dopamine agonists for D2/D3 dopamine receptor types that are highly represented along mesocorticolimbic dopamine reward pathways (Black et al., 2002). In fact, recent studies have revealed important differences in performance on reward and risk processing tasks between PD patients who do and do not develop ICB while taking agonists (Claassen et al., 2011; Voon et al., 2011; van Eimeren et al., 2010). Notably, there is no current direct evidence that these patients experience a reduction in inhibitory control over motor actions. Thus, an important second aim of the current study was to compare the effects of dopamine agonist medication on inhibitory action control among PD patients with active impulsive-compulsive symptoms and patients who have not developed these symptoms while taking dopamine agonists.
Measuring the Expression and Suppression of Action Impulses
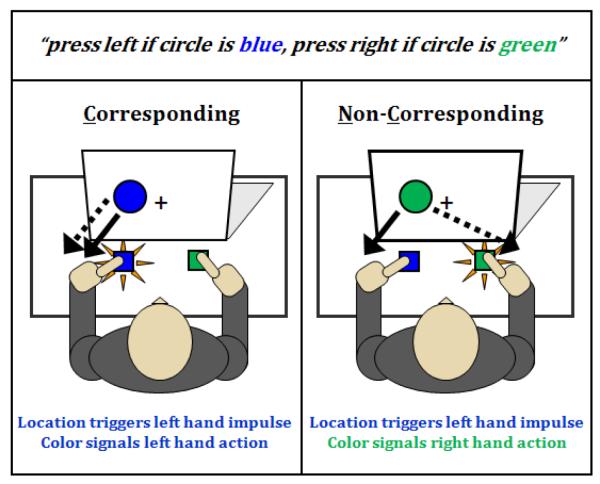
The well-known Simon task (Simon, 1969; Simon, 1990) and the Dual Process Activation-Suppression (DPAS) model (Ridderinkhof, 2002a,b; Kornblum et al., 1990) provide a powerful experimental and conceptual framework for investigating the expression and suppression of action impulses. In the well-known Simon task, selecting a designated action based on some physical attribute of a stimulus (e.g., its color) is influenced by two streams of processing, one encompassing a relatively fast, spontaneous activation of an action impulse triggered by the location of the stimulus and a second encompassing a relatively slower, deliberate translation of the goal-directed stimulus feature (color) into the designated action (see Figure 1). When these two streams correspond (e.g., the color of a circle appearing in the left hemi-field signals a left-hand response) the simultaneous, or dual-process, engagement of the same action yields fast reaction times (RTs) and high accuracy rates. Conversely, RT slows and accuracy rates decrease when the action signaled by the circle’s color and the action impulse triggered by its spatial location are non-corresponding (e.g., the color of a circle appearing in the right hemi-field signals a left-hand response), presumably reflecting the extra time required to suppress the interfering action impulse. This effect has been used with considerable success to study individual and group differences in cognitive control over interfering action impulses (Hommel, 2011; van den Wildenberg et al., 2010).
Figure 1.
Instructions and example of trial types in the Simon task. Left panel shows corresponding (Cs) trials in which an initial response impulse signaled by the goal-irrelevant location of a stimulus corresponds to the same action signaled by the goal-relevant color feature of the stimulus; this facilitates reaction time and accuracy. Right panel depicts non-corresponding (Nc) trials in which the stimulus location activates an action impulse that conflicts with the correct action signaled by the stimulus color, which produces impulsive response errors and reaction time interference on correct trials. The detrimental influence of location-driven response impulses on the reaction times and accuracy rates of Nc trials relative to the facilitative influence on Cs trials is called the Simon effect.
The DPAS model specifies an analytical framework for dissociating two essential and temporally distinct cognitive processes that are masked in analyses of mean Simon effects. The first process, henceforth referred to as impulse capture, is assumed to occur very shortly after the onset of the imperative stimulus and to reflect the degree to which an individual’s response system is susceptible to capture by the activation of the location-driven action impulse. The strength of impulse capture is reflected in the proportion of fast, impulsive errors evident in plots of accuracy rates against RT (i.e., a conditional accuracy function) (Kornblum et al., 1990; van den Wildenberg et al., 2010; Wylie et al., 2010a). The second process is assumed to reflect top-down inhibitory control that is engaged more slowly and builds up to suppress the interference induced by an incorrect action impulse (Ridderinkhof, 2002a). Proficient inhibitory control is assumed to be most evident at the slow end of the RT distribution because it takes time for this control to emerge after it has been triggered by the incorrect action impulse. Plotting the magnitude of the Simon interference effect as a function of response speed (i.e., a delta plot) yields a pattern of increasing interference across fast to intermediate response latencies that is followed by a dramatic and statistically deviant reduction (c.f., Luce, 1986) in interference toward the slow end of the distribution (Proctor et al., 2011). The DPAS model asserts that the slope of the interference reduction at the slowest segment of the delta plot provides the most sensitive metric of the proficiency of inhibitory control over prepotent motor impulses.1 Empirical support for this assertion comes from several studies using both non-clinical and clinical populations (Burle et al., 2002; Ridderinkhof et al., 2005; Bub et al., 2006; Wijnen & Ridderinkhof, 2007; Wylie et al., 2007, 2009, 2010; for review, see Ridderinkhof, Wylie, & van den Wildenberg, 2011).
The Current Study
The aims of the study were to determine how the expression and suppression of impulsive motor actions among PD patients are (i) affected by dopamine agonist medication and (ii) differentially altered in individuals with current ICB. Patients with and without agonist-induced ICB completed the Simon task on two occasions, once under the influence of their agonist medication and once after being withdrawn from it temporarily. In our primary analyses we tested two competing hypotheses. First, if the effects of dopamine agonists on the cognitive control over actions are ameliorative, patients should show more proficient suppression of incorrect action impulses (i.e., less impulsive errors and a reduced Simon effect). If, however, agonists impair cognitive control and predispose to impulsive-compulsive behaviors, this predisposition should manifest itself through an increased commission of impulsive action errors (i.e., stronger impulse capture) and/or a reduced proficiency in suppressing interference from impulsive action tendencies. This pattern should be more pronounced among patients with active ICB than among patients who do not have these symptoms. In a secondary analysis, we studied the association of agonist dosage on the expression and suppression of action impulses. Last, we completed a third set of analyses inspired by growing evidence which suggests that cognitive effects of dopamine medication may depend on baseline cognitive performance (Cools and D’Esposito, 2011). Specifically, patients who perform proficiently while off their dopamine medication may show a decline in performance while on their dopamine medication, whereas patients who perform poorly while off their medication may show an improvement in performance while on their medication. If this differential pattern is demonstrated, it would suggest that treatment decisions may enhance or diminish cognitive control depending on inter-individual variation in baseline performance levels.
Methods
Participants
Thirty-eight (38) individuals with idiopathic PD participated in this study, all of whom were recruited from the patient population in the Movement Disorders Clinics at the University of Virginia and Vanderbilt University. They all met the following inclusion criteria: no history of (i) other neurological condition besides PD; (ii) bipolar affective disorder, schizophrenia, or other psychiatric condition known to compromise executive cognitive functions; or (iii) untreated or unstable mood disorder or medical condition known to interfere with cognition (e.g., diabetes, pulmonary disease). Participants were evaluated and diagnosed with idiopathic PD by a movement disorder neurologist, were being treated with the dopamine agonists pramipexole or ropinorole, and performed at a level on the Mini-Mental Status Exam (MMSE; Folstein et al., 1975) that revealed no evidence of dementia (all scores > 25). The severity of their motor symptoms was graded using the Unified Parkinson’s Disease Rating Scale (UPDRS) motor subscore; additionally, they all received a stage III rating or less using the Hoehn & Yahr scale (Hoehn and Yahr, 1967). Based on rating scale scores and neurological evaluation, each patient was considered to be experiencing mild to early moderate disease presentation. In addition to being treated with dopamine agonist medication to control their motor symptoms, 23 of the 38 patients were receiving levodopa co-therapy. The remaining 15 patients were on agonist monotherapy. All of the patients showed a positive medication response (i.e., a clinically-observed reduction in motor symptoms). Dosages for the dopamine medications were converted to levodopa equivalent daily dose (LEDD) values (Weintraub et al., 2006a). Six (6) patients were also being treated with anti-depressant medication. These patients, as well as the other participants, reported stable mood functioning. They all denied symptoms associated with major depression, both during the clinical interview and when they completed the experimental portions of the study. All participants had corrected-to-normal vision. They all provided informed consent prior to participating in the study in full compliance with the standards of ethical conduct in human investigation as regulated by the University of Virginia. Thirty-four of these patients completed additional cognitive tasks that are reported elsewhere (Claassen et al., 2011). The demographic characteristics of the participants in the current study are listed in Table 1.
