Abstract
Axon degeneration is a common and often early feature of neurodegeneration that correlates with the clinical manifestations and progression of neurological disease. Nicotinamide mononucleotide adenylytransferase (NMNAT) is a neuroprotective factor that delays axon degeneration following injury and in models of neurodegenerative diseases suggesting a converging molecular pathway of axon self-destruction. The underlying mechanisms have been under intense investigation and recent reports suggest a central role for axonal mitochondria in both degeneration and NMNAT/WLDS (Wallerian degeneration slow)-mediated protection. We used dorsal root ganglia (DRG) explants and Drosophila larval motor neurons (MNs) as models to address the role of mitochondria in Wallerian degeneration (WD). We find that expression of Drosophila NMNAT delays WD in human DRG neurons demonstrating evolutionary conservation of NMNAT function. Morphological comparison of mitochondria from WLDS-protected axons demonstrates that mitochondria shrink post-axotomy, though analysis of complex IV activity suggests that they retain their functional capacity despite this morphological change. To determine whether mitochondria are a critical site of regulation for WD, we genetically ablated mitochondria from Drosophila MN axons via the mitochondria trafficking protein milton. Milton loss-of-function did not induce axon degeneration in Drosophila larval MNs, and when axotomized WD proceeded stereotypically in milton distal axons although with a mild, but significant delay. Remarkably, the protective effects of NMNAT/WLDS were also maintained in axons devoid of mitochondria. These experiments unveil an axon self-destruction cascade governing WD that is not initiated by axonal mitochondria and for the first time illuminate a mitochondria-independent mechanism(s) regulating WD and NMNAT/WLDS-mediated axon protection.
INTRODUCTION
Neurodegeneration is a hallmark of several inherited and acquired diseases of the nervous system. Though great attention in the past has been given to understanding and mitigating neuron cell body loss in these diseases, efforts at targeting neuronal death have yielded modest success in preventing or delaying disease onset (1,2). Often preceding neuronal death, axon degeneration is a common pathological feature of many neurodegenerative processes (3), and may therefore provide a therapeutic target chronologically upstream of neuronal loss (4,5). Though formerly believed to be a passive process, recent observations concerning the highly orchestrated cascade of molecular events that occur following separation of an axon from its cell body, known as Wallerian degeneration' (WD) (6), suggest axon degeneration is an actively regulated process akin to, but molecularly distinct from apoptosis (5,7). Though the precise mechanism(s) governing axonal self-destruction remain elusive, insights have been afforded by the discovery of a naturally occurring mutant mouse strain in which WD is significantly delayed, the Wallerian degeneration slow (WldS) mouse (8,9). The neuroprotective properties of this strain have been mapped to a chimeric gene Ube4b/NMNAT producing a fusion, WLDS protein. Though the precise protective function of WLDS remains controversial (10–13) it is clear that nicotinamide mononucleotide adenylyltransferase (NMNAT) is an important neuronal maintenance factor (14) and the axon protective moiety of WLDS (15,16). To date, the mechanisms underlying NMNAT/WLDS-mediated delay of WD are not well understood, however, the role of NMNAT as an NAD+ synthase has led to the prevailing hypothesis that its axon protective properties are likely mediated through mitochondria (17).
Mitochondria consume a large proportion of cellular NAD+ in its reduced form to generate ATP (18). Energy deprivation, by uncoupling the electron transport chain (ETC) for example, is sufficient to induce Wallerian-like axon degeneration (19–21) and depolarization of the mitochondria membrane potential (MMP) with a precipitous decline in axonal ATP are early events in degeneration following axotomy (13,22). Overexpression of NMNAT/WLDS is not only capable of sustaining ATP levels and preventing MMP depolarization after axotomy (13,20,22), but also enhances the basal ATP synthesizing capacity of mitochondria (23). These functional findings, as well as the correlation between axon protection and the enrichment of WLDS (23) and NMNAT3 (24) with mitochondria, suggest that NMNAT/WLDS may exert axon protection, in part by preventing local energy deprivation (13,20,22). A recent report has also demonstrated that WLDS-expression can delay WD potentially through enhanced mitochondria Ca2+-buffering and motility (25). The ability of NMNAT/WLDS to prevent energy deprivation and alter the Ca2+ handling dynamics of mitochondria strongly suggests an axon self-destruction program that converges upon axonal mitochondria. However, it has yet to be determined whether mitochondria play a direct role in the sequence of events leading to or protecting from axon degeneration.
Here, we explore the ability of NMNAT/WLDS to preserve axonal mitochondria following axotomy, and directly address whether axonal mitochondria are required for axon-protection using WD as a model of axon degeneration. Using dorsal root ganglia (DRG) explants, we analyzed the ability of NMNAT/WLDS to protect mammalian DRG neurons and specifically axonal mitochondria. Using Drosophila larval motor neurons (MNs) we determined the requirement of axonal mitochondria for the initiation of WD and NMNAT/WLDS-mediated delay of WD. Our results unveil an axon self-destruction cascade not initiated by axonal mitochondria and a mitochondria-independent mechanism(s) regulating both Wallerian degeneration and NMNAT/WLDS-mediated axon protection.
RESULTS
Characterization of mitochondria in WLDS-protected axons
As changes in mitochondria morphology and function are early events in axotomy-induced axon degeneration and neurodegenerative diseases (17), we utilized a mammalian embryonic DRG explant model of WD (10) (Fig. 1A) to document mitochondria morphology and function, specifically in NMNAT/WLDS-expressing axons. Using lentiviral vector-mediated transduction, we found that mRFP-tagged Drosophila melanogaster NMNAT (DmNMNAT) and mCherry-tagged WLDS delayed WD in DRG explants derived from either rat or human embryos compared with mCherry expressing control cultures (Fig. 1A, Supplementary Material, Fig. S1). Notably, WD in human DRG cultures was rapid, with axon fragmentation complete as early as 5 h post-axotomy compared with rat DRG in which fragmentation was observed within 24 h (Supplementary Material, Fig. S1). Despite this rapid progression, however, both DmNMNAT and WLDS were capable of delaying WD in human DRG axons through 48 h (Fig. 1A, Supplementary Material, Fig. S1), highlighting the efficacy and evolutionary conservation of this axon-protective mechanism (26). We also assayed the axon protective efficacy of other WLDS and NMNAT transgenes in rat DRG axons, including a WLDS nuclear localization mutant (WLDS ΔNLS) and Homo sapiens NMNAT1 (HsNMNAT1) and HsNMNAT3. We found that both WLDS ΔNLS and HsNMNAT3 significantly delayed WD through 72 h, similar to that of DmNMNAT and WLDS, while HsNMNAT1 delayed degeneration of ∼50% of rat DRG axons through 24 h (Fig. 1B, Supplementary Material, Fig. S1), consistent with previous reports demonstrating that NMNAT-mediated axon protection occurs via a local mechanism within the axon inasmuch as HsNMNAT1 is enriched in the nucleus (27,28). WLDS (23,25) and HsNMNAT3 (24) have reported mitochondria localization, and we have observed DmNMNAT to be distributed throughout the cytoplasm of Drosophila neurons (14). The potent axon protective efficacy of WLDS and HsNMNAT3 and their localization to mitochondria further suggest that mitochondria might be a critical subcellular locale for axon protection.
Figure 1.
