Abstract
Aims
Hazardous environmental and genetic factors can damage endothelial cells to induce atherosclerotic vascular disease. Recent studies suggest that class III deacetylase SIRT1 may promote cell survival via novel antioxidative mechanisms. The current study tested the hypothesis that SIRT1, specifically overexpressed in the endothelium, is atheroprotective.
Methods and results
Human umbilical vein endothelial cells (HUVECs) were used to study the effects of oxidized low-density lipoprotein (LDL) on SIRT1 expression. Endothelial cell-specific SIRT1 transgenic (SIRT1-Tg) mice were used to study the effects of SIRT1 on aortic vascular tone. SIRT1-Tg mice were crossed with apolipoprotein E null (apoE−/−) mice to obtain SIRT1-Tg/apoE−/− mice for the analysis of atherogenesis in the presence of endothelial overexpression of SIRT1. SIRT1 expression in HUVECs was increased by the treatment with oxidative LDL. Adenoviral-mediated overexpression of SIRT1 was protective of apoptosis of HUVECs. Calorie restriction increased, whereas high-fat diet decreased, the SIRT1 expression in mouse aortas. In SIRT1-Tg mice, high fat-induced impairment in endothelium-dependent vasorelaxation was improved compared with that of wild-type littermates. This was accompanied by an upregualtion of aortic endothelial nitric oxide synthase expression in the SIRT1-Tg mice. The SIRT1-Tg/apoE−/− mice had less atherosclerotic lesions compared with apoE−/− controls, without affecting blood lipids and glucose levels.
Conclusion
These results suggest that endothelium-specific SIRT1 overexpression likely suppresses atherogenesis via improving endothelial cell survival and function.
Keywords: Endothelial cell, SIRT1, Atherosclerosis, eNOS, Apolipoprotein E
1. Introduction
Endothelium covers the inner surface of the vascular system and is a very important ‘organ’ to modulate vascular tone by sensing signals from blood and secreting active molecules.1 Upon exposures to harmful factors, endothelial cells undergo a series of changes, including increased permeability and decreased integrity, increased pro-inflammation and decreased anti-inflammation, collectively known as endothelial dysfunction.2 Endothelial cell dysfunction is often associated with endothelial cell apoptosis, deteriorating endothelial function to promote vascular pathogenesis.3 Nitric oxide (NO) is one of the most important factors that regulate vascular homeostasis.4 Endothelial NO is produced by endothelial nitric oxide synthase (eNOS), whose function is often regulated by vascular-damaging factors.4 Of interest, some therapeutic interventions or natural products can improve endothelial function via modulations of eNOS expression and/or function. For example, one of the mechanisms of statins to ameliorate vascular function is upregulation of eNOS expression and its NO-producing activity.5,6 Resveratrol, a component in grape and red wine that has cardiovascular protective effects, enhances both protein availability and activity of eNOS.7
It is interesting that resveratrol is also a potent SIRT1 activator.8 SIRT1 is the human orthologue of yeast Sir2, belonging to class III deacetylase. The deacetylating substrates of SIRT1 include histones, a variety of important transcription factors and some transcription co-factors.9 It has also been demonstrated that SIRT1 mediates the effects in extending life span of calorie restriction (CR) in yeast Saccharomyces cerevisiae, worm Caenorhabditis elegance, fly Drosophila melanogaster and possibly, mammals.9 Besides life span extension, CR has also been shown to have cardiovascular protective effects. Calorie restriction ameliorates endothelial cell function, lowers blood pressure, and decreases atherosclerosis.10,11 Based on these intriguing observations, we hypothesize that SIRT1 may serve as an endothelial protective factor and that upregulation of SIRT1 in endothelial cells may mimic CR’s beneficial effect on vascular health.
Recent report has shown that SIRT1 is highly expressed in the vasculature during blood vessel growth and disruption of SIRT1 gene expression results in defective blood vessel formation while blunting ischaemia-induced neovascularization.12 SIRT1 has also been shown to exert protective effects against endothelial dysfunction by preventing stress-induced senescence13 and to mediate the effects of CR on endothelium-dependent vasomotor tone by deacetylating eNOS and increasing nitric oxide (NO) bioavailability.14 Although SIRT1 has been shown to play a critical role in the regulation of vascular function,12–14 little information is available on the function of endothelial SIRT1 during hypercholesterolaemia-induced atherosclerosis. The aim of the present study was to test whether SIRT1 can promote endothelial cell function and exert atheroprotective function. The results show that SIRT1 inhibits the oxidized low-density lipoprotein (oxLDL)-induced endothelial cell apoptosis in vitro. Endothelial cell-specific transgenic mouse lines were then established and found beneficial in improving endothelium-dependent vasodilatation that was damaged by high-fat diet. These mice were crossed with an apolipoprotein E knockout (ApoE−/−) background to produce SIRT1-Tg/ApoE−/− mice. The extent of atherosclerosis was compared between SIRT1-Tg/ApoE−/− mice and ApoE−/− mice after 10 weeks of high-fat diet. The results show that SIRT1 overexpression in the vascular endothelium protects against atherogenesis.