Table 1.
Sample Characteristics
| All Patients | ICB Subgroups | |||
|---|---|---|---|---|
| ICB | No ICB | p-value | ||
| Sample Size | 38 | 19 | 19 | |
| Age (yrs) | 62.1 (7.0) | 60.9 (5.6) | 63.2 (8.1) | >.10 |
| Gender (M:F) | 19:19 | 10:9 | 9:10 | >.10 |
| Education (yrs) | 16.6 (2.6) | 16.8 (2.3) | 16.4 (2.9) | >.10 |
| MMSE | 28.8 (1.5) | 28.8 (1.5) | 28.8 (1.5) | >.10 |
| Disease Duration (yrs) | 7.6 (5.9) | 9.4 (6.8) | 5.9 (4.3) | .06 |
| UPDRS Motor Score | 16.8 (8.1) | 18.4 (7.4) | 15.3 (8.6) | >.10 |
| Agonist LEDD (mg) | 247 (138) | 277 (145) | 218 (128) | >.10 |
| Agonist Duration (yrs) | 3.7 (3.6) | 4.6 (4.0) | 2.8 (2.9) | >.10 |
| Daily Levodopa (mg) | -- | 584 (270) (n = 13) |
618 (220) (n = 10) |
>.10 |
MMSE = Mini-Mental Status Exam; UPDRS = Unified Parkinson’s Disease Rating Scale; LEDD = Levodopa Equivalent Daily Dosage (in mg); ICB = Impulse-Compulsive Behaviors
Patients were also classified with respect to the emergence of ICB coincident with dopamine agonist treatment. First, patients and their spouse/caregiver completed the self and informant screening versions of the Questionnaire for Impulsive-Compulsive Symptoms in Parkinson’s Disease (QUIP) that screens for the presence of any of several ICBs related to gambling, buying, sexual behavior, eating, hobbyism, punding, or medication use (Weintraub et al., 2009). A follow-up clinical interview firmly established the presence or absence of any ICBs, coincidence of ICBs with initiation of agonist pharmacotherapy, and the disruptive impact of these behaviors on daily functioning according to established criteria (Voon et al., 2006; Weintraub et al., 2009; Weintraub et al., 2006b). Patients were assigned to the ICB group if they were experiencing at least one of these behaviors, whereas patients who were not included in this group reported no such behaviors and did not meet criteria for ICB. The sample of ICB patients presented with compulsive gambling (16%), compulsive buying/shopping (53%), hypersexuality (53%), compulsive eating (42%), and compulsive hobbyism (68%). All but one patient presented with two or more ICBs.
Importantly, participants with ICB completed the study before withdrawing from or decreasing their agonist medication; thus, they performed the study while presenting with active ICB. After study completion, follow-up neurological visits confirmed that all patients with ICB showed a marked decline in ICB coincident with discontinuation or reduction of agonist medication. We recruited equal numbers of patients in both groups (n=19), each of whom completed 2 test sessions that took place on 2 separate visits. On one visit, they were tested while taking all of their dopamine enhancing medications and were in the optimal “on” phase of their medication cycle. On the other visit, they completed testing after an 18-24 hour withdrawal from their dopamine agonist medication. The order of visits was counterbalanced across participants. Patients on levodopa co-therapy were not withdrawn from this medication at either visit. Importantly, no changes in levodopa or agonist dosages or addition or discontinuation of either drug for clinical purposes were made at any time during study participation. As depicted in Table 1, the subgroups displayed similar disease characteristics.
Task and Procedures
The Simon task was administered using an IBM-compatible computer and a 17-inch monitor located at eye level approximately 1 meter in front of the participant. Participants were seated comfortably and grasped a handheld response grip in each hand that registered responses via a left or right thumb press made on a button at the end of each grip. They completed 4 blocks of 60 experimental trials that were preceded by a block of 60 practice trials. The beginning of a block of trials was signaled by the appearance of a small black, square-shaped fixation mark in the center of a light gray background on the computer screen. It remained on the screen for the entire duration of the block of trials. Within a variable duration of 1750 to 2250 milliseconds (ms) following the initial appearance of the fixation mark, a blue or green circle (diameter 2.1 cm; visual angle 1.20°) appeared .6 cm (.34° visual angle) to the left or to the right of fixation and remained on the screen until the participant either made a response or a 1500 ms time limit elapsed. Another variable interval of 1750 to 2250 ms passed following a response or expiration of the time limit before the next trial was initiated by the appearance of another blue or green circle. The end of a block of trials was indicated by the offset of the fixation mark. It took approximately 3-4 minutes to complete a block of trials. Participants were instructed to respond on the basis of a predetermined mapping between the color of the circle and a response hand (e.g., green circle = right-thumb press; blue circle = left-thumb press). The mappings between color and response hand were counterbalanced across participants. Participants were encouraged to maintain their gaze on the fixation mark because it helped them sustain their attention, and to try to respond as quickly as they could while maintaining a high level of accuracy (90-95%).
To elicit the Simon effect, two trial types were configured to manipulate the correspondence between the spatial location of the circle and the response signaled by its color (see Figure 1). For Corresponding (Cs) trials, the circle appeared to the side of fixation that matched the response side signaled by the color of the stimulus (e.g., a green circle calling for a right-hand response appeared to the right side of fixation). For Non-corresponding (Nc) trials, the circle appeared on the side of fixation opposite the side of the response signaled by the circle’s color (e.g., a green circle calling for a right-hand response appeared on the left side of fixation). Cs and Nc trial types were presented randomly, but with equal probability, within each block of trials. In total, participants completed 120 corresponding and 120 non-corresponding trials.
Statistical Techniques
Data outliers were addressed using methods described previously (Wylie et al., 2010a). Mean RT and square-root transformed accuracy data were submitted to separate repeated-measures ANOVAs. The within-subject experimental factors in the initial analysis were Correspondence (Corresponding, Non-corresponding) and Agonist (Off, On), with a between-subjects factor of Impulsive-Compulsive Behaviors (Present, Absent).
The strength of response capture by incorrect action impulses was inferred from the proportion of fast errors revealed in conditional accuracy functions (CAFs) that plot accuracy rates as a function of the entire RT distribution for each level of Correspondence. Accuracy rates for the fastest RT bin of the CAFs have been demonstrated to be the most sensitive measure of capture (van den Wildenberg et al., 2010). The proficiency of suppression was inferred from delta plots, which plot the Simon effect (i.e., mean RT for the non-corresponding condition minus mean RT for the corresponding condition) as a function of RT. The slope between the delta values of the two slowest RT bins was the primary dependent measure because this value has been demonstrated to be the most sensitive measure of the proficiency of inhibitory control over action impulses and associated with systematic differences in the activation of prefrontal areas (e.g., right inferior frontal cortex) which have been linked empirically to inhibitory action control and have known projections to basal ganglia structures (Davelaar, 2008; Forstmann et al., 2008a, b; Ridderinkhof et al., 2002a; for review, see van den Wildenberg et al., 2010).
The aforementioned values derived from the CAFs and the delta plots were then submitted to separate repeated-measures ANOVAs to examine factor effects on the expression and suppression of action impulses, respectively. Our detailed methods for computing and analyzing CAFs and delta plots derived from the Simon task can be found elsewhere (Wylie et al., 2010a, b; van den Wildenberg et al., 2010). Pearson correlations were computed to test associations between agonist dosage and key performance variables. Statistical techniques were also used to assess the impact of baseline inhibitory control performance in the off medication state on the effects of performance in the on agonist state. These techniques tested the alternative explanation of baseline effects in terms of regression to the mean.