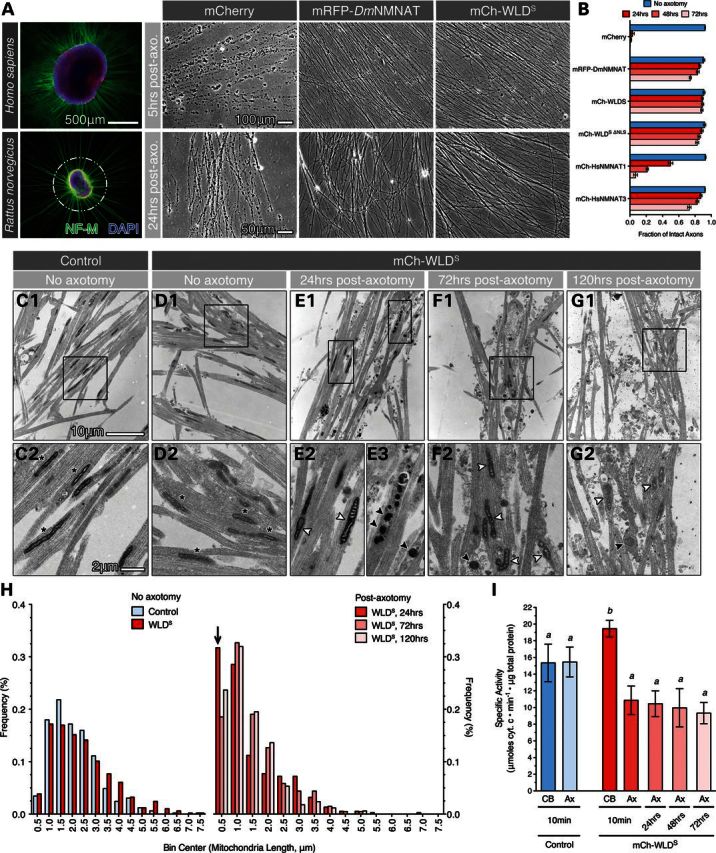
WLDS preserves complex IV activity, but not mitochondria morphology in axotomized DRG axons. (A) E15.5 rat and GW 8–8.5 human DRG explants 7 days in vitro extend neurofilament-medium positive (NF-M) axons radially from explant heads (white outline) containing Islet1/2-positive (red) sensory neuron cell bodies. Axotomy is performed via a circumferential cut and removal of the explant head. Scale bar, 500 μm for human and rat immunofluorescence micrographs. Wallerian degeneration of human and rat DRG explant axons was assayed morphologically in phase-contrast micrographs (right panels). Overexpression of RFP-tagged D. melanogaster NMNAT (mRFP-DmNMNAT) and mCherry-tagged WLDS (mCh-WLDS) delayed fragmentation of human (top panels) and rat (bottom panels) DRG axons 5 and 24 h post-axotomy, respectively. Scale bar, 100 μm for human and 50 μm for rat phase-contrast micrographs. (B) Quantification of axon protection in rat DRG axons expressing NMNAT and WLDS fusion proteins 24, 48 and 72 h post-axotomy. n ≥ 3 DRG for each transgene and timepoint. (C–G) Representative electron micrographs of DRG explant axons from non-axotomized controls (C) and mCh WLDS-expressing cultures prior to (D), 24 h (E), 72 h (F) and 120 h (G) after axotomy. Mitochondria within non-axotomized control and WLDS axons exhibited an elongated, spindle-like morphology with organized cristae (C2 and D2, asterisks); no difference in mitochondria morphology was observed between WLDS-expressing and control cultures. Following axotomy, mitochondria either maintained their spindle shape—though were shorter than non-axotomized controls (E2, F2 and G2, white arrowheads)—or became rounded and electron-dense (black arrowheads) (E3, F2 and G2). Bottom panels are magnified view of boxed area in the panel above. (H) Frequency histogram of mitochondria length in non-axotomized and axotomized DRG axons. Total number of mitochondria quantified per group: control, 495; WLDS, 496; WLDS, 24 h, 375; WLDS, 72 h, 214; WLDS, 120 h, 169. The distribution of mitochondria from WLDS axons 24 h post-axotomy is statistically different from WLDS, no axotomy controls at α < 0.2 (K–S P = 0.000207), K–S test (significance results summarized in Supplementary Material, Fig. S1C). Arrow marks the pool of mitochondria that displayed a transient increase at 24 h post-axotomy. (I) Quantification of mitochondria complex IV activity from axons (Ax) at 10 min, 24, 48 and 72 h post-axotomy as well as the explant heads containing DRG cell bodies (CB) collected at the time of axotomy. Mean ± SEM is shown, n ≥ 3 sample plates per group. Bars with different superscripts are statistically significant at P < 0.05, one-way ANOVA with Tukey's multiple comparison post-test.
We next examined whether axonal mitochondria undergo morphological or functional changes in WLDS-expressing rat DRG axons following axotomy. Using electron microscopy (EM), we found that WLDS expression had no impact on mitochondria morphology (Fig. 1C1 and D2). In WLDS-protected axons, mitochondria fell into two morphological classes after axotomy: those that maintained their morphology, but were shorter in length compared with those in uninjured axons (Fig. 1E2, F2 and G2), and those that were round, short and electron-dense, most likely representing degenerating mitochondria (Fig. 1E3). A decrease in median mitochondria length was observed as early as 24 h post-axotomy and this shorter length persisted through 120 h (Fig. 1H and Supplementary Material, Fig. S2). Kolmogorov–Smirnov (K–S) analysis of the distribution of mitochondria lengths revealed no significant difference between WT and WLDS expressing uncut axons (K–S P-value = 0.80976; significance level α > 0.01) (Fig. 1H and Supplementary Material, Fig. S1C). While there was no significant difference in the median mitochondria length in WLDS protected axons among 24, 72 and 120 h (Supplementary Material, Fig. S1B), the distribution of mitochondria length differed in WLDS expressing axons at 24 h after axotomy from that in WLDS uncut axons (K–S P = 0.000207, significance level α < 0.2), after which the distribution in WLDS expressing axons at 72 or 120 h was found to be no different from that of WLDS, uncut axons (K–S P = 0.87075 and 0.71491, respectively; significance level α > 0.01, Supplementary Material, Fig. S1C). The shift in mitochondria distribution in WLDS expressing axons at 24 h after axotomy came from a transient increase in the frequency of mitochondria with length ≤0.5 µm (arrow in Fig. 1H), likely due to the degeneration and clearance of non-WLDS protected axons in our culture preparations, as lentiviral vector transduction efficiency is <100%. To further confirm the finding of reduced mitochondria length, we used time-lapse fluorescence microscopy and directly observed the shrinking of mitochondria 30 min post-axotomy (Supplementary Material, Video S1). These morphological changes in mitochondria were similar to those observed in both neurodegenerative disease (29,30) and in isolated neurons following Ca2+-induced activation of the mPTP (31) or excitotoxic injury (32,33). This reduction in median mitochondria length might be due to post-axotomy fission events or an un-tethering of mitochondria from cellular transport machinery (34,35), either of which could affect mitochondria function (36). We therefore tested whether these morphological changes also reflected changes in axonal mitochondria function by assaying the enzyme activity of the terminal component in the ETC, cytochrome c oxidase (complex IV), at several timepoints post-axotomy (Fig. 1I). The specific activity of complex IV in mitochondria isolated from rat DRG axons was not significantly different between acutely axotomized WLDS-expressing and control axons, though complex IV activity was significantly greater in mitochondria isolated from WLDS-expressing DRG cell bodies. Surprisingly, complex IV activity was preserved in WLDS-expressing axons up to 72 h post-axotomy. Although we do not discard additional effects of axotomy on overall respiratory chain and oxidative phosphorylation (OXPHOS) activities, preservation of complex IV function suggests that axotomy-induced morphological changes in mitochondria do not necessarily reflect a change in the amount and function of mitochondria respiratory enzymes in WLDS-protected axons.
milton33-853 is a loss-of-function milton allele
We next determined whether preservation of mitochondria in WLDS-expressing axons was responsible for the overall delay of WD by testing the axon-protective efficacy of NMNAT and WLDS in mitochondria-devoid axons in vivo. To accomplish this, we utilized a loss-of-function mutation in milton, an adaptor protein linking mitochondria and the kinesin-mediated anterograde transport machinery (37), to genetically ablate mitochondria from axons in Drosophila. Here, we used milton33-853, an allele generated in a previously reported EMS mutagenesis screen (38) that phenocopies several reported milton loss-of-function alleles (Supplementary Material, Fig. S2). In Drosophila, loss of milton is lethal at the first-instar/early second-instar larvae stage (L1/L2) (39); our milton33-853 allele was also homozygous lethal at the L1/L2 stage and failed to complement two previously reported milton alleles (milt92 and P{lacW}miltK06704) as well as several deficiency (Df) alleles containing genomic deletions spanning the milton gene (Supplementary Material, Fig. S2A). To further confirm the mitochondria trafficking defect in milton33-853-mutant neurons, we examined the distribution of mitochondria by expressing the label mitoGFP under the motor neuron-specific D42-Gal4 driver (D42-Gal4>UAS-mitoGFP) in live (non-fixed) homozygous milton33-853 L2 larvae. As shown in Supplementary Material, Figure S2B, mitoGFP signal was excluded from homozygous mutant MN axons and dendrites, in contrast to heterozygous controls, demonstrating the loss of anterograde mitochondria transport in these mutant neurons. These results demonstrate that milton33-853 is a loss-of-function allele of milton.
milton33-853 results in the early loss of adult Drosophila olfactory receptor neurons that cannot be rescued by WLDS
To test the axon-protective efficacy of NMNAT/WLDS in the milton mutant neurons, we first employed a well-established antenna transection model to study WD of olfactory receptor neurons (ORNs) in adult Drosophila (Fig. 2A) (15,40). We used eyeless-flippase (eyFLP)-mediated mitotic recombination to generate homozygous milton33-853ORNs in the background of a heterozygous adult fly (41) and used Or22a-Gal4-driven mCD8-GFP to label the ORNs that project axons to the dorsomedial-2 (DM2) glomeruli of the midbrain antenna lobe (AL). Surprisingly, we found that milton resulted in the loss of Or22a ORN cell bodies and axons from the antennae of adult flies by 10 h post-eclosion (Fig. 2B). In the brain, we also noticed a complete loss of mCD8-GFP labeling in the DM2 glomeruli as well as gross anatomical defects of the AL in which individual glomeruli were poorly formed, or absent, as revealed by anti-bruchpilot (nc82) staining (Fig. 2C). This early loss of ORN neurons and lack of defined AL glomeruli suggest that milton and/or axonal mitochondria are likely required for the development of these sensory neurons, inasmuch as ORN precursor axons are targeted to the AL between 18 and 32 h after puparium formation (APF) (42). Overexpression of WLDS did not mitigate the loss of ORN or the anatomical defects caused by milton33-853 (Fig. 2), indicating that the developmental defects associated with the loss of axonal mitochondria are regulated by a mechanism that is distinct from that of axon degeneration suppressed by WLDS. Interestingly, it has been demonstrated in both mammalian and Drosophila systems that degeneration programs activated during neurodevelopment, such as those governing axon branch pruning or synapse elimination (9,43,44) are also likely regulated by mechanisms distinct from that which occur pathologically in a mature nervous system. These physiological degeneration programs are also unaffected by the expression of WLDS, as the nervous system in these animals develop without exuberant or supernumerary connections (9,43,44). The regulation of developmental axon elimination is therefore likely regulated by a pathway distinct from pathological, WLDS-sensitive, axon degeneration. Our findings of milton-induced loss of ORNs demonstrate a potentially novel role of milton and mitochondria in the development of this sensory neuron population, the aberration of which was not rescued by WLDS. These results are similar to recent findings in adult Drosophila wing chemosensory neurons, where WLDS was unable to rescue the die-back degeneration of axons induced by RNAi-mediated knockdown of milton (45). While an interesting avenue for future investigations, the loss of ORNs induced by milton precluded the use of ORNs as a model for investigating the role of mitochondria in the regulation and delay of WD by NMNAT/WLDS in these studies.