2. Methods
2.1. Plasmids and constructs
The plasmid containing coding sequence of human SIRT1 was a kind gift of Professor Fuyuki Ishikawa.15 To generate endothelium-specific transgenic mouse, full-length human SIRT1 cDNA was ligated with mouse VE-Cadherin promoter,16 and this clone was named as VE-S (Figure 4A).
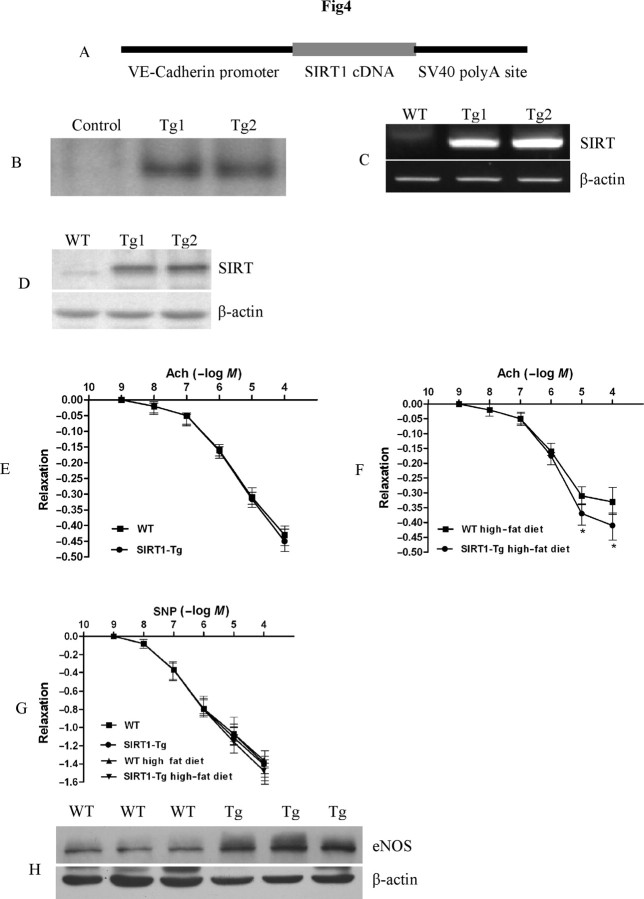
Figure 4.
SIRT1 overexpression in endothelial cells in vivo promotes endothelium-dependent vasodilation and aortic eNOS expression in high-fat diet-fed mice. (A) Diagram of the construct used for the generation of SIRT1-Tg mice. (B) Southern blot confirmation of SIRT1-Tg mice. (C) RT–PCR detection of human SIRT1 transcription in mouse aortas. (D) Western blot analysis of human SIRT1 expression in mouse aortas. (E) The dose–response curves of aortic rings to ACH in SIRT1-Tg mice and WT littermates under normal conditions. Magnitudes of relaxation are mean ± SEM of percentage reversal of PE-induced contractile responses. (F) The dose-response curves of aortic rings to ACH in SIRT1-Tg mice and WT littermates with high fat diet for 6 months. Magnitudes of relaxation are mean ± SEM of percentage reversal of PE-induced contractile responses (n = 5). *P < 0.05 SIRT1-Tg mice with high fat diet for 6 months vs. WT littermates with high fat diet for 6 months. (G) The dose-response curves of aortic rings to SNP in SIRT1-Tg mice and WT littermates with or without high fat diet for 6 months. Magnitudes of relaxation are mean ± SEM of percentage reversal of PE-induced contractile responses. (H) Western blot analyses of eNOS expression in aorta of SIRT1-Tg mice and WT littermates with high fat diet for 6 months (n = 5).
2.2. Preparation of human umbilical vein endothelial cells
Human umbilical vein endothelial cells (HUVECs) were freshly isolated using a collagenase IV method as described previously,17 and cultured in medium M200 (Cascade Biologics Inc.). Cells between third and sixth generations were studied.
2.3. Immunofluorescence
Immunofluorescence was carried out according to standard methods. The rabbit anti-SIRT1 polyclonal antibody (Santa Cruz Inc.) was used. Briefly, cells were fixed with 4% formaldehyde and pre-treated with 0.5% NP-40 in phosphate-buffered saline for 15 min. After blocking with 3% bovine serum albumin, the primary antibody and fluorescence-conjugated secondary antibody were incubated at room temperature for 1 h, respectively. After stain with 4′,6-diamidino-2-phenylindole, both antibodies were analysed, and photos were taken under fluorescent microscope.
2.4. Flow cytometry
Flow cytometry was used to detect HUVEC apoptosis induced by oxLDL according to the method described previously.18
2.5. Adenovirus
Coding sequence of SIRT1 was subcloned with pAdtrack-CMV vector and recombined with pAdeasy-1 in BJ5183 E. coli to generate the recombined adenoviral plasmids. After PacI digestion, adenoviral plasmids were transfected into HEK293 cells to generate infectious adenovirus particles.19 Amplification were conducted thrice to obtain enough active adenovirus, which was then used to transfect HUVECs at MOI 100.