Patients prescribed levodopa co-therapy took their levodopa medication as usual during on and off dopamine agonist testing sessions. Thus, in the off agonist state, some patients performed under the acute influence of levodopa whereas patients prescribed agonist monotherapy performed the off agonist session completely withdrawn from dopamine modifying medications. To determine the effect of levodopa status on performance variables and its potential interaction with agonist state, we re-analyzed mean and distributional data with the factor Levodopa (none, present) included as a between-subjects variable. As the presence or absence of Levodopa did not influence the pattern of results, we present data that collapses across Levodopa status (see analyses in Supplementary Material).
Results
Table 1 lists overall patient characteristics as well as characteristics for subgroups based on the presence or absence of ICB. Notably, these subgroups did not differ in any of the listed clinical features; the only exception was a trend toward longer disease duration among patients presenting with ICB compared to those without ICB. We next present the results from the Simon task.
Dopamine Agonist Medication and Impulsive-Compulsive Behaviors
Mean Interference Effects on RT and Accuracy
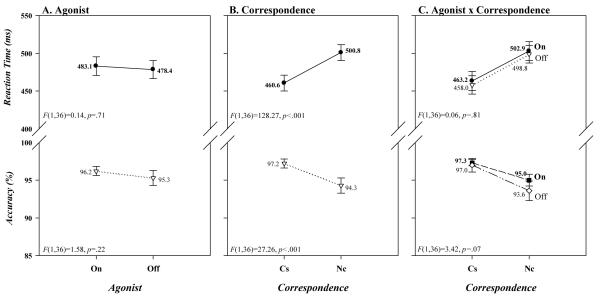
It is apparent in Figure 2A that the mean response latencies and accuracy rates of PD patients were unaffected by their dopamine agonist medication state ([On vs. Off: RT 483 vs. 478 ms; Accuracy 96.2 vs. 95.3%] Agonist: RT, F(1,36)=0.14, p=.71; Accuracy, F(1,36)=1.58, p=.22). Similarly, as depicted in the upper panels of Figure 2B and C, a robust Simon effect was produced among these subjects on RT ([40 ms] Correspondence, F(1,36)=128.27, p<.001) that was unaffected by agonist state ([On 40 ms; Off 41 ms] Agonist x Correspondence, F(1,36)=0.06, p=.81). Varying somewhat from this pattern, as illustrated in the bottom panels of Figure 2B and C, the strong Simon effect on accuracy ([-3%] Correspondence: F(1,36)=27.26, p<.001) tended to increase when patients were off agonist medication ([On, −2.3%; Off, −3.4%]; Agonist x Correspondence, F(1,36)=3.42, p=.07). Overall, treatment with a dopamine agonist had no effect on mean response latency and accuracy, and had minimal, if any, influence on the Simon effect.
Figure 2.
Mean RTs and accuracy rates (% correct) for the entire sample of PD patients as a function of (A) agonist state (on, off), (B) simon correspondence (corresponding [Cs], non-corresponding [Nc]), and (B) the interaction between agonist state and simon correspondence. Error bars reflect standard error of the means.
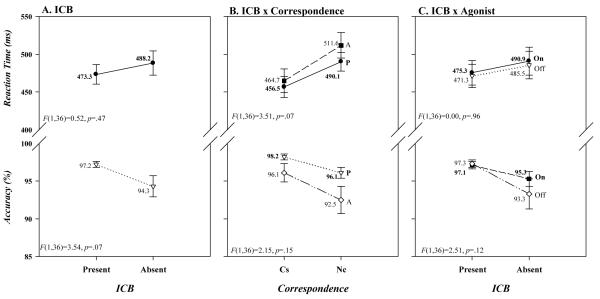
In Figure 3A it can be seen that although the presence or absence of ICB had no influence on overall mean RT (Present 473; Absent 488 ms), patients with these symptoms did tend to have higher overall mean accuracy rates ([97.2 vs. 94.3%] ICB: RT, F(1,36)=0.52, p=.47; Accuracy, F(1,36)=3.54, p=.07); and, as shown in Figure 3C, these patterns were not altered by agonist state (ICB x Agonist: RT, F(1,36)=0.00, p=0.96; Accuracy, F(1,36)=2.51, p=.12). Similarly, the magnitude of the Simon effect on accuracy, depicted in Figure 3B, was not related to the presence of ICB (Present −2.1%, Absent −3.6%), whereas the effect tended to be reduced on RT among patients who were symptomatic ([Present 34 ms vs. Absent 47 ms] ICB x Correspondence: RT, F(1,36)=3.51, p=.07; Accuracy, F(1,36)=2.14, p=.15). Notably, these patterns were not influenced by agonist medication state for either RT or accuracy (ICB x Agonist x Correspondence: RT, F(1,36)=0.31, p=.58; Accuracy, F(1,36)=1.44, p=.24).
Figure 3.
Mean RTs and accuracy rates (% correct) for the entire sample of PD patients as a function of (A) impulsive-compulsive behaviors (ICB; present, absent), (B) the interaction between ICB (P=present, A=absent) and simon correspondence (Cs, Nc), and (C) the interaction between ICB and agonist state (on, off). Error bars reflect standard error of the means.
We next turn to distributional analyses for the more focused insights they may provide about the expression and suppression of action impulses.
Response Capture by Incorrect Action Impulses
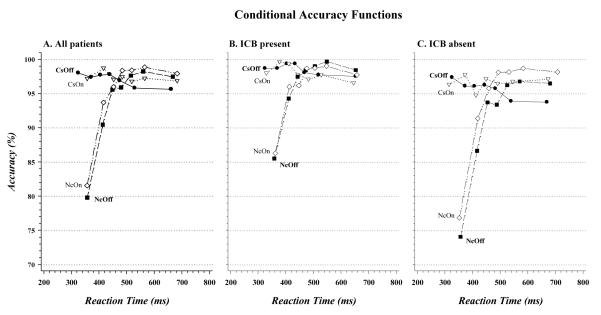
Visual examination of the conditional accuracy functions (CAF) in Figure 4A highlights, as we have reported in our previous work, the absence of uniformity in accuracy across the RT distribution. To analyze these patterns, we first included all bins of the CAF in the analysis (Bins factor) before focusing on patients’ accuracy rates at the fastest RT bin for corresponding and non-corresponding trials when they were either on or off agonist medication. We report the interaction terms containing the Bins factor as the relationships between the other factors remained consistent with the mean accuracy analyses.
Figure 4.
Conditional accuracy functions for corresponding (Cs) and non-corresponding (Nc) trial types as a function of dopamine agonist state (off versus on) for (A) all patients, (B) patients with impulsive-compulsive behaviors (ICB), and (C) patients without ICB. Across panels, it is clear that errors are associated with the fastest reaction times on non-corresponding (Nc] trials, but that the pattern of error rates does not differ between agonist states. Panels B and C indicate that patients with ICB made fewer fast, impulsive action errors than patients without ICB.
Figure 4A reveals a striking difference in the percentage of errors for corresponding and non-corresponding trials across the bins of the RT distribution (Bins x Correspondence: F(6,31)=10.76, p<.001). On non-corresponding trials, a pronounced pattern of fast errors was followed by a dramatic reduction in errors at intermediate and slow speeds. In contrast, the entire range of response latencies for corresponding trials was associated with low error rates. It is apparent as well that the patterns of errors across bins were not influenced by agonist state (Agonist x Bins: F(6,31)=1.15, p=.36; Agonist x Bins x ICB: F(6,31)=0.36, p=.90; Agonist x Bins x Correspondence: F(6,31)=1.01, p=.44; Agonist x Bins x ICB x Correspondence: F(6,31)=0.97, p=.46). Even a focused analysis on the fastest bin of accuracy rates confirmed that the higher percentage of fast errors on non-corresponding than on corresponding trials (Correspondence, F(1,36)=58.13, p<.001) was unaltered by agonist state (Agonist x Correspondence, F(1,36)=0.69, p=.41). Within the conceptual framework of the DPAS model, these results support the conclusion that patients experienced early response capture by incorrect action impulses on non-corresponding trials that was not influenced by dopamine agonist medication.