Figure 2.
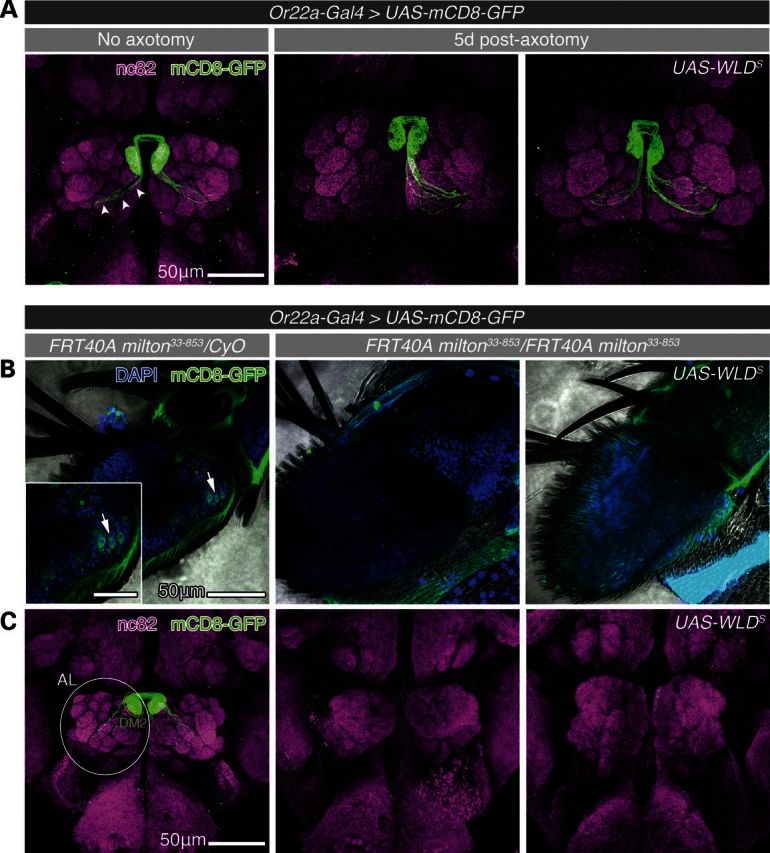
WLDS prevents degeneration of axotomized ORN axons but fails to rescue milton-induced loss of ORNs. (A) Confocal projections of whole-mount adult fly brains (1–2 days after eclosion). Or22a-Gal4-driven expression of mCD8-GFP illuminates ORN axons (white arrows). Five days following transection of the left antenna, ORN axons underwent Wallerian degeneration that was suppressed by the expression of WLDS, as has been previously reported (40). (B) Confocal projections of whole-mount antennae with heterozygous (control), homozygous milton, and homozygous milton, WLDS-expressing ORNs from adult flies ≤10 h after eclosion. mCD8-GFP labeled ORN cell bodies (white arrows) and axons are present in the antennae of control flies (left panel) while ORNs were absent from homozygous milton antennae (middle panel). Furthermore, milton-induced ORN loss was not suppressed by the expression of WLDS (right panel). (C) Confocal projections of whole-mount brains revealed gross anatomical defects in the organization of antenna lobe (AL) neuropil (nc82, white outline) that included the loss of defined glomeruli in milton33-853 and WLDS-expressing milton33-853 ORN flies. Scale bars, 50 μm for (A), (B) and (C), and 25 μm for inset in (B).
milton33-853 Drosophila larva motor neurons develop normally
We therefore sought to identify a neuron population where differentiation and development are not affected by mitochondria mislocalization, to enable our study of WD in axons devoid of mitochondria. As the development of MNs is unaffected by the loss of milton (Supplementary Material, Fig. S3A) (39), we used this neuron population to employ an in vivo segmental nerve crush model of WD (46). Here, we used the mosaic analysis with a repressible cell marker (MARCM) method to generate single MN ‘MARCM clones’ that were either wild-type (FRT40A iso) or homozygous for milton (FRT40A milton33-853) in the background of a heterozygous larva (47). This strategy had several advantages including: (ii) bypassing the early lethality of milton in the whole organism; (ii) affording single MN resolution in the morphological analysis of degenerating axons; and (iii) allowing chromosomal recombination with UAS-transgenes to overexpress cell markers, mitochondria labels and NMNAT proteins in both wild-type and milton MN backgrounds. To test the feasibility of this approach, we first generated milton33-853 MN clones that expressed mCD8-GFP under the pan-neuronal driver C155-Gal4 (C155-Gal>UAS-mCD8-GFP). Similar to wild-type MNs, we found that milton33-853 MNs developed cell bodies, dendrites and axons traceable to synapses at neuromuscular junctions (NMJs) (Supplementary Material, Fig. S3A). In late third-instar larvae (L3), milton33-853 MNs did not exhibit morphological signs of die-back degeneration such as retraction from NMJs or retrograde fragmentation of the distal axon. Wild-type and milton33-853 MNs both displayed smooth, continuous mCD8-GFP+ axons often punctuated by focal varicosities throughout their uninjured length (Supplementary Material, Fig. S3A, insets). While focal axonal swelling has been associated with axon damage (48), we believe varicosities here are representative of normal axon morphology, as they were commonly observed in both wild-type and milton33-853 MNs (49), and are likely the result of transient accumulations of organelles or other transported material. These experiments confirm that loss of milton had no detrimental effects on the development of MNs, and that milton MNs display normal axon and synaptic morphology.
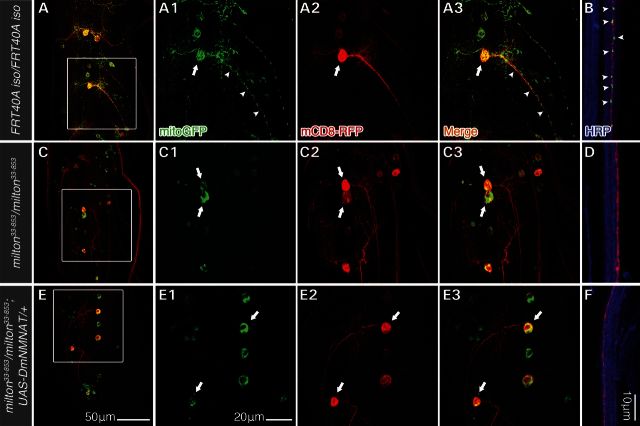
We next tested whether milton MN clones exhibited the same mitochondria localization defect as MNs from homozygous milton33-853 larvae (Supplementary Material, Fig. S2B). Here, we generated MNs that expressed the cell label mCD8-RFP as well as the mitochondria label mitoGFP under the D42-Gal4 driver (D42-Gal4>UAS-mCD8-RFP, UAS-mitoGFP) (Fig. 3). MitoGFP was observed in the cell bodies (Fig. 3A, arrows), dendritic fields and axons (Fig. 3A and B, arrowheads) of wild-type MNs, while mitoGFP in milton33-853 MNs was restricted to cell bodies (Fig. 3C) and not observed in distal axons (Fig. 3D) or NMJs (data not shown). These results confirmed that anterograde trafficking of mitochondria was defective specifically in milton33-853 MNs. Furthermore, overexpression of UAS-DmNMNAT did not rescue the mitochondria localization defect in milton33-853 MNs (Fig. 3E and F). Collectively, these results indicate that axonal mitochondria are not required for proper morphological development of larvae MNs, and that generation of milton MNs via MARCM was suitable for further investigating the role of mitochondria in WD in vivo.
Figure 3.
milton33-853results in a loss of mitochondrial anterograde transport in larva motor neuron MARCM clones. Third-instar larval MN MARCM clones and their mitochondria were simultaneously labeled by expressing UAS-mCD8-RFP and UAS-mitoGFP under the MN-specific driver D42-Gal4 (D42-Gal4>UAS-mCD8-RFP, UAS-mitoGFP). HRP immunostaining was used to highlight far distal segmental nerves containing mCD8 RFP-expressing axons (B, D, F, ∼800 μm from the VNC). MitoGFP was distributed throughout the cell body (A, arrows), dendrites, as well as proximal (A, arrowheads) and distal axons (B) of wild-type MNs. In milton33-853MNs, mitoGFP signal was restricted to MN cell bodies (C, arrows) and absent from dendrites and axon (C and D). Overexpression of DmNMNAT (D42-Gal4>UAS-DmNMNAT) in milton MNs did not suppress the mitochondria trafficking defect, as mitoGFP was also restricted to cell bodies (E, arrows) and absent from dendrites and axons (E and F). Scale bars, 50 μm in (E) for (A), (C) and (E), 20 μm in E1 for A1–A3, C1–C3, and E1–E3, and 10 μm for (B), (D) and (F).