2.6. Extraction of RNA and reverse transcription polymerase chain reaction
Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacture’s protocol. Two micrograms of total RNA were used to synthesize first-strand cDNA with M-MuLV reverse transcriptase (New England Biolabs) using random primers. Semi-quantitative reverse transcriptive–PCR (RT–PCR) was used to analyse SIRT1 mRNA level in cells. Primers used in PCR were: human SIRT1 sense 5′-CTTCAGGTCAAGGGATGGTAT-3′, antisense 5′-GCGTGTCTATGTTCTGGGTAT-3′. The results were normalized to β-actin mRNA.
2.7. Western blot analysis
Cellular and mouse tissue proteins were extracted with Radio Immuno Precipitation Assay (RIPA) buffer (25 mM Tris–HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% SDS). After complete homogenization on ice rotator, samples were centrifuged at 12 000 g for 30 min at 4°C to precipitate cell debris. The supernatants were transferred into fresh tubes and protein concentrations were determined by BCA method. Proteins were fractionated by 10% SDS–PAGE and electro-transferred onto a polyvinylidene fluoride membrane. After blocking with Tris-buffered saline (TBS) containing 5% non-fat milk, the membranes were probed with primary antibody for SIRT1 (1:500 dilution, Santa Cruz Inc.), eNOS (1:500 dilution, Santa Cruz Inc.), or Bax (1:500 dilution, Santa Cruz Inc.) at 4°C over night. Horseradish peroxidase-conjugated secondary antibody was used for luminochemical detection. Band intensities were quantified by densitometry. The results were normalized to β-actin (1:2000 dilution, Sigma).
2.8. RNA interference
Chemically synthesized small interfering SIRT1 RNAs were transfected into HUVECs with Lipofectamin 2000 according to the manufacture’s protocol. The sequence utilized was 5′-GATGAAGTTGACCTCCTCA-3′, as described previously.20
2.9. Animals and diets
The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). One set of mice was used to study the effect of CR on SIRT1 expression. Ten male C57BL/6 mice were randomly divided into two groups with five in each group. From 4 weeks of age, one group was fed ad libitum, another group was put on caloric restriction (CR) diet (70% of calorie intake adjusted to body weight) for 1 year. With identical design of splitting, control and experimental groups were tested for the effect of high-fat diet on SIRT1 expression. A group of five males was put on high-fat diet (composition: each 10 kg—basal food 8875 g, triglyceride 1000 g, and cholesterol 125 g) for 6 months. At the end of 1 year (for CR and control group) or half a year (for high-fat diet and control group), the two sets of mouse were sacrificed and full-length aortas were homogenized using RIPA buffer to extract proteins for western blot analysis.
Endothelial cell-specific transgenic mouse lines were established by microinjecting VE-S plasmid into fertilized C57BL/6 eggs. Positive transgenic mice were identified by PCR and further confirmed by Southern blot. PCR primers used here are the same as in RT-PCR. The mice were given free access to food and water, except when fasting blood specimens were obtained. Positive transgenic mice and WT littermates were fed with high-fat diet (each 10 kg containing basal food 8875 g, triglyceride 1000 g, and cholesterol 125 g) for 6 months from 1 month of age. At the end of high-fat diet, the aortic vascular tone of these mice was detected (see Section 2.7). The blood of these mice was collected to determine the levels of plasma lipids and glucose.
SIRT1-Tg/apoE−/− mouse line was established by breeding SIRT1-Tg mice with apoE−/− mice. Three primers were used for PCR genotyping of the apoE allele: oIMR180 (5′-GCCTAGCCGAGGGAGAGCCG-3′), oIMR181 (5′-TGTGACTTGGGAGCTCTGCAGC-3′), and oIMR182 (5′-GCCGCCCCGACTGCATCT-3′). Primer pair oIMR180 and oIMR181 amplified a 155-bp WT band, whereas primer pair oIMR180 and oIMR182 amplified a 243-bp apoE-targeted allele band. Male SIRT1-Tg/apoE−/− and apoE−/− controls were fed with high-fat diet to induce atherosclerosis. These mice were put on high-fat diet for 10 weeks from 4 weeks of age.21
2.10. Ex vivo studies of aortic vascular tone
Upon sacrificing, descending aorta was carefully removed and exercised into 4 mm segments. After equilibration in an organ bath for 30 min under a resting tension of 0.3 g in carbongenated (95% O2/5% CO2) Krebs bicarbonate solution [in mmol/L, NaCl 120, KCl 5.2, CaCl2 2.4, MgSO4·7H2O 1.2, NaHCO3 25, Na2-EDTA 0.03, and dextrose (pH 7.4)], the bath temperature was kept at 37°C. Subsequently, aortic ring contraction was induced with phenylephrine (PE) (Sigma Chemicals, USA), and the relaxation was induced with a cumulative dose of acetylcholine (ACH) or sodium nitroprusside (SNP). The relaxation responses were expressed as mean ± SEM (in %), showing reversal of the PE-induced contractile responses.
2.11. Analysis of atherosclerotic lesions
At the end of high-fat diet feeding, aortic atherosclerotic lesions were assessed by Oil Red O staining as published previously.22,23 Briefly, after perfusion with 4% formaldehyde–PBS, hearts and aortas were dissected out. The aorta was cut 2 mm distal from the heart and opened longitudinally using microscissors and pinned flat on a black wax surface. The en face preparation was fixed overnight and stained with Oil Red O. The photo images of the aortas were captured with digital camera mounted on the dissecting microscope. The atherosclerotic lesion area and the total area of the aorta were measured with Image-Pro Plus software.