As is apparent in Figure 4B and C, the presence or absence of ICB produced differential patterns of error rates for non-corresponding and corresponding trials across the RT distribution (ICB x Bins x Correspondence: F(6,31)=2.86, p<.05). Guided by the DPAS model, analyzing accuracy rates for the fastest bin of trials showed that the percentage of fast errors was lower among patients with ICB present as opposed to patients without ICB (ICB, F(1,36)=4.40, p<.05). Importantly, this effect varied with the correspondence of the stimulus-response mapping (ICB x Correspondence: F(1,36)=5.03, p<.05). Specifically, both patient groups had comparably low fast error rates on corresponding trials (~2-3%), whereas patients without ICB had a much higher percentage of fast errors on non-corresponding trials than did patients with ICB (25 vs. 14%)(F(1,36)=4.93, p<.05). This suggests that patients with ICB experienced less initial response system capture by the activation of incorrect action impulses than did patients who were not manifesting ICB symptoms. Notably, these differences in fast errors were unaffected by agonist state (ICB x Agonist: F(1, 36)=.05, p=.83), irrespective of stimulus-response correspondence (ICB x Agonist x Correspondence: F(1, 36)=.13, p=.72).
Suppressing Interfering Action Impulses
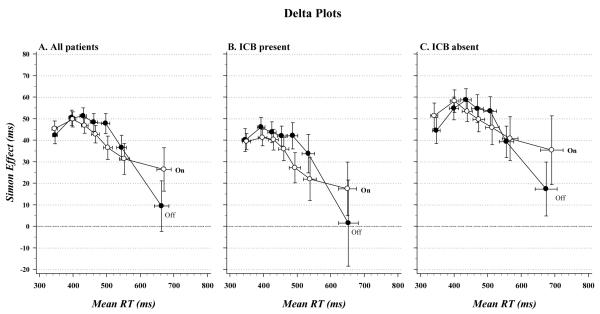
Consistent with our previous work, the delta plots in Figure 5A reveal variations in the size of the Simon effect across the RT distribution. As predicted by the DPAS model, the magnitude of the Simon effect produced by the initial activation of an incorrect action impulse was modulated by the hypothesized gradual buildup of inhibitory control, the result of which is a precipitous reduction in the Simon effect for the slowest RTs. The slope of the segment connecting the final two delta values of the delta plot provides the most sensitive metric of the proficiency of inhibitory control. We first included slopes from all segments of the delta plot in the analysis (Segment factor) before focusing on a comparison of the slopes from the final delta segment.
Figure 5.
RT delta plots as a function of agonist state for (A) all patients, (B) patients with impulsive-compulsive behaviors (ICB), and (C) patients without ICB. Across panels, delta slopes diverge at the slow end of the distribution, indicating more proficient suppression (steeper negative-going delta slope) of action impulses in the off agonist state and less proficient suppression under the acute influence of dopamine agonist medication. Suppression slopes were uninfluenced by the presence or absence of ICB.
As can be seen in Figure 5A, early in processing the slopes of the delta plot were positive, indicating an initial increase in interference, whereas later in processing the slopes were negative, reflecting suppression of that early interference (Segment, F(5,32)=14.20, p<.001). This overall form of the delta plot was not influenced by agonist medication state (Agonist, F(1,36)=0.02, p=.89). However, agonist medication state did produce differential effects on the slopes of the segments across the RT distribution (Agonist x Segment, F(5,32)=3.09, p=.007). It is evident in Figure 5A that the slope of the final segment of the delta plot, which is most sensitive to inhibitory proficiency, is less negative-going when patients were on their agonist (m = −0.05, SEM = .05) as opposed to when they off their agonist (m = −0.23, SEM = .06) F(1,36)=4.40, p<.05). According to the DPAS model, this suggests that patients were less effective at suppressing interference when they were under the influence of their agonist medication.
The delta plots derived separately for patients with and without ICB are shown in Figure 5B and Figure 5C. The presence or absence of these symptoms did not influence the forms of the delta plots when all slopes were included in the analysis (all ps>.25). Moreover, final delta slope values were similar between patients with ICB (m = .15) and patients without ICB (m = .13) (ICB: F(1,36)=0.08, p=.78), and this pattern was not affected differentially by agonist state (ICB x Agonist: F(1,36)=0.31, p=.58). This pattern of effects confirms that the temporal dynamics of response suppression did not vary with the presence or absence of ICB.
Association of Agonist Dosage to the Expression and Suppression of Action Impulses
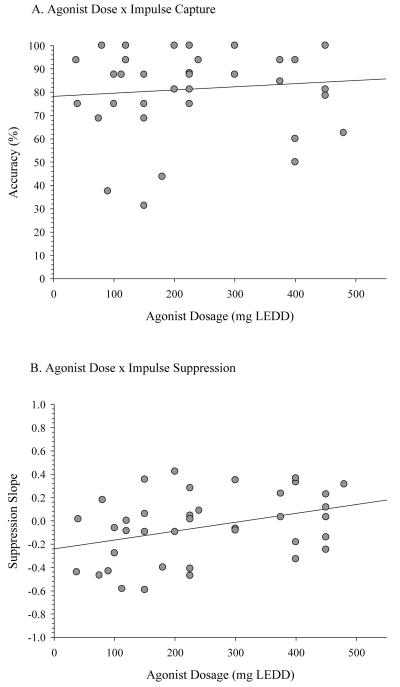
Correlational analyses focused on the association between daily agonist dose (expressed in milligrams of levodopa equivalent daily dosage or LEDD) and both the strength of response capture by action impulses (i.e., fast errors) and the proficiency of suppression (i.e., final delta slope) during the on agonist state. Agonist dosage was unrelated to the strength of response capture (r=.11, p=.52), but it was positively associated with the final slope value (r=.37, p=.02) (Figure 6). The latter is consistent with the interpretation that higher daily doses of dopamine agonist are associated with more positive delta slope values, and by inference, less proficient suppression of action impulses.
Figure 6.
Scatterplots depicting (A) the absence of an association between agonist dosage and fast, impulsive errors, and (B) the significant positive association (r=.37) between agonist dosage and the suppression slope from the final segment of the delta plot. In panel B, more positive slope values reflect less proficient inhibitory control. Agonist dosage is expressed in milligrams levodopa equivalent daily dose (LEDD).
Dependence of Agonist Effects on Baseline Performance
We tested whether agonist effects are dependent on baseline performance in the off agonist state. We predicted that individuals with low baseline proficiency of suppression in the off agonist state would show improved suppression under the influence of dopamine agonist medication, whereas individuals with high baseline proficiency of suppression in the off state would show a reduction in suppression in the on agonist state. This prediction was supported across the entire sample by a pronounced negative correlation between change and initial value (r=−.82; p<.001), indicating that high and low suppression values in the off agonist state were associated with reversed patterns in the on agonist state.
Ruling Out Alternative Explanations
It is tempting to interpret the strong correlation between initial value and change as an indication that effects of agonist medication depend on individual baseline performances. However, an alternative explanation for this correlation is that it is due to regression to the mean. That is, a correlation of −0.68 is already expected even in the absence of a genuine relationship between initial value and change (cf. Tu and Gilthorpe, 2007, p. 446). In order to rule out regression to the mean, we applied a conservative testing procedure that yields a more reliable test of the genuine relationship between initial value and change (Greenen and de Vijver, 1993; Tu and Gilthorpe, 2007). If in fact poor suppressors benefit and good suppressors worsen on agonist medication, it follows that the variance of the suppression measure should be significantly lower in the on than in the off medication state (cf. Myrtek and Foerster, 1986).2 The test of the equality of variances between the two conditions (Tu and Gilthorpe, 2007, equation 4) revealed that the variance of the suppression measure in the on medication state (variance = .09) was significantly lower than in the off state (variance = .16) (t(36)=1.68, p=.05), thus strengthening our confidence in the predicted relationship between change and initial value. This set of additional analyses shows that the observed agonist effects on suppression are driven by individual baseline differences and cannot be explained in terms of regression to the mean.
It is also possible that baseline differences in suppression in the off agonist state can be accounted for by individual differences in clinical or general performance factors. However, the proficiency of suppression in the off agonist state did not correlate with motor symptom severity, age, disease duration, agonist and total LEDD, time on agonist, mean RT, or fast errors (all ps>.15).