Wallerian degeneration is mildly delayed in milton33-853 motor neurons
The ability to generate morphologically intact wild-type and milton33-853 MN clones allowed us to use a tractable larvae nerve crush model of in vivo axotomy (46). Early L3 larvae containing mCD8-GFP labeled MNs were anesthetized on CO2 and pinched around the ventral cuticle resulting in a nerve crush injury of segmental nerves distal to the ventral nerve cord (Fig. 4A). Horseradish peroxidase (HRP), a Drosophila axon membrane marker (50,51), was used to identify whole segmental nerves in which single MN axons traverse; axotomy sites were outlined by a reduction in HRP signal, which enabled the identification of both proximal and distal axon segments from an axotomized MN within a crushed, but still continuous segmental nerve (Fig. 4B and G). To avoid assessing incompletely transected axons, we considered a crush injury successful if a clear gap in mCD8-GFP signal ≥25 μm between the proximal and distal axon stumps could be observed from the resulting axotomy. To better define the progression of WD in MNs, we established a method for morphologically categorizing axons as either ‘non-degenerated’ (intact) or ‘degenerated’ in this injury model system (Supplementary Material, Fig. S3B). Following axotomy, distal axons degenerated anterogradely from the site of injury toward the NMJ with mCD8-GFP-labeled axons appearing fragmented as early as 8 h post-crush in both wild-type and milton33-853 MNs (Fig. 4D and H). As WD occurs rapidly and often asynchronously along a distal axon (52), we based our degeneration criterion on the fragmentation status of an axon over a 200 μm length of segmental nerve starting 300 μm away from the severed distal stump; this distance was sufficiently far from cellular debris and axon fragments resulting from either the crush itself or acute axon degeneration (AAD) of the immediate proximal and distal axon stumps (53). Fragmented axons were scored by the presence of ‘breaks’ reflected as gaps in mCD8-GFP signal ≥1 μm in length. Axons were classified as either (i) ‘continuous’ (non-degenerated) if they contained ≤5 breaks or (ii) ‘fragmented’ (degenerated) if they contained ≥10 breaks (Supplementary Material, Fig. S3B) over the 200 μm length of segmental nerve (Fig. 4B). Over the course of this study, we did not observe any axons with >5 but <10 breaks pre- or post-axotomy over the 200 μm nerve segment scored. Following axotomy, we also noted endbulbs (e.g. Figs 4D1 and 3F1) and supernumerary branching (e.g. Figs 4C1 and 3J1) of the proximal axon segments, likely indicative of a regenerative response (46). WD followed a similar time course for both wild-type and milton33-853 MN axons, with axon continuity being maintained at 4 h and a heterogeneous population of continuous and fragmented axons appearing 8 h post-crush. WD in this model follows a biphasic progression similar to that in mammalian systems, in which axons remain intact over a variable latent phase and rapidly fragment, or ‘catastrophe,’ in an anterograde, segmental wave (52). Interestingly, a significantly smaller fraction of milton33-853axons were fragmented at 8 h post-crush compared with wild-type axons (P < 0.01), though all milton33-853 and wild-type axons were fragmented by 16 h (Fig. 4K). This observation has two implications. First, as the absence of mitochondria does not completely arrest axon degeneration, mitochondria are not absolutely required to activate a putative injury-induced axon self-destruction program, despite mitochondria dysfunction being an early event following axotomy in wild-type axons (17). Secondly, the mild axon protective effect caused by the exclusion of mitochondria from distal axons at 8 h suggests that mitochondria dysfunction following injury may contribute incrementally to axon self-destruction, expediting the degeneration process.
Figure 4.
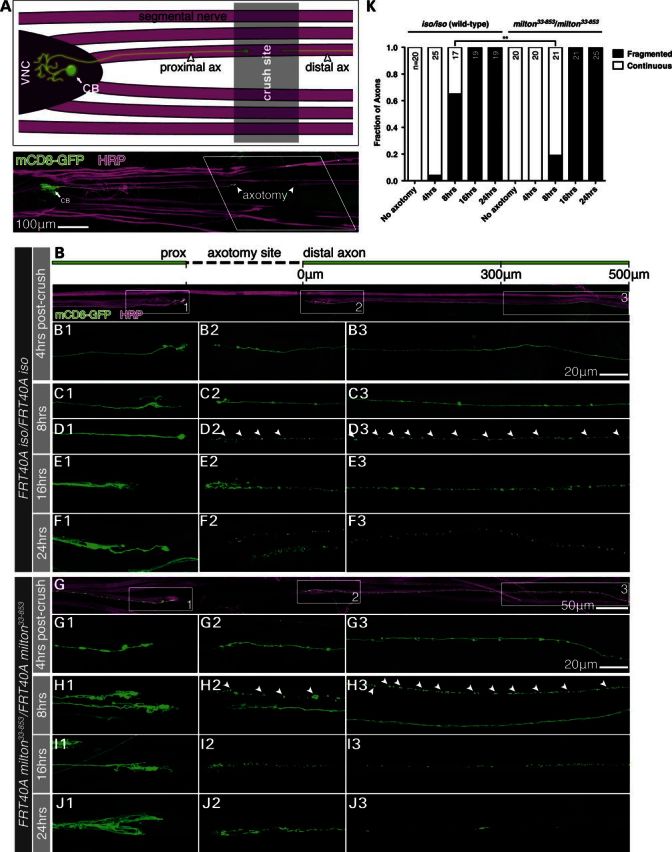
Wallerian degeneration in wild-type and milton MN axons. (A) Anatomical schematic of the larvae segmental nerve crush injury model (early third-instar larvae, 4 h post-crush). Pinching the ventral cuticle with forceps crushed the segmental nerves within a third-instar larva. The crush site was outlined by a break or reduction in horseradish peroxidase (HRP) labeling in the segmental nerves containing mCD8-GFP (C155-Gal4)-expressing axons from MN MARCM clones. Proximal and distal MN axons were readily traceable following axotomy, providing a tractable method of assessing single axon morphology and degeneration in vivo. (B–F) Montage of wild-type proximal, distal and far distal (300–500 μm from crush) axons at 4, 8, 16 and 24 h post-axotomy. The numbered panels display images of corresponding boxed areas in (B). Distal axon continuity was maintained at 4 h post-crush (B) with fragmentation of mCD8-GFP signal beginning 8 h post-crush (C and D, arrowheads demarcate mCD8-GFP axon fragments). By 16 h, all axons observed were fragmented (E) with a significant reduction in mCD8-GFP signal at 24 h (F). (G–J) Montage of milton33-853 proximal, distal and far distal axons at 4, 8, 16 and 24 h post-axotomy. The numbered panels display images of corresponding boxed areas in (G). Wallerian degeneration followed a time course similar to wild-type MNs, with axon fragmentation beginning 8 h post-crush following a 4 h period of latency and completed by 16 h post-crush (G–H). (K) Morphological quantification of Wallerian degeneration in (B–J). Axon fragmentation was mildly delayed by milton33-853, with a smaller fraction of fragmented axons observed 8 h post-axotomy, though all milton33-853 axons were fragmented by 16 h as observed in wild-type controls. n = total number of MNs quantified per timepoint for each genotype. **P < 0.01, Fisher's exact test. Scale bars, 100 μm in (A), 50 μm for (B) and (G) and 20 μm in B3 and G3 for B1–F3 and G1–J3.
Axonal mitochondria are not required for NMNAT/WLDS-mediated delay of Wallerian degeneration
To determine whether axonal mitochondria are required for NMNAT/WLDS-mediated axon protection, we overexpressed wild-type Drosophila NMNAT (UAS-DmNMNAT), the substrate binding mutant DmNMNATWR (UAS-DmNMNATWR, enzymatic activity <1% of wild-type protein) (14) or WLDS (UAS-WLDS) in wild-type and milton33-853 MN clones. DmNMNAT and WLDS protected both wild-type and milton33-853 axons at 16 h post-crush, a timepoint at which control wild-type and control milton33-853 axons were completely degenerated (Fig. 5A–I). We also found that WLDS delayed WD in wild-type and milton33-853 axons 24 h post-crush, indicating that the absence of mitochondria from axons did not reduce the protective efficacy of WLDS (Fig. 5J). While the requirement for NAD+ (10,11) and NMNAT enzymatic activity for axon protection remains controversial (15,26,54), here DmNMNATWR was as neuroprotective as DmNMNAT and WLDS in both wild-type and milton33-853 MN backgrounds. These results further suggest an additional protective mechanism independent of modulating axonal NAD+ levels and collectively demonstrate that the axon protection mediated by NMNAT and WLDS following injury are not solely dependent upon the presence of mitochondria in the axon.
Figure 5.