2.12. Plasma lipid analysis
Plasma was separated from 0.5 mL of blood immediately after collection. The levels of plasma lipids and glucose were determined according to the methods described elsewhere.23
2.13. Statistical analysis
Data are expressed as mean ± SD, and unpaired Student’s t test was used to compare the statistical difference between the groups (for paired or unpaired means; if multifactorial, ANOVA would be better). Differences were considered significant at a value of P < 0.05.
3. Results
3.1. Endothelial SIRT1 expression was regulated by different stimuli in vitro and in vivo
SIRT1 is expressed at a relatively high level in HUVECs as detected by RT-PCR and western blot (Figure 1A and C, lane 1), consistent with the findings of Potente et al.12 Immunofluorescent labelling with a specific antibody demonstrated a predominant nuclear localization of SIRT1 in HUVECs (Figure 1B). To determine whether SIRT1 expression can be regulated by different stimuli, we treated HUVECs with oxLDL and hydrogen peroxide (H2O2). As shown in Figure 1, SIRT1 level was upregulated by both oxLDL and H2O2 (Figure 1C–F). Of note, oxLDL or H2O2 did not alter the subcellular localization of SIRT1 (data not shown).
Figure 1.
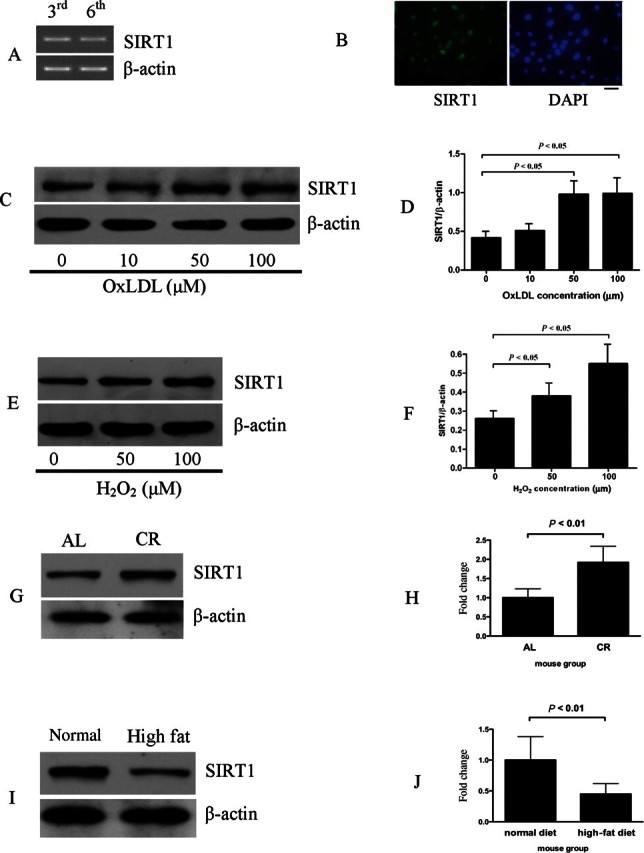
Regulation of endothelial SIRT1 expression by different stimuli. (A) Detection of SIRT1 expression in human umbilical vein endothelial cells (HUVECs) of third and sixth generation by RT–PCR. (B) Immunoflorescence showed that SIRT1 localizes in the nuclear of HUVECs. The bar indicates 5 µm. (C and D) SIRT1 expression was upregulated by oxLDL treatment in HUVECs. (E and F) SIRT1 expression was upregulated by H2O2 treatment in HUVECs. HUVEC was treated with indicated concentration of oxLDL or H2O2 for 24 h and protein was harvested for western blot analysis. Each lane represents three times of repeat. (G and H) SIRT1 level was increased in aorta of mice under CR for 1 year. (I and J) SIRT1 level was decreased in aorta of mice fed with high fat diet for 6 months. Full length of aortas were homogenized to extract protein by RIPA buffer for western blot analysis (n = 5). In all western blots, β-actin was used as loading control.
Since SIRT1 is an important factor that mediates the beneficial effects of CR,9 we examined SIRT1 expression in aortas of mice receiving CR for 1 year. Compared with WT littermates that fed ad libitum for 1 year, CR doubled the aortic SIRT1 expression (Figure 1G and H). On the contrary, after high-fat diet for 6 months, aortic SIRT1 expression was decreased by approximately 60% (Figure 1I and J).
3.2. SIRT1 inhibited oxLDL-induced human umbilical vein endothelial cell apoptosis
Next, we set out to explore the role of SIRT1 in oxLDL-induced endothelial cell apoptosis. oxLDL induced HUVEC apoptosis as determined by flow cytometry. SIRT1 overexpression significantly decreased the percentage of apoptotic cells compared with GFP overexpression. Of note, the treatment with the endogenous SIRT1 inhibitor NAM significantly diminished the anti-apoptotic effect of SIRT1 (Figure 2A). Consistent with this finding, oxLDL significantly upregulated pro-apoptotic protein Bax expression in HUVECs, which was decreased by adenovirus-mediated SIRT1 overexpression (Figure 2B).