Discussion
The activation of incorrect motor impulses in the Simon task produced clear interference on non-corresponding trials as evidenced by a slowing of mean RT and a reduction in mean accuracy rates compared to corresponding trials. More importantly, the distributional analyses revealed patterns of effects that were not evident in the mean results and conformed to the predictions of the DPAS model. First, the conditional accuracy functions were characterized by a pattern of predominantly fast errors on non-corresponding trials that is consistent with rapid response system capture by incorrect action impulses which breech the threshold for response execution and escape inhibitory control. Second, on conflict trials in which incorrect activation did not produce an overt response error (i.e., a correct response was made) the delta plots nonetheless exposed RT interference that increased across the faster segments and then reversed dramatically at the slow end of the RT distribution. This steep reduction is consistent with a late effect of top-down inhibitory control that takes time to buildup within a trial and is most effective on slower reactions. These patterns, which replicate previous studies, provided the opportunity to directly test the effects of dopamine agonist state and susceptibility to impulsive-compulsive behaviors on both the expression and suppression of action impulses in PD patients.
Dopamine Agonists Can Impair Suppression of Action Impulses
The current data reveal differential effects of dopamine agonist medication on response capture by action impulses and inhibitory control engaged to suppress interference produced by this capture. As revealed by the conditional accuracy functions, susceptibility to making fast, impulsive errors on non-corresponding trials did not vary with agonist state. This argues against the interpretation that dopamine agonists alter the motor system by making it more susceptible to capture by stimulus-driven action impulses. In contrast, dopamine agonists disrupted cognitive control processes that are engaged reactively to suppress motor system interference by the activation of impulsive actions. Specifically, patients under the acute influence of their agonist were less proficient at suppressing interference (i.e., display a less negative-going delta slope toward the slow end of the RT distribution) compared to when they were temporarily withdrawn from agonist medication. Additionally, higher doses of agonist tended to correlate with less proficient suppression ability. Taken together, these findings suggest that agonists do not predispose speeded decision-making to stronger capture by stimulus-driven response impulses, but instead impair the ability to inhibit these actions from interfering with goal-directed behavior. Previous studies show that PD impairs inhibitory action control (Wylie et al., 2010a; Praamstra et al., 1998), but the current study demonstrates that a medication intended to ameliorate clinical motor symptoms can further disrupt this key component of the brain’s cognitive control system.
PD Agonist Effects on Inhibitory Action Control Depend on Baseline Performance
The effects of agonist medication on inhibitory control were sensitive to baseline performance when the patient was withdrawn from agonist medication. Specifically, patients who were most proficient at suppressing action impulses in the off agonist state experienced a significant reduction in inhibitory control when they were under the influence of the agonist. In contrast, patients who were relatively poor at suppressing action impulses when off of their agonist showed improved inhibitory control under the influence of an agonist. Analyses indicated that these effects could not be accounted for solely by regression to the mean. These paradoxical effects of agonist medication on cognitive control resemble patterns observed in other studies of dopamine effects on cognition that suggest that an inverted U-shaped curve accounts for some relationships between cognitive performance and dopamine function (Cools and D’Esposito, 2011). This account rests on the assumption that peak performance on cognitive measures sensitive to dopamine function depends on optimal dopamine levels, but that reductions (e.g., due to pathology) or overdoses (e.g., due to medications) of dopamine lead to suboptimal cognitive performance (Cools et al., 2001a; Gotham, Brown, & Marsden, 1988; Swainson et al., 2000). Based on this account, patients with dopamine pathology that affects inhibitory control circuits of the basal ganglia would be expected to show poor inhibitory control in the off agonist state that could be partially restored when dopamine levels are increased by agonist medication. In contrast, proficient inhibitory control in the off agonist state suggests a relative sparing of dopamine pathology in inhibitory control circuits of the basal ganglia. With the addition of agonist medication, the enhanced dopamine levels would then “overdose” these circuits and impair inhibitory control performance. Similar patterns of effects in PD have been demonstrated in studies of working memory and reversal learning (Cools and D’Esposito, 2011; Cools et al., 2001a, b; Costa et al., 2001).
Patients With Impulsive-Compulsive Behaviors Are Not More Susceptible to Motor Impulses
A complementary aim of the study was to determine if the emergence of ICB involves a specific deficit in impulse control over prepotent action impulses? Our results indicate that patients displaying ICB did not experience either stronger activation of action impulses or a greater reduction in inhibitory control under the influence of a dopamine agonist relative to patients without ICB. These patterns argue against the notion that the emergence of ICB involves increased susceptibility to impulsive motor behavior (see also Djamshidian et al., 2011). Quite to the contrary, patients with ICB showed a trend toward reduced interference and committed fewer fast errors on non-corresponding trials compared to patients without ICB, suggesting that these patients were actually less susceptible to impulse capture by the processing of irrelevant stimulus information. In fact, despite similar RTs, the ICB patients in this study committed fewer fast, impulsive action errors than healthy controls without PD whose data were reported in two previous studies that used a similar experimental task and design (Wylie et al., 2010a, b). Given that the DPAS model asserts that early response capture reflects the strength of bottom-up activation of a conflicting response, this might suggest that ICB patients are either more effective at fast action selection under conflict or experience less buildup of stimulus-driven response activation. While this finding requires additional investigation, it seems to suggest that control over impulsive manual actions as revealed by the Simon task may not be sensitive to the impulsive behaviors displayed by patients with ICB.
Other forms of impulsivity outside of the motor domain may be more important to understanding the mechanisms underlying ICB in PD, such as an inclination toward taking higher risks, difficulties restraining pursuit of immediate rewards and delaying action to obtain higher rewards, pursuing immediate pleasures with little forethought about potential negative consequences, or issuing decisions before all relevant information is obtained (Evenden, 1999). In fact, “impulsive action” and “impulsive decision-making” are argued to involve distinct time courses, mediating influences (e.g., reward, risk), and underlying neural mechanisms (Eagle and Baunez, 2010). The emergence of ICB in PD appears to be driven largely by impulsive decision-making as ICB patients show a greater propensity for anticipating and learning from rewarding experiences, preferring smaller immediate rewards over larger delayed rewards, and taking greater risks to obtain reward under the influence of agonists (Claassen et al., 2011; Voon et al., 2011, Housden et al., 2010; Milenkova et al., 2011; Voon et al., 2010). Imaging studies of patients with ICB also point to distinguishing features in mesolimbic function, which is implicated in reward, risk, and outcome-based learning and decision-making (van Eimeren et al., 2010, Rao et al., 2010; Steeves et al., 2009; Thiel et al., 2003; O’Sullivan et al., 2011). Thus, the absence of an ICB influence on inhibitory action control may be due to the fact that the specific vulnerability in this group of patients is linked closely to abnormal mesolimbic activity.
Potential Agonist Effects on the Neural Mechanisms of Inhibitory Control
Animal studies indicate that dopamine’s influence on inhibitory control over motor behavior involves its modulating influence in dorsal (via nigrostriatal pathways) as opposed to ventral (via mesocorticolimbic pathways) striatal regions (Eagle and Robbins, 2003; Eagle et al., 2011). The posterior regions of the dorsal striatum (e.g., the putamen), which are tightly linked to basic motor processes, are affected earliest by the dopamine pathology in PD (Kaasinen & Rinne, 2002; McAuley, 2003). Cognitive control functions, including inhibitory action control, are linked to relatively anterior regions of the dorsal striatum (e.g., caudate nucleus), which can be impacted quite variably across early to moderate stages of PD (Kaasinen & Rinne, 2002; Lewis, Dove, Robbins, Barker, & Owen, 2003). Across individuals, differential degrees of dopamine depletion in this region may explain the paradoxical response to agonist medication described above (Rowe et al., 2008). Alternatively, the detrimental effect of agonist medication on inhibitory control may reflect its differential impact on D1-mediated pathways (i.e., direct or go routes), which give rise to action selection, and D2-mediated pathways (indirect or no-go routes), which are involved in the selective suppression of actions (Aron, 2007; Aron and Verbruggen, 2008; Claffey et al., 2010). Dopamine agonists have a higher affinity for D2-like receptors, potentially driving the bias of activity toward the inhibition of the indirect or no-go pathways (Dodd et al., 2005; Black et al., 2002; Frank, 2005). Since the activation of D2 receptors putatively inhibits the indirect pathway, the net effect would be a reduction in selective suppression.