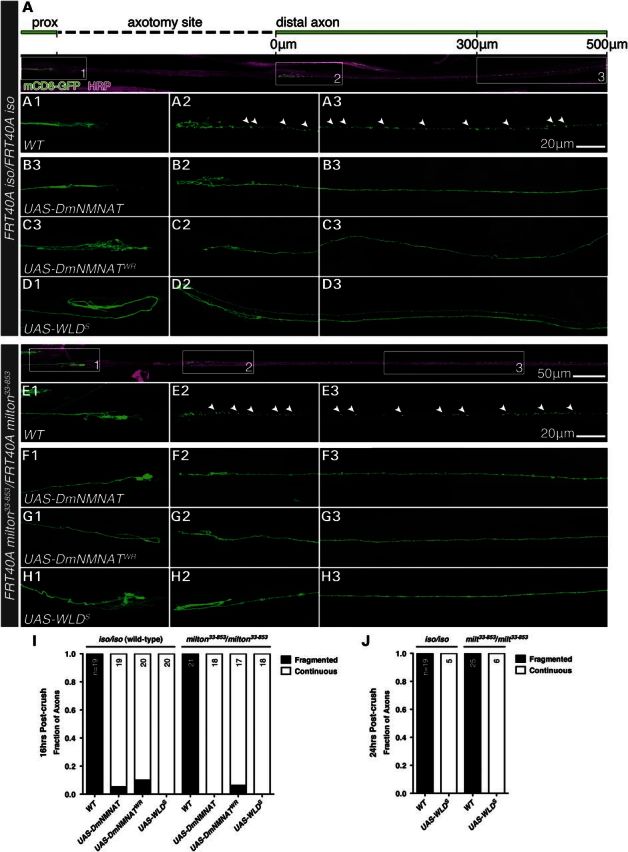
DmNMNAT and WLDS delay Wallerian degeneration independent of axonal mitochondria. (A–H) Expression of DmNMNAT, enzymatically inactive DmNMNATWR and WLDS preserved axon continuity 16 h post-axotomy in wild-type (A–D) and milton33-853 (E–H) MN axons. (I and J) Morphological quantification of Wallerian degeneration in (A–H) 16 h (I) and 24 h post-axotomy (J). n = total number of MNs quantified per genotype. Arrowheads in A2–3 and E2–3 demarcate mCD8-GFP axon fragments. Scale bars, 50 μm in (E) for (A) and (E), and 20 μm in A3 for A1–D3 and in E3 for E1–H3.
DISCUSSION
In this report, we find that WLDS has the striking ability to preserve the functional integrity of mitochondria in axotomized axons despite minor changes in their morphology. However, our findings that WD proceeds in axons devoid of mitochondria with a mild delay, compared with that of axons with mitochondria, suggest the existence of (i) an axon self-destruction cascade that is not initiated by axonal mitochondria and (ii) an NMNAT/WLDS-sensitive pathway of axon self-destruction that operates in parallel with a putative mitochondria-mediated pathway.
Increase in intra-axonal Ca2+ concentration is both necessary and sufficient for the progression of WD (55) and is likely the initiation signal for axon destruction. Sources of this Ca2+ include channel-mediated influx (56,57) and potentially release from intra-axonal organelles, such as mitochondria (58). The mild delay in axon degeneration observed in milton axons in this report could be explained by a reduction in the axonal Ca2+ burden post-axotomy, for example as a result of mPTP-mediated release of mitochondria Ca2+ stores (58). Avery et al. (25) have recently implicated enhanced Ca2+ buffering by axonal mitochondria as a potential mechanism behind WLDS-mediated delay of WD, with WLDS capable of preventing the acute rise in intra-axonal Ca2+ following axotomy. While we show that NMNAT/WLDS are protective in milton axons, it would be interesting to know if NMNAT/WLDS are also capable of preventing the rise in intra-axonal Ca2+ in this mitochondria-devoid background. Such studies could clarify the potential role for mitochondria Ca2+ buffering and might allude to an alternative mechanism of NMNAT/WLDS-mitigated Ca2+ entry.
The ability of NMNAT/WLDS to delay WD in milton motor axons suggests a protection mechanism that is independent of axonal mitochondria. In a concurrent study, Ocampo et al. also demonstrated that NMA1 and NMA2, the yeast homologs of NMNAT, are protective against cellular toxicity in models of Huntington's and Parkinson's disease in yeast devoid of mitochondrial DNA and functional OXPHOS pathways (59). Both studies have used genetic approaches to ablate either the OXPHOS function of mitochondria (Ocampo et al.) or the entire organelle (this report), and revealed the capability of NMNAT proteins to protect against cellular degeneration without mitochondria-dependent functions. This mechanism could be consistent with the previously reported chaperone activity of DmNMNAT and human NMNAT3 that confer neuroprotection in models of spinocereballar ataxia 1 (SCA-1) (60) and tau-induced neurodegeneration (61). In both models, NMNAT is capable of binding substrate targets (e.g. ataxin and tau), preventing their aggregation and mitigating their proteotoxicity. Previous studies have demonstrated that inhibition of the ubiquitin proteasome system can delay WD in both mammalian and Drosophila models (44,62,63). An exciting hypothesis is that NMNAT/WLDS protect axons by chaperoning or shielding critical WD substrates from proteolytic degradation. Further identification of NMNAT chaperone targets might therefore provide greater insight into its protection as well as a better understanding of the proteins that regulate WD.
The ability to preserve mitochondria and protect against degeneration independent of mitochondrial function raises the possibility of parallel mechanisms of NMNAT/WLDS-neuroprotection, especially in neurodegenerative disease linked to mitochondria dysfunction (64). It will be important to dissect the mitochondria-preserving properties from other functional roles of NMNAT/WLDS, the understanding of which could provide new avenues for therapeutic design in neurodegenerative disease.
MATERIALS AND METHODS
Plasmids and generation of lentiviral particles
The cDNAs for lentiviral vector-mediated transgene expression were sublconed into the lentiviral transfer vector pLentiMP2 (pRRLsin.PPT.Th.CMV.MCS.Wpre) carrying the cytomegalovirus (CMV) promoter (65,66). The following cDNAs were cloned: a monomeric red fluorescent protein (mRFP) and D. melanogaster NMNAT (mRFP-DmNMNAT) fusion, mCherry-WLDS, mCherry-WLDS ΔNLS, mCherry-Homo sapiens NMNAT1, mCherry-HsNMNAT3 and mitoGFP (GFP-cytochrome c oxidase, GFP-COx).
For lentiviral particle production, we used the four-plasmid method as described previously (65,66). Lentivirus was concentrated by ultracentrifugation at 21 000 rpm and stored at −80°C in the presence of phosphate-buffered saline (PBS) and 0.5% bovine serum albumin (BSA). The viral titers were determined by ELISA for the HIV Gag capsid protein p24 (Perkin Elmer, Waltham, MA, USA). Viral titers were expressed in transducing units (TU) and ranged from 108 to 109 TU/ml.
DRG explant culture, axotomy protocol and quantification of axon degeneration
Whole DRG explants were dissected aseptically from E15.5 rat or gestation week (GW) 8–8.5 human spinal cord tissue, rinsed in cold L15 media, and seeded on poly-d-lysine (0.1 mg/ml in 0.1 m boric acid, pH 8.0) (Sigma-Aldrich, St Louis, MO, USA) and laminin-coated (5 μg/ml in 0.1 m PBS) (Sigma-Aldrich) plates. Cells were grown in a culture medium consisting of Neurobasal, 2% B-27 serum-free supplement, 0.5% GlutaMAX (Gibco), nerve growth factor (NGF, 10 ng/ml) (PeproTech, Rocky Hill, NJ, USA), neurotrophin-3 (NT-3, 10 ng/ml) (PeproTech) and 1 μm 5-fluoro-2′-deoxyuridine (FudR) (Sigma-Aldrich) at 37°C, 5% CO2. One day after plating, cultures were transduced by adding concentrated lentivirus directly to the culture medium at a multiplicity of infection of ∼100. Media was refreshed 24 h post-transduction and every 2 days thereafter. Transduced neurons were examined under a fluorescence microscope to confirm detectable lentiviral-vector mediated transgene expression. In Figure 1A, rat and human DRG explants were immunostained using the primary antibodies rabbit anti-islet1/2 (Developmental Studies Hybridoma Bank, University of Iowa) 1:1000, mouse anti-neurofilament medium (NF-M) (EnCore Biotechnologies, Gainesville, FL, USA) 1:200. Goat anti-rabbit Alexa 594 and goat anti-mouse Alexa 488 (Invitrogen Life Technologies) secondary antibodies were used at 1:1000 as well as a nuclear 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen Life Technologies) counter-stain at 1:1000.
For ex vivo axotomy, DRG explants extend axons 1-week post-transduction at which point axons were severed using the sharpened end of a glass capillary tube to remove explant heads containing the neuronal cell bodies. Cultures were fixed in 4% paraformaldehyde (PFA) in PBS (pH 7.4) and embedded at various timepoints post-axotomy in Kaiser's glycerol gelatin (8% gelatin, 50% glycerol, 1% carbolic acid). To quantify axon degeneration, phase-contrast images were taken from ≥3 random fields per explant (n ≥ 3 DRG explants per transgene and time point) at a position ∼800 μm from the axotomy edge. Images were randomized for non-biased analysis. Axons that were fragmented or exhibited varicosities greater than twice the diameter of the axon shaft were scored as ‘degenerated’. The fractions of intact axons per field were determined by dividing the total number of non-degenerated axons by the total number of axons in that field.