Figure 2.
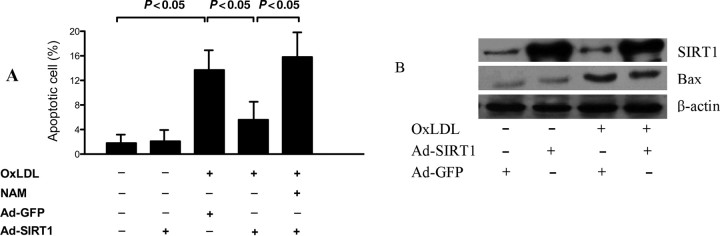
Inhibition of human umbilical vein endothelial cell (HUVEC) apoptosis by adenoviral SIRT1 overexpression. (A) SIRT1 inhibited oxLDL induced HUVEC apoptosis as determined by flow cytometry. (B) SIRT1 decreased pro-apoptotic protein Bax expression stimulated by oxLDL. β-Actin was used as loading control. Each assay was done in triplicate.
3.3. SIRT1 regulated endothelial nitric oxide synthase expression
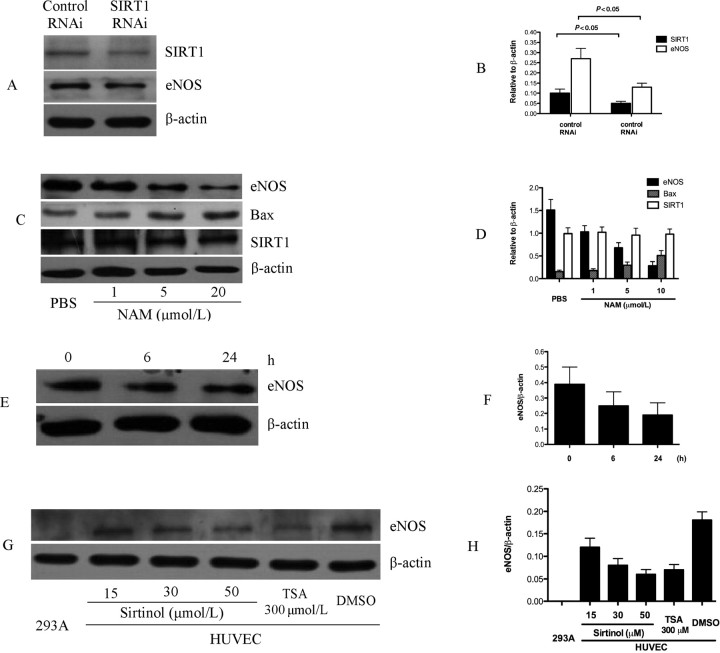
Previous studies have provided multiple lines of evidence that endothelial cell apoptosis is counteracted by NO synthesized by eNOS.24 We detected whether SIRT1 regulates eNOS protein expression. RNAi attenuation of HUVEC SIRT1 expression resulted in the decreased eNOS protein level (Figure 3A and B). Inhibition of SIRT1 with NAM decreased eNOS expression level in a dose- and time-dependent manner (Figure 3C–F). Accordingly, we found that inhibition of SIRT1 with NAM increased pro-apoptotic protein Bax expression in a dose-dependent manner (Figure 3C and D), providing evidence that decreased eNOS expression induced by inhibitor of SIRT1 is associated with cell survival function of SIRT1 in HUVECs. Another inhibitor of SIRT1, sirtinol, also decreased the eNOS expression level in a dose-dependent manner (Figure 3G and H).
Figure 3.
Regulation of eNOS expression in human umbilical vein endothelial cells by SIRT1. (A and B) SIRT1 RNAi effectively knock down endogenous SIRT1 level about 50%. eNOS expression was decreased when SIRT1 was knock down. (C and D) SIRT1 inhibition by NAM decreased eNOS level in dose-dependent manner. Also, Bax protein is upregulated in dose-dependent manner when endothelial cells treated with NAM. (E and F) NAM treatment decreased eNOS level in time-dependent manner. (G and H) SIRT1 inhibition by sirtinol decreased eNOS level in dose-dependent manner. HEK293A cell is used here as negative control. TSA treatment used as positive control. In all western blot experiment, β-actin was used as loading control and each assay was done in triplicate.
3.4. SIRT1 overexpression in endothelial cells in vivo promoted endothelium-dependent vasodilation in high-fat diet-fed mice
To investigate the function of vascular endothelial SIRT1 in vivo, we made transgenic mouse lines with endothelial cell-specific expression of human SIRT1 using VE-Cadherin promoter (Figure 4A). Positive transgenic mice were identified with PCR and confirmed by Southern blot (Figure 4B). SIRT1 transgenic mice were phenotypically normal and fertile. Genotypes of descendents of transgenic mice appeared as Mendelian rule. The expression of human SIRT1 in mouse aortas was determined by RT–PCR and western blot (Figure 4C and D). Two independent transgenic mouse lines were used for further experiments. No difference was observed between these two mouse lines.