At a broader network level, studies of neural activation patterns associated with response interference trials on conflict tasks (e.g., Simon and Flanker tasks) highlight the involvement of fronto-parietal and fronto-striatal networks (e.g., Casey et al., 2000; Pardo et al., 1990; Peterson et al., 2002; Hazeltine et al., 2003; Schumacher et al., 2007; for a meta-analysis see Nee et al., 2007; for reviews see Ridderinkhof et al., 2004b). Conflict trials afford two competing actions, one prepotent and the other goal-directed. These action affordances are likely instantiated by circuits connecting posterior parietal to premotor cortices, which are thought to form the basis of association-driven visuomotor transformations (Ridderinkhof et al., 2011; Sturmer et al., 2007). The resolution of this conflict may come from different sources, including lateral inhibition within motor areas or by an enhancement of activation in brain areas involved in directing action selection, such as the pre-supplementary motor area (pre-SMA)(Cisek, 2007; Ullsperger & von Cramon, 2001). In fact, individual differences in the susceptibility to capture by the prepotent response in the Simon task are accompanied by stronger activation in the pre-supplementary motor area (pre-SMA), which is consistent with a heightened demand on action selection (Forstmann et al., 2008a).
The resolution of conflict in the Simon task also depends critically on the engagement of neural circuitry involved in top-down inhibitory action control (Burle et al., 2004; van den Wildenberg et al., 2010). For example, healthy adults who are more proficient at suppressing impulsive actions in the Simon task (i.e., have a steeper negative-going final delta slope) show greater activation of the right inferior frontal cortex (rIFC) (Forstmann et al., 2008a). Interestingly, activation patterns in the pre-SMA that were linked to fast response capture were unrelated to variations in the proficiency of suppression. Other imaging studies have also highlighted involvement of rIFC in selective inhibitory control in situations of action conflict (Davelaar, 2008; Jahfari et al., 2011; Forstmann et al., 2008b). These findings are consistent with existing models describing a central role for rIFC and its efferent projections to the basal ganglia in inhibitory action control (see Aron et al., 2007). The current findings also suggest the possibility that these putative inhibitory control circuits may be modulated directly by dopamine agonist medication in PD patients.
Study Limitations and Extant Issues
There are a few limitations and extant issues worth addressing. We measured the acute influence of dopamine agonists, thus the chronic effects of agonists on impulse and inhibitory control remain unknown. Although an 18-24 hour withdrawal period was sufficient to reveal agonist effects in the current study, it remains an open question as to how washout periods of different durations impact cognitive performance in PD. We did not manipulate levodopa administration, and the majority of patients was taking levodopa and remained under the influence of this dopamine medication during both testing sessions. Thus, dopaminergic activity was still impacted by levodopa even when patients were withdrawn from their agonists. Notably, the between-subjects factor accounting for levodopa status did not influence the dynamics of response capture or suppression of action impulses (see Supplementary Material). In animal work, levodopa has been shown to alter the expression of impulsive behavior (Pattij and Vanderschuren, 2008). Neuropsychological studies comparing levodopa and agonist effects have produced mixed results (Brusa et al., 2003). Future studies of cognitive control in PD would clearly benefit from within-subject designs that test patient performance under the selective influence of dopamine modifying medications. The ICB grouped included patients presenting with various forms of impulsive-compulsive behaviors, which could introduce an important source of variability in impulsive motor control. The mechanisms underlying the variable expressions of ICB and the contextual factors that play a role in the development of ICB remain poorly understood. We considered potential differences between two broad subgroups of ICB patients, specifically patients presenting with primary problems controlling gambling and buying behaviors (n=10) versus patients presenting primarily with compulsive sexuality, eating, and/or hobbyism (n=9), but these subgroups showed no significant differences in performance. In fact, both groups showed a similar reduction in fast, impulsive errors compared to patients without ICB in the present study and healthy controls from previous work.
In a previous study, we showed that deep brain stimulation (DBS) of the subthalamic nucleus (STN) in PD patients with moderate motor symptoms improved the proficiency of inhibitory control over action impulses in the Simon task (Wylie et al., 2010b) and prepotent responses (van den Wildenberg et al., 2006). How can we reconcile the apparent discrepancies between the effects of agonist medication and of STN DBS on suppression? It is important to point out that patients in the STN DBS study showed poor suppression when stimulation was not being delivered; in fact, the performance of this group of patients was similar to the patients in the current study who were poor suppressors when they were not taking their agonist medication. Thus, both of these groups of poor suppressors benefitted from their respective treatment. This suggests that these treatments may result in a common final effect, but this improvement in suppression is best realized among patients who are poor at suppressing when their treatment is withdrawn and likely suffering from advanced dopamine depletion in cognitive control circuits of the basal ganglia (Wylie et al., 2010a).
Conclusions
Individuals with PD are generally less proficient at suppressing involuntarily activated action impulses. This study offers empirical demonstration that dopamine agonists alter the proficiency of inhibiting action impulses, and these effects depend on baseline performance. The presence of ICB did not exacerbate impulsive response errors or difficulties inhibiting interfering response impulses; in fact, ICB patients showed reduced susceptibility to acting on motor impulses. The development of tools to measure motor and cognitive functions that are sensitive to dopamine medication is essential to formulating treatment decisions that optimize basic motor and cognitive control processes in PD patients. This is particularly true of dopamine agonist medication, which can impact motor, cognitive, affective, and reward processing functions, as well as predispose to rather dramatic and disruptive behavioral changes.
Supplementary Material
Acknowledgements
We thank Bert van Beek for programming the computer task.
Funding
National Institute on Aging (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health) grant (K23AG028750 to S.A.W.); American Academy of Neurology Clinical Research Fellowship (to D.O.C.); The Netherlands Organization for Scientific Research grants (to K.R.R. and to W.P.M.v.d.W); Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. grant (to T.R.B.).
Footnotes
The DPAS model is agnostic about what happens precisely at intermediate bins of the RT distribution apart from the general expectation that increasing interference effects across early and middle latencies of the RT distribution will turn into a negative-going slope at the slow end of the distribution. The DPAS model specifies that the dynamic change in the magnitude of the interference effect as a function of time is best captured by the slope of the delta plot for slow RTs (i.e. the final delta slope). For a detailed review of empirical evidence supporting this conjecture, see van den Wildenberg et al. (2010).
Since this analysis method is sensitive to differences in measurement error between conditions, we first tested the underlying assumption that the measurement error was equally distributed across medication state conditions. For each participant, we computed the standard deviation of RT for each level of Bin, Correspondence, and Agonist and submitted these values to a repeated-measures ANOVA. This analysis verified that dopamine agonist administration did not systematically affect RT variability (F < 1 for main effect of Agonist and all interactions with Agonist).