EM and quantification of mitochondria morphology
For EM analysis, cultures were prepared as described previously (67). Briefly, DRG explant cultures were fixed in 2% glutaraldehyde (in 0.1 M phosphate buffer, PB, plus 100 mm sucrose) overnight at 4°C and post-fixed in 2% osmium tetroxide (in 0.1 m PB) for 1 h at 4°C. Cultures were rinsed with 0.1 m PB and dehydrated in ethanol. Axons were embedded in EMbed 812 resin (Electron Microscopy Sciences, Hatfield, PA, USA) and cured overnight at 64°C. Resin blocks were thin-sectioned at silver/gold thickness, mounted on copper grids, and stained with uranyl acetate and lead citrate. EM images were taken using a Philips CM10 electron microscope at 60 kV. Axons were imaged at a ×5200 magnification for comparison of mitochondria morphology.
For quantification of axonal mitochondria length, 20 random EM fields were imaged per grid for each treatment and timepoint. From these 20 micrographs, mitochondria length was determined in non-degenerated axons based on the following criteria: (i) an axon was deemed non-degenerated if it had an intact microtubule network similar to that of uncut controls, lacked any breaks or discontinuations in the axolemma besides those due to axons leaving the plane of the image and contained no axonal swellings or ‘beads’ greater than twice the diameter of the axon itself; (ii) structures were classified as mitochondria based on the presence of a double membrane and a visible mitochondrial matrix/cristae; (iii) structures lacking a visible mitochondrial matrix but containing a double membrane otherwise distinguishable from other organelles, such as multi-vesicular bodies, were counted as mitochondria; (iv) mitochondria length was determined by measuring the longest dimension. Statistical analyses were performed by K–S test for frequency histogram (Fig. 1H and Supplementary Material, Fig. S1C) and Kruskal–Wallis test with Dunn's multiple comparison post-test for box plots (Supplementary Material, Fig. S1B).
Live imaging of mitochondria in DRG axons
DRG co-transduced with pLenti-mitoGFP and mCherry-WLDS ΔNLS and imaged live 30 min post-axotomy on an Olympus IX70 inverted microscope using a ×60 oil, 1.40 NA objective in a temperature-controlled chamber (37°C, 5% CO2). Movies were taken over 5 min and recorded at 40–60 frames per minute.
Cytochrome c oxidase (complex IV) activity assay
To determine the functional state of mitochondria in WLDS-protected axons, we directly measured the cytochrome c oxidase (COx, Complex IV) activity from WLDS-infected DRG explants 10 min, 24, 48 and 72 h post-axotomy. Complex IV activity was determined spectrophotometrically as the decrease in absorbance at 550 nm resulting from the oxidation of reduced cytochrome c (68). Cell bodies (explant heads) and axons from DRG explants were collected in 40 μl of phosphate buffered saline (PBS, pH 7.4) and snap frozen in liquid nitrogen. Fifteen DRG explants were used per experiment (n ≥ 3 experiments per group). To disrupt cell membranes, samples underwent three freeze/thaw cycles followed by the addition of 2.5 mm lauryl maltoside to permeablize outer mitochondrial membranes. To measure Complex IV activity, samples were subsequently added to a 1 ml solution containing 10 mm KH2PO4 (pH 6.5), 0.25 M sucrose, 1 mg/ml BSA and 10 μm of reduced cytochrome c and the absorbance was immediately recorded for 1 min. Complex IV specific activity was expressed as the μmoles of cytochrome c oxidized/min/μg of total protein and calculated by the equation:
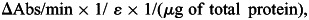
where ɛ = 18.5, the mm extinction coefficient for cytochrome c. Total sample protein was determined using the CBQCA fluorescent protein quantitation kit following the manufacturer's protocol (Invitrogen Life Technologies, Carlsbad, CA, USA). Statistical analysis was performed by one-way analysis of variance (ANOVA) with Tukey's multiple comparison post-test.
Drosophila stocks and complementation assay
All Drosophila stock strains were maintained on a cornmeal-molasses-yeast media at room temperature (∼22°C) with 60–65% ambient humidity. The following Drosophila strains were used in these studies: ;;Or22a-Gal4, UAS-mCD8-GFP, P{lacW}miltK06704, Df(2L)BSC291, Df(2L)ED441 and Df(2L)spd2were obtained from Bloomington Drosophila Stock Center (University of Indiana, Bloomington, IN, USA). hsFLP, UAS-mCD8-RFP; FRT40A tub-Gal80/CyO was obtained from Dr Jay Parrish, PhD (Department of Biology, University of Washington). ;;D42-Gal4, UAS-mitoGFP and FRT40A milton33-853 was obtained from Dr Hugo J. Bellen, DVM, PhD (Duncan Neurological Research Institute, Baylor College of Medicine). FRT40A milton92/CyO, y+ was obtained from Dr Thomas Schwarz (Children's Hospital, Harvard Medical School). UAS-WLDS was obtained from Dr Marc R. Freeman (Department of Neurobiology, University of Massachusetts Medical School). eyFLP; FRT40A cl- w+/CyO, UAS-DmNMNAT (wild-type Drosophila NMNAT) and UAS-DmNMNATWR (<1% enzymatic activity) strains were generated in the laboratory and have been reported elsewhere (14).
To visualize wild-type and milton adult ORNs or larvae MNs and at the same time overexpress DmNMNAT, DmNMNATWR, WLDS or mitoGFP, we generated the following strains by recombination using the stocks listed above:
eyFLP; FRT40A cl- w+/CyO; Or22a-Gal4/TM6, Tb, Hu
FRT40A iso/CyO; UAS-DmNMNAT
FRT40A iso/CyO; UAS-DmNMNATWR
FRT40A iso/CyO; UAS-WLDS
FRT40A milton33-853/CyO; UAS-DmNMNAT
FRT40A milton33-853/CyO; UAS- DmNMNATWR
FRT40A milton33-853/CyO; UAS- WLDS
FRT40A milton33-853/CyO; D42-Gal4, UAS-mitoGFP
‘MARCM set’:
hsFLP, C155-Gal4,UAS-mCD8-GFP; FRT40A tub-Gal80/CyO
‘MARCM set’:
hsFLP, UAS-mCD8-RFP; FRT40A tub-Gal80/CyO; D42-Gal4, UAS-mitoGFP
eyFLP; FRT40A cl- w+/CyO; Or22a-Gal4/TM6, Tb, Hu
FRT40A iso/CyO; UAS-DmNMNAT
FRT40A iso/CyO; UAS-DmNMNATWR
FRT40A iso/CyO; UAS-WLDS
FRT40A milton33-853/CyO; UAS-DmNMNAT
FRT40A milton33-853/CyO; UAS- DmNMNATWR
FRT40A milton33-853/CyO; UAS- WLDS
FRT40A milton33-853/CyO; D42-Gal4, UAS-mitoGFP
The milton33-853 allele used in this study was generated in a previously reported EMS mutagenesis screen (38). To characterize this loss-of-function allele and ensure that the larval lethality and mitochondria trafficking defects were not the result of a second-site mutagenesis hit, we performed lethality-based complementation mapping using a previously reported larvae lethal milton92 allele (39), the loss-of-function p-element insertion line P{lacW}miltK06704, and the deficiency lines Df(2L)BSC291, Df(2L)ED441, and Df(2L)spd2 containing genomic deletions spanning the milton gene (Bloomington Stock Center Deletion Project) (Supplementary Material, Fig. S3A). milton33-853/CyO flies were crossed with each of these flies and lethality was determined by counting the number of non-balanced progeny flies following eclosion. milton33-853 failed to complement all alleles and Df lines tested.
Imaging of live (non-fixed) whole Drosophila larvae
Early second-instar larvae were mounted alive on a microscope slide using double-sided tape, immersed in glycerol, and immobilized with a coverslip so that the ventral side of larvae was facing up. Confocal microscopy was performed through the cuticle to image the distribution of mitoGFP signal in the nervous system of control and homozygous milton33-853 larvae (D42-Gal4>UAS-mitoGFP).
Generation of single MN mosaic analysis with a repressible cell marker (MARCM) clones
MARCM set flies were crossed with flies previously generated containing either a wild-type chromosome (FRT40A iso) or a milton allele (FRT40A milton33-853). Embryos from this cross were collected and heat shocked at 38°C for 1 h, 3–5 h after egg laying. Larvae are allowed to mature at 25°C until early L3 at which point a nerve crush was performed.
Larvae nerve crush protocol and quantification of axon degeneration
To axotomize MN MARCM clones, a larvae nerve crush injury was performed as described previously (46). Briefly, early third-instar larvae were sorted using a fluorescence stereomicroscope and those larvae containing mCD8-GFP+ MARCM clones were briefly anesthetized on a CO2 pad. The ventral nerve cord (VNC) and segmental nerves were visualized through the translucent cuticle under a standard dissection stereomicroscope and the segmental nerves were crushed by pinching around the cuticle for 5 s using a Dumont number 5 forceps (Fine Science Tools, Foster City, CA, USA). Larvae were transferred to grape juice plates with yeast paste and maintained at 25°C until dissection.