To determine whether SIRT1 plays important role in regulating endothelium-dependent vascular tone, vasomotor function of aortic rings was measured. The aortic strips of SIRT1-Tg mice and WT littermates were sensitive to ACH and SNP, and the relaxation started from the concentration of 10−8 mol/L of the vasodilators (Figure 4E). However, no significant difference of ACH-induced, endothelium-dependent vasorelaxation was observed in SIRT1-Tg mice and WT littermates (Figure 4E). To further observe whether SIRT1 overexpression in endothelial cells improved endothelium function under stress, we first challenged the endothelium function with high-fat diet for 6 months. Endothelium-dependent vasodilation was significantly impaired in mice after 6 months of high-fat diet, as assessed by stimulation with ACH (Figure 4F). After 6 months of high-fat diet, the relaxation of the aortic strips for ACH was apparently higher in SIRT1-Tg mice compared with WT littermates (Figure 4F). The relaxation of the aortic strips for SNP in SIRT1-Tg was similar to that in WT littermates under both normal and high-fat diet (Figure 4G). This indicated that the endothelial cell-dependent aortic relaxation function is improved in SIRT1-Tg mice compared with WT littermates under hypercholesterolaemic conditions. Then eNOS level, the hallmark of in vivo endothelium function, was detected. Consistent with the finding in aortic vascular tone that the level of eNOS in aorta of SIRT1-Tg mice was higher than that of WT littermates after feeding with high-fat diet but not under normal conditions (Figure 4H). These results indicate that upregulation of SIRT1 improved endothelium function in aortas in vivo under hypercholesterolaemic conditions.
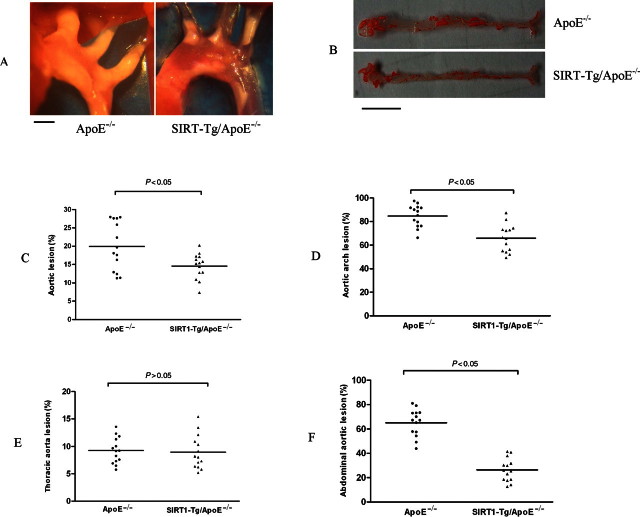
3.5. SIRT1-Tg/apoE−/− mice developed less atherosclerosis than that of control apoE−/− mice
Based on SIRT1’s vascular relaxation regulation effect, we hypothesized that endothelial SIRT1 may have atheroprotective function. To test this hypothesis, SIRT1-Tg mice were bred with apoE−/− mice to obtain SIRT1-Tg/apoE−/− mice. After 10 weeks of high-fat diet, atherosclerosis at aortic face and aortic root in SIRT1-Tg/apoE−/− mice and apoE−/− controls were analysed. SIRT1-Tg/apoE−/− mice have less atherosclerotic plaque at aortic root than that of apoE−/− controls (Figure 5A). Image analysis showed that atherosclerotic plaque to aortic face of SIRT1-Tg/apoE−/− mice was 14.6% compared with 19.6% in that of apoE−/− controls (P < 0.05) (Figure 5B and C). Detailed analysis of atherosclerosis plaque showed that the difference was mainly from the areas of aortic arch and abdominal aorta between SIRT1-Tg/apoE−/− and apoE−/− controls, while there was no significant difference in the areas of thoracic aorta (Figure 5D–F).
Figure 5.
Reduced atherosclerosis in SIRT1-Tg/apoE−/− mice compared with apoE−/− controls. Male SIRT1-Tg/apoE−/− mice and apoE−/− controls at 4 weeks of age were fed high fat diet for 10 weeks. The mice were then sacrificed and aortas from the aortic arch to the iliac arteries were examined for atherosclerotic lesion formation using the en face technique. Percentage of atherosclerotic lesions in the whole aorta was quantified. The mean atherosclerotic lesion area in each group of mice is indicated by a horizontal bar with adjacent mean value. Each point represents the mean atherosclerotic lesion area of one mouse. (A) SIRT1-Tg/apoE−/− mice developed less atherosclerotic plaques at aortic root. The bar indicates 1 mm. (B and C) The percentage of plaque area to aortic face in SIRT1-Tg/apoE−/− mice are less than that of apoE−/− controls. The bar indicates 1 cm in B. (D) Aortic atherosclerotic lesion area at aortic arch. (E) Aortic atherosclerotic lesion area at thoracic aorta. (F) Aortic atherosclerotic lesion area at abdominal aorta.