References
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27(14):3743–52. doi: 10.1523/JNEUROSCI.0519-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Verbruggen F. Stop the presses: dissociating a selective from a global mechanism for stopping. Psychol Sci. 2008;19(11):1146–53. doi: 10.1111/j.1467-9280.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- Aron AR. The neural basis of inhibition in cognitive control. Neuroscientist. 2007;13(3):214–28. doi: 10.1177/1073858407299288. [DOI] [PubMed] [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, et al. A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci U S A. 2002;99(26):17113–8. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Black KJ, Hershey T, Koller JM, Videen TO, Mintun MA, Price JL, Perlmutter JS. A possible substrate for dopamine-related changes in mood and behavior: Prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci USA. 2002;99(26):17113–17118. doi: 10.1073/pnas.012260599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusa L, Bassi A, Stefani A, Pierantozzi M, Peppe A, Caramia MD, et al. Pramipexole in comparison to l-dopa: a neuropsychological study. J Neural Transm. 2003;110(4):373–80. doi: 10.1007/s00702-002-0811-7. [DOI] [PubMed] [Google Scholar]
- Bub DN, Masson MEJ, Lalonde CE. Cognitive control in children: stroop interference and suppression of word reading. Psychol Sci. 2006;17:351–357. doi: 10.1111/j.1467-9280.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- Burle B, Possamai CA, Vidal F, Bonnet M, Hasbroucq T. Executive control in the Simon effect: an electromyographic and distributional analysis. Psychol Res. 2002;66(4):324–336. doi: 10.1007/s00426-002-0105-6. [DOI] [PubMed] [Google Scholar]
- Burle B, Vidal F, Tandonnet C, Hasbroucq T. Physiological evidence for response inhibition in choice reaction time tasks. Brain Cogn. 2004;56:153–164. doi: 10.1016/j.bandc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 2000;97:8728–8733. doi: 10.1073/pnas.97.15.8728. 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P. Cortical mechanisms of action selection: the affordance competition hypothesis. Philos Trans R Soc Lond B Biol Sci. 2007;362(1485):1585–99. doi: 10.1098/rstb.2007.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen DO, van den Wildenberg WP, Ridderinkhof KR, Jessup CK, Harrison MB, Wooten GF, et al. The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behav Neurosci. 2011;125(4):492–500. doi: 10.1037/a0023795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claffey MP, Sheldon S, Stinear CM, Verbruggen F, Aron AR. Having a goal to stop action is associated with advance control of specific motor representations. Neuropsychologia. 2010;48(2):541–8. doi: 10.1016/j.neuropsychologia.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001a;11(12):1136–43. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001b;124(12):2503–12. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69(12):113–25. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Peppe A, Dell’Agnello G, Carlesimo GA, Murri L, Bonuccelli U, et al. Dopaminergic modulation of visual-spatial working memory in Parkinson’s disease. Dement Geriatr Cogn Disord. 2003;15(2):55–66. doi: 10.1159/000067968. [DOI] [PubMed] [Google Scholar]
- Davelaar EJ. A computational study of conflict-monitoring at two levels of processing: reaction time distributional analyses and hemodynamic responses. Brain Res. 2008;1202:109–19. doi: 10.1016/j.brainres.2007.06.068. [DOI] [PubMed] [Google Scholar]
- Djamshidian A, O’Sullivan SS, Lees A, Averbeck BB. Stroop test performance in impulsive and non impulsive patients with Parkinson’s disease. Parkinsonism Relat Disord. 2011 Mar;17(3):212–4. doi: 10.1016/j.parkreldis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson disease. Arch Neurol. 2005 Sep;62(9):1377–81. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Baunez C. Is there an inhibitory-response-control system in the rat? Evidence from anatomical and pharmacological studies of behavioral inhibition. Neurosci Biobehav Rev. 2010;34(1):50–72. doi: 10.1016/j.neubiorev.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003;117(6):1302–17. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW. Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neurosci. 2011;31(20):7349–56. doi: 10.1523/JNEUROSCI.6182-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci. 2008a;28(39):9790–6. doi: 10.1523/JNEUROSCI.1465-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmann BU, van den Wildenberg WP, Ridderinkhof KR. Neural mechanisms, temporal dynamics, and individual differences in interference control. J Cogn Neurosci. 2008b;20(10):1854–65. doi: 10.1162/jocn.2008.20122. [DOI] [PubMed] [Google Scholar]
- Frank MC, Piedad J, Richards H, Cavanna AE. The role of impulse control disorders in Tourette syndrome: an exploratory study. J Neurol Sci. 2011;310:276–78. doi: 10.1016/j.jns.2011.06.032. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Dynamic dopamine modulation in the basal ganglia: a neurocomputational account of cognitive deficits in medicated and nonmedicated Parkinsonism. J Cogn Neurosci. 2005;17(1):51–72. doi: 10.1162/0898929052880093. [DOI] [PubMed] [Google Scholar]
- Gauggel S, Rieger M, Feghoff TA. Inhibition of ongoing responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2004;75(4):539–44. doi: 10.1136/jnnp.2003.016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen R, van de Vijver FJR. A simple test of the law of initial values. Psychophysiology. 1993;30:525–530. doi: 10.1111/j.1469-8986.1993.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. Frontal cognitive function in patients with Parkinson’s disease on and off levodopa. Brain. 1988;111:299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Grant JE, Mancebo MC, Pinto A, Eisen JL, Rasmussen SA. Impulse control disorder in adults with obsessive compulsive disorder. J Psychiatr Res. 2006;40(6):494–501. doi: 10.1016/j.jpsychires.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Hazeltine E, Diedrichsen J, Kennerley SW, Ivry RB. Bimanual cross-talk during reaching movements is primarily related to response selection, not the specification of motor parameters. Psychol Res. 2003;67(1):56–70. doi: 10.1007/s00426-002-0119-0. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Neural systems for control of voluntary action--a hypothesis. Adv Biophys. 1998;35:81–102. [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hommel B. Attention and spatial stimulus coding in the Simon task. Acta Psychol. 2011;136:265–268. doi: 10.1016/j.actpsy.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Housden CR, O’Sullivan SS, Joyce EM, Lees AJ, Roiser JP. Intact reward learning but elevated delay discounting in Parkinson’s disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology. 2010;35(11):2155–64. doi: 10.1038/npp.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahfari S, Waldorp L, van den Wildenberg WP, Scholte HS, Ridderinkhof KR, Forstmann BU. Effective connectivity reveals important roles for both the hyperdirect (fronto-subthalamic) and the indirect (fronto-striatal-pallidal) fronto-basal ganglia pathways during response inhibition. J Neurosci. 2011;31(18):6891–9. doi: 10.1523/JNEUROSCI.5253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasinen V, Rinne JO. Functional imaging studies of dopamine system and cognition in normal aging and Parkinson’s disease. Neurosci Biobehav Rev. 2002;26:785–793. doi: 10.1016/s0149-7634(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Kaladjian A, Jeanningros R, Azorin JM, Anton JL, Mazzola-Pomietto P. Impulsivity and neural correlates of response inhibition in schizophrenia. Psychol Med. 2011;41(2):291–9. doi: 10.1017/S0033291710000796. [DOI] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: cognitive basis for stimulus-response compatibility--a model and taxonomy. Psychol Rev. 1990;97(2):253–70. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Cogn Neurosci. 2003;23:6351–6356. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luce RD. Response Times: Their Role in Inferring Elementary Mental Organization. Oxford Science Publications; New York: 1986. [Google Scholar]
- McAuley JH. The physiological basis of clinical deficits in Parkinson’s disease. Prog Neurobiol. 2003;69:27–48. doi: 10.1016/s0301-0082(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Milenkova M, Mohammadi B, Kollewe K, Schrader C, Fellbrich A, Wittfoth M, et al. Intertemporal choice in Parkinson’s disease. Mov Disord. 2011;26:2004–2010. doi: 10.1002/mds.23756. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia intrinsic circuits and their role in behavior. Curr Opin Neurobiol. 1993;3(6):950–7. doi: 10.1016/0959-4388(93)90167-w. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Myrtek M, Foerster F. The law of initial value: a rare exception. Biol Psychol. 1986;22:227–237. doi: 10.1016/0301-0511(86)90028-1. [DOI] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- O’Sullivan SS, Wu K, Politis M, Lawrence AD, Evans AH, Bose SK, et al. Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive-compulsive behaviours. Brain. 2011;134(4):969–78. doi: 10.1093/brain/awr003. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Pardo PJ, Janer KW, Raichle ME. The anterior cingulate cortex mediates processing selection in the Stroop attentional conflict paradigm. Proc Natl Acad Sci U S A. 1990;87(1):256–9. doi: 10.1073/pnas.87.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29(4):192–9. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, et al. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Res Cogn Brain Res. 2002;13(3):427–40. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Stegeman D, Cools A, Horstink MW. Reliance on external cues for movement initiation in Parkinson’s disease: Evidence from movement-related potentials. Brain. 1998;121:167–177. doi: 10.1093/brain/121.1.167. [DOI] [PubMed] [Google Scholar]