Axon degeneration was quantitatively assessed by morphological categorization following nerve crush injury. Larvae were immunostained following dissection with the Drosophila axon membrane marker HRP (50,51) to trace proximal and distal axons of mCD8-GFP+ MN MARCM clones from their cell body of origin to their NMJ within segmental nerves. A successful axotomy contained a clear gap in mCD8-GFP signal ≥25 μm between proximal and distal axons. Axon degeneration status was scored in a 200 μm segment of distal axon starting ∼300 μm away from the start of the distal axon segment. Distal axons were categorized as either ‘continuous’ (non-degenerated) or ‘fragmented’ (degenerated) based on the presence of ‘breaks’ in mCD8-GFP signal ≥1 μm in length; axons were considered fragmented if they contained ≥10 breaks, and continuous if they contained ≤5 breaks. As varicosities were a feature common to both uninjured wild-type and milton axons, this morphological characteristic was not used in classifying WD in this model system. As the pan-neuronal driver C155-Gal4 was used to label individual clones, the following criteria were established to qualify distal axons for scoring following axotomy: (i) distal axons of MNs were identified by locating their proximal axons within the same HRP+ segmental nerve and tracing them back to a cell body within the VNC; (ii) for ‘mixed’ segmental nerves which contained mCD8-GFP+ axons from both motor and sensory neuron clones, distal MN axons were scored only if they could be distinguished from sensory neurons by tracing proximal sensory neuron axons to a cell body in the larvae body wall; (iii) segmental nerves containing axons from >5 MN clones were excluded from scoring owing to the difficulty in distinguishing the degeneration status of individual distal axons in these nerves; and (iv) axons from multiple MNs that traversed a segmental nerve as a single axon fascicle were scored as a single axon. Randomized confocal micrographs of distal axons were scored by an observer blinded to genotype and timepoint post-axotomy. The fraction of fragmented and continuous axons was organized into a contingency table and statistical analysis was performed by Fisher's exact test.
Antenna nerve transection
Axotomy of ORNs was performed as described previously (15). One week after eclosion, flies were briefly anesthetized on CO2 and the third segment of the antennae was transected using SuperFine Vannas scissors (World Precision Instruments, Sarasota, FL, USA). Post-axotomy, flies were returned to food vials and maintained at room temperature until dissection.
Dissection and immunolabeling
Larvae filet preparations and adult fly brains were dissected in ice cold phosphate-buffered saline (PBS) and fixed for 20 min in 4% formaldehyde (pH 7.4) diluted in PBS. Fixative was removed and larvae/brains were washed/permeablized ×3 with 0.4% Triton X-100 (Sigma-Aldrich) in PBS (PBST). Primary antibodies were diluted in 5% normal goat serum (NGS)/PBST and used at the following dilutions: rabbit anti-HRP (Jackson Immunoresearch) 1:1000; mouse anti-nc82 (Developmental Studies Hybridoma Bank, University of Iowa) 1:200. Tissues were incubated in primary antibody overnight at 4°C, washed ×3 with PBST and incubated in secondary antibody for 1 h at room temperature. Goat anti-rabbit or goat-anti-mouse Alexa 637 (Invitrogen Life Technologies) secondary antibodies were used at 1:200. Nuclear 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen Life Technologies) counter-stain was used at 1:1000. Tissues were washed ×3 with PBST following incubation in secondary antibodies and mounted on microscope slides in Vectashield Mounting Medium for Fluorescence (Vector Laboratories, Burlingame, CA, USA).
Confocal microscopy
Images from fluorescently labeled specimens were captured on an Olympus IX81 confocal microscope using an Olympus PlanApo N 60x/1.42 NA oil-immersion objective. Images were processed using FluoView10-ASW (Olympus, Tokyo, Japan) and Adobe Photoshop CS5 (Adobe Systems, San Jose, CA, USA).
SUPPLEMENTARY MATERIAL
FUNDING
This work was supported by a Lois Pope Neuroscience Research Fellowship (to B.M.K.), the Buoniconti Fund and the Miami Project to Cure Paralysis (to P.T.), the National Institute for Neurological Disorders and Stroke Grant R01NS64269 (to R.G.Z.), the University of Miami Neuroscience Center Fellowship (to R.G.Z.) and the Pew Charitable Trust (to R.G.Z.).
ACKNOWLEDGEMENTS
The authors thank the Bloomington Stock Center for flies and related reagents. We would like to thank Margaret Bates at the UM EM Core and Pingping Jia at the Miami Project to Cure Paralysis Viral Vector Core for technical support and Dr Brant Watson for critical reading of the manuscript. We also thank Dr Alejandro Ocampo and Dr Antonio Barrientos for constructive discussion of the project and sharing data.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Mattson M.P. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. doi:10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- 2.Yuan J., Yankner B.A. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. doi:10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 3.Saxena S., Caroni P. Mechanisms of axon degeneration: from development to disease. Prog. Neurobiol. 2007;83:174–191. doi: 10.1016/j.pneurobio.2007.07.007. doi:10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Medana I.M., Esiri M.M. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. doi:10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 5.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. doi:10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- 6.Waller A. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. 1850;140:423–429. doi:10.1098/rstl.1850.0021. [PMC free article] [PubMed] [Google Scholar]
- 7.Raff M.C., Whitmore A.V., Finn J.T. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. doi:10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- 8.Perry V.H., Brown M.C., Lunn E.R., Tree P., Gordon S. Evidence that very slow Wallerian degeneration in C57BL/Ola mice is an intrinsic property of the peripheral nerve. Eur. J. Neurosci. 1990;2:802–808. doi: 10.1111/j.1460-9568.1990.tb00472.x. doi:10.1111/j.1460-9568.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 9.Perry V.H., Lunn E.R., Brown M.C., Cahusac S., Gordon S. Evidence that the rate of Wallerian degeneration is controlled by a single autosomal dominant gene. Eur. J. Neurosci. 1990;2:408–413. doi: 10.1111/j.1460-9568.1990.tb00433.x. doi:10.1111/j.1460-9568.1990.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 10.Araki T., Sasaki Y., Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. doi:10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 11.Conforti L., Fang G., Beirowski B., Wang M.S., Sorci L., Asress S., Adalbert R., Silva A., Bridge K., Huang X.P., et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007;14:116–127. doi: 10.1038/sj.cdd.4401944. doi:10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- 12.Gillingwater T.H., Ingham C.A., Parry K.E., Wright A.K., Haley J.E., Wishart T.M., Arbuthnott G.W., Ribchester R.R. Delayed synaptic degeneration in the CNS of Wlds mice after cortical lesion. Brain. 2006;129:1546–1556. doi: 10.1093/brain/awl101. doi:10.1093/brain/awl101. [DOI] [PubMed] [Google Scholar]
- 13.Wang J., Zhai Q., Chen Y., Lin E., Gu W., McBurney M.W., He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J. Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. doi:10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhai R.G., Cao Y., Hiesinger P.R., Zhou Y., Mehta S.Q., Schulze K.L., Verstreken P., Bellen H.J. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006;4:e416. doi: 10.1371/journal.pbio.0040416. doi:10.1371/journal.pbio.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avery M.A., Sheehan A.E., Kerr K.S., Wang J., Freeman M.R. Wld S requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J. Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. doi:10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack T.G., Reiner M., Beirowski B., Mi W., Emanuelli M., Wagner D., Thomson D., Gillingwater T., Court F., Conforti L., et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. doi:10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- 17.Court F.A., Coleman M.P. Mitochondria as a central sensor for axonal degenerative stimuli. Trends Neurosci. 2012;35:364–372. doi: 10.1016/j.tins.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 18.Di Lisa F., Ziegler M. Pathophysiological relevance of mitochondria in NAD(+) metabolism. FEBS Lett. 2001;492:4–8. doi: 10.1016/s0014-5793(01)02198-6. doi:10.1016/S0014-5793(01)02198-6. [DOI] [PubMed] [Google Scholar]
- 19.Schlaepfer W.W. Effects of energy deprivation on Wallerian degeneration in isolated segments of rat peripheral nerve. Brain Res. 1974;78:71–81. doi: 10.1016/0006-8993(74)90354-0. doi:10.1016/0006-8993(74)90354-0. [DOI] [PubMed] [Google Scholar]