3.6. SIRT1 overexpression in endothelial cells did not change the levels of blood lipids and glucose
We further detected the levels of blood lipid and glucose to determine whether the anti-atherosclerosis effect of endothelial SIRT1 is due to its possible effect on lipid or glucose metabolism. Results showed that there were no differences in the levels of blood lipid and glucose between (i) SIRT1-Tg mice and WT littermates fed with high-fat diet for 6 months, (ii) SIRT1-Tg/apoE−/− mice and apoE−/− controls fed with high-fat diet for 10 weeks (Table 1), suggesting that the anti-atherosclerosis effect of endothelial SIRT1 does not depend on the regulation of glucose and lipid metabolism.
Table 1.
Plasma lipid and glucose levels in mice fed high fat diet (mmol/L)
| 6 Months |
10 Weeks |
|||
|---|---|---|---|---|
| WT (n = 6) | SIRT1-Tg (n = 8) | apoE−/− (n = 10) | SIRT1-Tg/apoE−/− (n = 12) | |
| TG | 0.33 ± 0.11 | 0.42 ± 0.13 | 0.41 ± 0.13 | 0.40 ± 0.09 |
| TC | 2.36 ± 0.60 | 3.72 ± 0.45 | 28.69 ± 7.04 | 25.55 ± 7.46 |
| HDL-C | 1.71 ± 0.44 | 3.08 ± 0.39 | 15.06 ± 2.92 | 14.19 ± 3.12 |
| LDL-C | 0.49 ± 0.23 | 0.52 ± 0.22 | 12.66 ± 4.66 | 12.40 ± 4.25 |
| VLDL-C | 0.14 ± 0.06 | 0.19 ± 0.06 | 0.96 ± 0.28 | 0.94 ± 0.30 |
| Glucose | 11.75 ± 2.68 | 9.12 ± 3.11 | 9.25 ± 2.64 | 9.02 ± 2.68 |
TG, triglyceride; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very low-density lipoprotein cholesterol.
4. Discussion
Multiple evidences suggest that SIRT1 is a potential anti-atherosclerosis factor. Population study revealed that CR can significantly reduce hallmarks of atherosclerosis.11 The biological effect of CR in mammals is, at least in part, mediated by SIRT1.25 Resveratrol and some structurally similar chemicals, collectively known as polyphenols that have a series of beneficial effects on cardiovascular system, have been proved to be potent activators of SIRT1.8 In this study we present direct evidence that endothelial SIRT1 is a bona fide anti-atherosclerosis factor in vivo. First, numerous studies point to SIRT1 as a key regulator of cell survival in response to oxidative stress, we showed that oxLDL and H2O2 treatment increased the SIRT1 level in HUVECs, which seems to be a compensation effect in protecting against oxidation-induced apoptosis. Consistent with this notion, HUVECs infected with adenoviral SIRT1 were more resistant to oxLDL-induced apoptosis. Then, we found that high-fat diet, the risk factor of atherosclerosis, decreased the aortic SIRT1 level, whereas CR increased it. Finally, at vascular levels, endothelium-specific overexpression of SIRT1 promotes endothelium-dependent vasodilation under hypercholesterolaemic conditions. In apoE−/− mice, endothelium-specific overexpression of SIRT1 can decrease about 25% (from 19.6% in non-transgenic mice to 14.6% in transgenic mice) of atherosclerosis without changing the system factors including the levels of blood lipids and glucose. Although this effect is not as robust as active participants of atherogenesis such as lipid metabolism and inflammation-related genes, it is of physiologically significance for a modulator of endothelial cell function and apoptosis. Through detailed analysis of atherosclerosis plaque areas, we found that the anti-atherosclerosis effects of SIRT1 was mainly reflected in decreasing atherosclerosis of SIRT1-Tg/apoE−/− mice in the aortic arch and the abdominal aorta, but not in the thoracic aorta, and this may offset anti-atherosclerosis effects of SIRT1 in the whole aorta. It remains to be elucidated whether the differential effects in the different parts of aorta are associated with the SIRT1 expression level in these regions.
The mechanism of SIRT1’s anti-atherosclerosis effect is related to inhibition of endothelial cell apoptosis and improvement of endothelium function, and these effects are probably mediated by the regulation of eNOS level. Former research found that CR induces eNOS expression and mitochondrial biogenesis.26 Here we give direct evidence that SIRT1 is a regulator of eNOS expression in vitro and in vivo. By using HUVECs as in vitro model, we show that inhibition of SIRT1 by NAM increased oxLDL-induced apoptosis. Inhibition of SIRT1 by NAM, sirtinol, or SIRT1 RNAi could effectively decrease eNOS expression, suggesting that SIRT1 is essential for the expression of eNOS expression, and a recent study gave the similar result.13 Endothelium-specific overexpression of SIRT1 upregulated eNOS expression and improved the endothelial cell-dependent aortic relaxation function under hypercholesterolaemic conditions. Taken together, these results suggest that the anti-apoptotic effect of SIRT1 is related to eNOS expression, which is well recognized for increasing NO to promote endothelial survival and improve endothelial function.24
SIRT1 was originally identified as a histone deacetylase and found to be localized in the nucleus.27 Consistent with previous findings, our results showed that SIRT1 lies in the nucleus in HUVECs and the predominant nuclear localization of SIRT1 cannot be altered with oxLDL or H2O2 treatment, suggesting that SIRT1 on the expression of eNOS seems to occur in the nucleus. However, we could not observe the change of eNOS expression at both the RNA and protein levels with either wild-type (WT) or dominant negative adenoviral SIRT1 overexpression in HUVECs (data not shown). Using chromatin immunoprecipitation (ChIP) assay, we failed to detect endogenous SIRT1 protein on the eNOS promoter and enhancer region in the HUVEC line ECV304 (data not shown), suggesting that the in vitro and in vivo regulation of eNOS expression is possible indirect effects of SIRT1. A recent paper reported that a fraction of SIRT1 colocalizes and binds to eNOS in the cytoplasm of endothelial cells, and SIRT1 deacetylates eNOS directly,14 suggesting that SIRT1 regulates eNOS activity depending on the different conditions. Thus, the exact mechanism(s) underlying the regulation of eNOS activity by SIRT1 remains to be further elucidated.