- Praamstra P, Plat EM, Meyer AS, Horstink MW. Motor cortex activation in Parkinson’s disease: dissociation of electrocortical and peripheral measures of response generation. Mov Disord. 1999;14(5):790–9. doi: 10.1002/1531-8257(199909)14:5<790::aid-mds1011>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group Pramipexole vs levodopa as initial treatment for Parkinson disease: A randomized controlled trial. JAMA. 2000;284(15):1931–8. doi: 10.1001/jama.284.15.1931. [DOI] [PubMed] [Google Scholar]
- Proctor RW, Miles JD, Baroni G. Reaction time distribution analysis of spatial correspondance effects. Psychon Bull Rev. 2011;18(2):242–66. doi: 10.3758/s13423-011-0053-5. [DOI] [PubMed] [Google Scholar]
- Rao H, Mamikonyan E, Detre JA, Siderowf AD, Stern MB, Potenza MN, et al. Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Mov Disord. 2010;25(11):1660–9. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE, 056 Study Group A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med. 2000;342(20):1484–91. doi: 10.1056/NEJM200005183422004. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89(4):1009–23. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56(2):129–40. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Micro- and macro-adjustments of task set: activation and suppression in conflict tasks. Psychol Res. 2002b;66(4):312–23. doi: 10.1007/s00426-002-0104-7. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR. Activation and suppression in conflict tasks: Empirical clarification through distributional analyses. In: Prinz W, Hommel B, editors. Common Mechanisms in Perception and Action. Attention & Performance. XIX. Oxford University Press; Oxford: 2002a. pp. 494–519. [Google Scholar]
- Ridderinkhof KR, Forstmann BU, Wylie SA, Burle B, van den Wildenberg WPM. Neurocognitive mechanisms of action control: resisting the call of the Sirens. Wylie Interdisciplinary Reviews Cognitive Science. 2011;2:174–192. doi: 10.1002/wcs.99. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Scheres A, Oosterlaan J, Sergeant J. Delta plots in the study of individual differences: new tools reveal response inhibition deficits in AD/HD that are eliminated by methylphenidate treatment. J Abnorm Psychol. 2005;114:197–215. doi: 10.1037/0021-843X.114.2.197. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cognition. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Winjnen J, Burle Boris, Posner M. Attention. Guillford Press; New York: 2004. Response inhibition is conflict tasks is revealed in delta plots. [Google Scholar]
- Ridderinkhof KR, Wylie SA, van den Wildenberg WPM. Action control in times of conflict: Analysis of reaction time distributions in healthy and clinical populations. In: Posner M, editor. Cognitive Neuroscience of Attention. 2nd Ed. Guilford Press; New York: 2011. [Google Scholar]
- Robbins TW, Brown VJ. The role of the striatum in the mental chronometry of action: a theoretical review. Rev Neurosci. 1990;2(4):181–214. doi: 10.1515/REVNEURO.1990.2.4.181. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BCP, Eckstein D, Williams-Gray CH, Fallon S, Barker RA, Owen AM. Parkinson’s disease and dopaminergic treatment—differential effects on movement, reward and cognition. Brain. 2008;131:2094–2105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher EH, Cole MW, D’Esposito M. Selection and maintenance of stimulus-response rules during preparation and performance of a spatial choice-reaction task. Brain Res. 2007;1136:77–87. doi: 10.1016/j.brainres.2006.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JR. Reactions toward the source of stimulation. J Exp Psychol. 1969;81(1):174–6. doi: 10.1037/h0027448. [DOI] [PubMed] [Google Scholar]
- Simon JR. The effects of an irrelevant directional cue on human information processing. In: Proctor RW, Reeve TG, editors. Stimulus-Response Compatibility: an integrated perspective. Elsevier Science Publishing Company, Inc.; New York: 1990. pp. 31–63. [Google Scholar]
- Steeves TD, Miyasaki J, Zurowski M, Lang AE, Pellecchia G, Van Eimeren T, et al. Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain. 2009;132(5):1376–85. doi: 10.1093/brain/awp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturmer B, Redlich M, Irlbacher K, Brandt S. Executive control over response priming and conflict: a transcranial magnetic stimulation study. Exp Brain Res. 2007;183(3):329–39. doi: 10.1007/s00221-007-1053-6. [DOI] [PubMed] [Google Scholar]
- Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38(5):596–612. doi: 10.1016/s0028-3932(99)00103-7. [DOI] [PubMed] [Google Scholar]
- Thiel A, Hilker R, Kessler J, Habedank B, Herholz K, Heiss WD. Activation of basal ganglia loops in idiopathic Parkinson’s disease: a PET study. J Neural Transm. 2003;110(11):d1289–301. doi: 10.1007/s00702-003-0041-7. [DOI] [PubMed] [Google Scholar]
- Tu YK, Gilthorpe MS. Revisiting the relation between change and initial value: A review and evaluation. STAT MED. 2007;26(16):3205–3206. doi: 10.1002/sim.2538. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: a dissociation of error processing and response competition revealed by event-related fMRI and ERPs. Neuroimage. 2001;14(6):1387–401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, van der Werf YD, Verhoef KM, de Wit S, Berendse HW, Wolters ECh, et al. Frontal-striatal abnormalities underlying behaviours in the compulsive-impulsive spectrum. J Neurol Sci. 2010;289:55–9. doi: 10.1016/j.jns.2009.08.043. [DOI] [PubMed] [Google Scholar]
- van den Wildenberg WP, Wylie SA, Forstmann BU, Burle B, Hasbroucq T, Ridderinkhof KR. To head or to heed? Beyond the surface of selective action inhibition: a review. Front Hum Neurosci. 2010;4:222. doi: 10.3389/fnhum.2010.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wildenberg WP, van Boxtel GJ, van der Molen MW, Bosch DA, Speelman JD, Brunia CH. Stimulation of the subthalamic region facilitates the selection and inhibition of motor responses in Parkinson’s disease. J Cogn Neurosci. 2006;18:626–636. doi: 10.1162/jocn.2006.18.4.626. [DOI] [PubMed] [Google Scholar]
- van Eimeren T, Pellecchia G, Cilia R, Ballanger B, Steeves TD, Houle S, et al. Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology. 2010;75:1711–6. doi: 10.1212/WNL.0b013e3181fc27fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Fox SH. Medication-related impulse control and repetitive behaviors in Parkinson disease. Arch Neurol. 2007;64(8):1089–96. doi: 10.1001/archneur.64.8.1089. [DOI] [PubMed] [Google Scholar]
- Voon V, Gao J, Brezing C, Symmonds M, Ekanayake V, Fernandez H, et al. Dopamine agonists and risk: impulse control disorders in Parkinson’s disease. Brain. 2011;134(5):1438–46. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254–7. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Pessiglione M, Brezing C, Gallea C, Fernandez HH, Dolan RJ, et al. Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron. 2010;65:135–142. doi: 10.1016/j.neuron.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Hoops S, Shea JA, Lyons KE, Pahwa R, Driver-Dunckley ED, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson’s disease. Mov Disord. 2009;24(10):1461–7. doi: 10.1002/mds.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol. 2010;67(5):589–95. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Potenza MN. Impulse control disorders in Parkinson’s disease. Curr Neurol Neurosci Rep. 2006b;6(4):302–6. doi: 10.1007/s11910-006-0022-y. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006a;63(7):969–73. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnen JG, Ridderinkhof KR. Response inhibition in motor and oculomotor conflict tasks: different mechanisms, different dynamics? Brain Cogn. 2007;63:260–70. doi: 10.1016/j.bandc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Bashore TR, van den Wildenberg WP. The effect of Parkinson’s disease on the dynamics of on-line and proactive cognitive control during action selection. J Cogn Neurosci. 2010a;22(9):2058–73. doi: 10.1162/jocn.2009.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WP, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, et al. The effect of speed-accuracy strategy on response interference control in Parkinson’s disease. Neuropsychologia. 2009a;47(8-9):1844–53. doi: 10.1016/j.neuropsychologia.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WP, Ridderinkhof KR, Bashore TR, Powell VD, Manning CA, et al. The effect of Parkinson’s disease on interference control during action selection. Neuropsychologia. 2009b;47(1):145–57. doi: 10.1016/j.neuropsychologia.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Elias WJ, Frysinger RC, Bashore TR, Downs KE, van Wouwe NC, van den Wildenberg WPM. Subthalamic nucleus stimulation influences expression and suppression of impulsive behavior in Parkinson’s disease. Brain. 2010b;133:3611–3624. doi: 10.1093/brain/awq239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Eckerle MK, Manning CA. Inefficient response inhibition in individuals with mild cognitive impairment. Neuropsychologia. 2007;45:1408–19. doi: 10.1016/j.neuropsychologia.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Ye Z, Hammer A, Camara E, Munte TF. Pramipexole modulates the neural network of reward anticipation. Hum Brain Mapp. 2011;32(5):800–11. doi: 10.1002/hbm.21067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.