- 20.Ikegami K., Koike T. Non-apoptotic neurite degeneration in apoptotic neuronal death: pivotal role of mitochondrial function in neurites. Neuroscience. 2003;122:617–626. doi: 10.1016/j.neuroscience.2003.08.057. doi:10.1016/j.neuroscience.2003.08.057. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez S., Moldovan M., Krarup C. Acute energy restriction triggers Wallerian degeneration in mouse. Exp. Neurol. 2008;212:166–178. doi: 10.1016/j.expneurol.2008.03.022. doi:10.1016/j.expneurol.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Sievers C., Platt N., Perry V.H., Coleman M.P., Conforti L. Neurites undergoing Wallerian degeneration show an apoptotic-like process with Annexin V positive staining and loss of mitochondrial membrane potential. Neurosci. Res. 2003;46:161–169. doi: 10.1016/s0168-0102(03)00039-7. doi:10.1016/S0168-0102(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 23.Yahata N., Yuasa S., Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. doi:10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger F., Lau C., Dahlmann M., Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. doi:10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 25.Avery M.A., Rooney T.M., Pandya J.D., Wishart T.M., Gillingwater T.H., Geddes J.W., Sullivan P.G., Freeman M.R. WldS prevents axon degeneration through increased mitochondrial flux and enhanced mitochondrial Ca2+ buffering. Curr. Biol. 2012;22:596–600. doi: 10.1016/j.cub.2012.02.043. doi:10.1016/j.cub.2012.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki Y., Vohra B.P., Lund F.E., Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J. Neurosci. 2009;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. doi:10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beirowski B., Babetto E., Gilley J., Mazzola F., Conforti L., Janeckova L., Magni G., Ribchester R.R., Coleman M.P. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J. Neurosci. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. doi:10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasaki Y., Vohra B.P., Baloh R.H., Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J. Neurosci. 2009;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. doi:10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knott A.B., Perkins G., Schwarzenbacher R., Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat. Rev. Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. doi:10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perkins G., Bossy-Wetzel E., Ellisman M.H. New insights into mitochondrial structure during cell death. Exp. Neurol. 2009;218:183–192. doi: 10.1016/j.expneurol.2009.05.021. doi:10.1016/j.expneurol.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dubinsky J.M., Levi Y. Calcium-induced activation of the mitochondrial permeability transition in hippocampal neurons. J. Neurosci. Res. 1998;53:728–741. doi: 10.1002/(SICI)1097-4547(19980915)53:6<728::AID-JNR10>3.0.CO;2-U. doi:10.1002/(SICI)1097-4547(19980915)53:6<728::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 32.Rintoul G.L., Filiano A.J., Brocard J.B., Kress G.J., Reynolds I.J. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J. Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brustovetsky T., Li V., Brustovetsky N. Stimulation of glutamate receptors in cultured hippocampal neurons causes Ca2+-dependent mitochondrial contraction. Cell Calcium. 2009;46:18–29. doi: 10.1016/j.ceca.2009.03.017. doi:10.1016/j.ceca.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H., Chan D.C. Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum. Mol. Genet. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. doi:10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H., Chan D.C. Emerging functions of mammalian mitochondrial fusion and fission. Hum. Mol. Genet. 2005;14:R283–R289. doi: 10.1093/hmg/ddi270. doi:10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- 36.Rintoul G.L., Reynolds I.J. Mitochondrial trafficking and morphology in neuronal injury. Biochim. Biophys. Acta. 2010;1802:143–150. doi: 10.1016/j.bbadis.2009.09.005. doi:10.1016/j.bbadis.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Glater E.E., Megeath L.J., Stowers R.S., Schwarz T.L. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. doi:10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mehta S.Q., Hiesinger P.R., Beronja S., Zhai R.G., Schulze K.L., Verstreken P., Cao Y., Zhou Y., Tepass U., Crair M.C., et al. Mutations in Drosophila sec15 reveal a function in neuronal targeting for a subset of exocyst components. Neuron. 2005;46:219–232. doi: 10.1016/j.neuron.2005.02.029. doi:10.1016/j.neuron.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 39.Stowers R.S., Megeath L.J., Gorska-Andrzejak J., Meinertzhagen I.A., Schwarz T.L. Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron. 2002;36:1063–1077. doi: 10.1016/s0896-6273(02)01094-2. doi:10.1016/S0896-6273(02)01094-2. [DOI] [PubMed] [Google Scholar]
- 40.MacDonald J.M., Beach M.G., Porpiglia E., Sheehan A.E., Watts R.J., Freeman M.R. The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. doi:10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Hummel T., Vasconcelos M.L., Clemens J.C., Fishilevich Y., Vosshall L.B., Zipursky S.L. Axonal targeting of olfactory receptor neurons in Drosophila is controlled by Dscam. Neuron. 2003;37:221–231. doi: 10.1016/s0896-6273(02)01183-2. doi:10.1016/S0896-6273(02)01183-2. [DOI] [PubMed] [Google Scholar]
- 42.Chou Y.H., Zheng X., Beachy P.A., Luo L. Patterning axon targeting of olfactory receptor neurons by coupled hedgehog signaling at two distinct steps. Cell. 2010;142:954–966. doi: 10.1016/j.cell.2010.08.015. doi:10.1016/j.cell.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parson S.H., Mackintosh C.L., Ribchester R.R. Elimination of motor nerve terminals in neonatal mice expressing a gene for slow wallerian degeneration (C57Bl/Wlds) Eur. J. Neurosci. 1997;9:1586–1592. doi: 10.1111/j.1460-9568.1997.tb01516.x. doi:10.1111/j.1460-9568.1997.tb01516.x. [DOI] [PubMed] [Google Scholar]
- 44.Hoopfer E.D., McLaughlin T., Watts R.J., Schuldiner O., O'Leary D.D., Luo L. Wlds protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. doi:10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Fang Y., Soares L., Teng X., Geary M., Bonini N.M. A novel Drosophila model of nerve injury reveals an essential role of Nmnat in maintaining axonal integrity. Curr. Biol. 2012;22:590–595. doi: 10.1016/j.cub.2012.01.065. doi:10.1016/j.cub.2012.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiong X., Wang X., Ewanek R., Bhat P., Diantonio A., Collins C.A. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J. Cell Biol. 2010;191:211–223. doi: 10.1083/jcb.201006039. doi:10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee T., Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. doi:10.1016/S0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 48.Nikic I., Merkler D., Sorbara C., Brinkoetter M., Kreutzfeldt M., Bareyre F.M., Bruck W., Bishop D., Misgeld T., Kerschensteiner M. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2011;17:495–499. doi: 10.1038/nm.2324. doi:10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- 49.Kim M.D., Wen Y., Jan Y.N. Patterning and organization of motor neuron dendrites in the Drosophila larva. Dev. Biol. 2009;336:213–221. doi: 10.1016/j.ydbio.2009.09.041. doi:10.1016/j.ydbio.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jan L.Y., Jan Y.N. Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc. Natl Acad. Sci. USA. 1982;79:2700–2704. doi: 10.1073/pnas.79.8.2700. doi:10.1073/pnas.79.8.2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paschinger K., Rendic D., Wilson I.B. Revealing the anti-HRP epitope in Drosophila and Caenorhabditis. Glycoconj. J. 2009;26:385–395. doi: 10.1007/s10719-008-9155-3. doi:10.1007/s10719-008-9155-3. [DOI] [PubMed] [Google Scholar]
- 52.Beirowski B., Adalbert R., Wagner D., Grumme D.S., Addicks K., Ribchester R.R., Coleman M.P. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. doi:10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kerschensteiner M., Schwab M.E., Lichtman J.W., Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 2005;11:572–577. doi: 10.1038/nm1229. doi:10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 54.Jia H., Yan T., Feng Y., Zeng C., Shi X., Zhai Q. Identification of a critical site in Wld(s): essential for Nmnat enzyme activity and axon-protective function. Neurosci. Lett. 2007;413:46–51. doi: 10.1016/j.neulet.2006.11.067. doi:10.1016/j.neulet.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 55.Wang J.T., Medress Z.A., Barres B.A. Axon degeneration: molecular mechanisms of a self-destruction pathway. J. Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. doi:10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.George E.B., Glass J.D., Griffin J.W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 1995;15:6445–6452. doi: 10.1523/JNEUROSCI.15-10-06445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knoferle J., Koch J.C., Ostendorf T., Michel U., Planchamp V., Vutova P., Tonges L., Stadelmann C., Bruck W., Bahr M., et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc. Natl Acad. Sci. USA. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. doi:10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasola A., Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. doi:10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 59.Ocampo A., Liu J.J., Barrientos A. NAD+ salvage pathway proteins suppress proteotoxicity in yeast models of neurodegeneration by promoting the clearance of misfolded/oligomerized proteins. Hum. Mol. Genet. 2013 doi: 10.1093/hmg/ddt016. doi:10.1093/hmg/ddt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhai R.G., Zhang F., Hiesinger P.R., Cao Y., Haueter C.M., Bellen H.J. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;452:887–891. doi: 10.1038/nature06721. doi:10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ali Y.O., Ruan K., Zhai R.G. NMNAT suppresses tau-induced neurodegeneration by promoting clearance of hyperphosphorylated tau oligomers in a Drosophila model of tauopathy. Hum. Mol. Genet. 2012;21:237–250. doi: 10.1093/hmg/ddr449. doi:10.1093/hmg/ddr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai Q., Wang J., Kim A., Liu Q., Watts R., Hoopfer E., Mitchison T., Luo L., He Z. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. doi:10.1016/S0896-6273(03)00429-X. [DOI] [PubMed] [Google Scholar]
- 63.MacInnis B.L., Campenot R.B. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol. Cell. Neurosci. 2005;28:430–439. doi: 10.1016/j.mcn.2004.10.003. doi:10.1016/j.mcn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Schon E.A., Przedborski S. Mitochondria: the next (neurode)generation. Neuron. 2011;70:1033–1053. doi: 10.1016/j.neuron.2011.06.003. doi:10.1016/j.neuron.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dull T., Zufferey R., Kelly M., Mandel R.J., Nguyen M., Trono D., Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Follenzi A., Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. doi:10.1016/S0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- 67.Ratner N., Elbein A., Bunge M.B., Porter S., Bunge R.P., Glaser L. Specific asparagine-linked oligosaccharides are not required for certain neuron-neuron and neuron-Schwann cell interactions. J. Cell Biol. 1986;103:159–170. doi: 10.1083/jcb.103.1.159. doi:10.1083/jcb.103.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barrientos A. In vivo and in organello assessment of OXPHOS activities. Methods. 2002;26:307–316. doi: 10.1016/S1046-2023(02)00036-1. doi:10.1016/S1046-2023(02)00036-1. [DOI] [PubMed] [Google Scholar]