In summary, the results of the present study showed that endothelial SIRT1 is anti-atherosclerosis factor and the possible mechanism may be related to inhibit oxLDL-induced apoptosis, upregulate eNOS expression, and improve endothelium relaxation function. Combining with recent studies revealing that SIRT1 mediated the life span effect of CR9 and CR can decrease atherosclerosis,10 we infer that SIRT1 may be the underlying mediator of CR’s atheroprotective effect and that SIRT1 may be a useful target for atherosclerosis prevention and treatment.
Funding
This work was supported by the grants from the National Basic Research Program of China (Nos: 2005CB522402, 2006CB503801), National 863 project (No: 2006AA02A406), and National Natural Science Foundation of China (No: 30721063).
Acknowledgements
The authors thank Zhi-gang She, Ran Zhang, Li-hong Sun, Li Li, Hui-na Zhang, Yun-biao Lu, and Yue Wang for technical assistant.
Conflict of interest: none declared.
References
- 1.Aird WC. Endothelial Cells in Health and Disease. Boca Raton, FL: Taylor & Francis; 2005. Endothelium as an organ; pp. p1–32. [Google Scholar]
- 2.Goligorsky MS. Endothelial cell dysfunction: can’t live with it, how to live without it. Am J Physiol Renal Physiol. 2005;288:F871–F880. doi: 10.1152/ajprenal.00333.2004. [DOI] [PubMed] [Google Scholar]
- 3.Dimmeler S, Haendeler J, Zeiher AM. Regulation of endothelial cell apoptosis in atherothrombosis. Curr Opin Lipidol. 2002;13:531–536. doi: 10.1097/00041433-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Braam B, Verhaar MC. Understanding eNOS for pharmacological modulation of endothelial function: a translational view. Curr Pharm Des. 2007;13:1727–1740. doi: 10.2174/138161207780831275. [DOI] [PubMed] [Google Scholar]
- 5.Martínez-González J, Badimon L. Influence of statin use on endothelial function: from bench to clinics. Curr Pharm Des. 2007;13:1771–1786. doi: 10.2174/138161207780831220. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto E, Yamashita T, Tanaka T, Kataoka K, Tokutomi Y, Lai ZF, et al. Pravastatin enhances beneficial effects of olmesartan on vascular injury of salt-sensitive hypertensive rats, via pleiotropic effects. Arterioscler Thromb Vasc Biol. 2007;27:556–563. doi: 10.1161/01.ATV.0000254855.24394.f9. [DOI] [PubMed] [Google Scholar]
- 7.Wallerath T, Deckert G, Ternes T, Anderson H, Li H, Witte K, et al. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.cir.0000029925.18593.5c. [DOI] [PubMed] [Google Scholar]
- 8.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 9.Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- 11.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci USA. 2004;101:6659–6663. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ota H, Akishita M, Eto M, Iijima K, Kaneki M, Ouchi Y. Sirt1 modulates premature senescence-like phenotype in human endothelial cells. J Mol Cell Cardiol. 2007;43:571–579. doi: 10.1016/j.yjmcc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, et al. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takata T, Ishikawa F. Human Sir2-related protein SIRT1 associates with the bHLH repressors HES1 and HEY2 and is involved in HES1- and HEY2-mediated transcriptional repression. Biochem Biophys Res Commun. 2003;301:250–257. doi: 10.1016/s0006-291x(02)03020-6. [DOI] [PubMed] [Google Scholar]
- 16.Gory S, Vernet M, Laurent M, Dejana M, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–192. [PubMed] [Google Scholar]
- 17.Jaffe EA, Nachmann RL, Becker CG, Minick R. Culture of human endothelial cells derived from umbilical veins—identification by morphological and immunological criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X-S, Xin L, Yin W-X, Shang X-Y, Lu L, Watt RM, et al. Increased efficiency of oligonucleotide-mediated gene repair through slowing replication fork progression. Proc Natl Acad Sci USA. 2005;102:2508–2513. doi: 10.1073/pnas.0406991102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He T-C, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, de Oliveira RM, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vikramadithyan RK, Hu Y, Noh H-L, Liang C-P, Hallam K, Tall AR, et al. Human aldose reductase expression accelerates diabetic atherosclerosis in transgenic mice. J Clin Invest. 2005;115:2434–2443. doi: 10.1172/JCI24819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, et al. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95:1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 24.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 25.Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- 26.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 27.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]