Abstract
BACKGROUND
In a prior study of patients with diabetes, diastolic function was similarly impaired in masked hypertension (MHT) and sustained hypertension (SHT). We evaluated whether MHT is associated with impaired diastolic function compared with SHT and sustained normotension (NT) in the general population.
METHODS
From February 2005 to December 2010, 798 participants without a history of cardiovascular disease or treated hypertension, were enrolled in the Masked Hypertension Study. Participants underwent clinic blood pressure (CBP) and 24-hour ambulatory blood pressure (ABP) measurements. A 2-dimensional Doppler echocardiogram was performed to evaluate diastolic function,s cardiac structure, volume, and systolic function. The 9 CBPs obtained across 3 clinic visits and awake ABP measurements were averaged. Clinic hypertension was defined as systolic/diastolic blood pressure (SBP/DBP) ≥ 140/90 mmHg. Ambulatory hypertension was defined as awake SBP/DBP ≥ 135/85mm Hg. MHT was defined as having ambulatory but not clinic hypertension. White-coat hypertensives (n = 8) were excluded from the analysis.
RESULTS
Of the 790 participants, 116 (14.7%) participants had MHT, 37 (4.7%) participants had SHT, and 637 (80.6%) participants had NT. After age, sex, race/ethnicity, and body mass index adjustment, compared with NT, E’-velocities were significantly lower in MHT (P < 0.01) and SHT (P < 0.05), and E/E’ ratios were significantly higher MHT (P < 0.05) and SHT (P < 0.05). These associations were independent of left ventricular mass. Diastolic function parameters did not significantly differ between MHT and SHT.
CONCLUSIONS
Diastolic function was impaired in MHT compared with NT independent of changes in left ventricular mass.
Keywords: ambulatory blood pressure monitoring, blood pressure, echocardiography, hypertension.
The term masked hypertension (MHT)1 is used to describe individuals with clinic blood pressure (CBP) in the normal range (<140/90mm Hg) but elevated awake ambulatory blood pressure (ABP; ≥135/85mm Hg). The prevalence of MHT is estimated to be between 8% and 20% in the general adult population.2 Individuals with MHT have been shown to have a higher risk of cardiovascular events than individuals with sustained normotension (NT), defined as having normal CBP and normal ABP (<135/85mm Hg) and similar cardiovascular risk compared with individuals with sustained hypertension (SHT), defined as having clinic hypertension (HT; ≥140/90mm Hg) and elevated ABP (≥135/85mm Hg).3–6 Previous cross-sectional studies have shown that participants with MHT have higher levels of left ventricular mass (LVM)7, 8 and a higher likelihood of concentric remodeling,4, 9 compared with individuals with NT.
Impaired diastolic function is a common finding in HT.10 It may occur in the absence of left ventricular hypertrophy (LVH) in HT11 and may even occur before the development of HT and LVH.12 In a sample of 71 clinic outpatients with diabetes, Marchesi et al.13 showed that LVM increased progressively from NT to MHT to SHT, whereas diastolic function was found to be similarly impaired in MHT and SHT compared with NT. Because individuals with MHT may be at increased risk of developing SHT,14 we hypothesized that an alteration of diastolic function may be present in MHT compared with NT in a population with a wider range of cardiovascular disease risk. We also hypothesized that diastolic function may be similarly impaired in MHT compared with SHT. The primary aim of this study was to evaluate left ventricular (LV) diastolic function as well as cardiac structures, volumes, and systolic function in MHT compared with NT and SHT among participants from the Masked Hypertension Study, an ongoing, worksite-based study.
METHODS
Study population
The Masked Hypertension Study, an ongoing, worksite-based study of the prevalence, predictors, and prognosis of MHT, is comprised of adult employees who work for > 20 hours/week, including at least 2 consecutive days. Participants were recruited from 2 large universities with medical schools (Stony Brook University and Columbia University) and affiliated teaching hospitals, as well as a private hedge fund management organization. The current analysis includes 798 participants who were enrolled between February 2005 and December 2010. Exclusion criteria included any of the following: a screening clinic systolic blood pressure (SBP) > 160mm Hg or diastolic blood pressure (DBP) > 105mm Hg, evidence of secondary hypertension other than a history of pregnancy-induced hypertension, taking antihypertensive medications or other medications that are known to affect blood pressure (e.g., steroids, tricyclic antidepressants), overt cardiovascular disease, history of chronic renal disease, liver disease, adrenal disease, thyroid disease, were pregnant, or reported active substance abuse or a severe debilitating psychiatric disorder. The blood pressure eligibility criterion for this study was chosen to obtain a sample with a wide distribution of untreated blood pressures. For safety reasons, we referred participants immediately to their physicians for further management if their screening CBP was > 160/105mm Hg. Information about demographics (age, sex, race, ethnicity), height, weight, cardiovascular risk factors, and family history of risk factors were ascertained from all participants. Written informed consent was obtained from all participants. The study was approved by the institutional review boards of Columbia University and Stony Brook University.
Blood pressure assessments
Participants attended 5 visits over a 4-week period. During the first 3 visits (visit 1–3), which usually occurred within a 3-week period, the participant was escorted into an examination room and asked to rest comfortably in the seated position, with legs uncrossed, for at least 5 minutes. A research nurse/technician then obtained 3 consecutive CBP readings, separated by at least 1 minute, using a mercury sphygmomanometer (W.A. Baum, Copiague, NY) and stethoscope. Thus, a total of 9 CBP readings were available for each participant. On visit 3, the participant was fitted with an appropriate-sized arm cuff for a Spacelabs ambulatory blood pressure monitor (Model 90207; Redmond, WA). ABP measurements were taken at 28-minute intervals throughout the subsequent 24-hour monitoring period. The recording was analyzed to obtain average awake SBP and DBP levels based on sleep/wake times defined by data obtained from an actigraphy monitor worn on the wrist (ActiWatch; Phillips Respironics, Murrayville, PA), supplemented by diary reports of the times participants woke up and went to sleep. The next day (visit 4) participants returned the ambulatory blood pressure monitor and the actigraphy monitor.
Hypertension classification
For purposes of the present analyses, participants were classified as having NHT, MHT, and SHT by CBP and ABP measurements. For the primary analysis, the 9 CBP readings from the 3 clinic visits were averaged. Clinic HT was defined as mean SBP ≥ 140mm Hg or mean DBP ≥ 90mm Hg. Ambulatory HT, based on mean awake ABP, was defined as mean SBP ≥ 135mm Hg or mean DBP ≥ 85mm Hg, which are internationally accepted limits.15, 16 MHT was defined as having CBP in the normal range (SBP < 140mm Hg and DBP < 90mm Hg) and ambulatory HT. SHT was defined as having both clinic HT and ambulatory HT. NT was defined as having both normal CBP and normal ABP. White-coat hypertension (WCHT) was defined as having clinic HT and normal ABP.
Two-dimensional and Doppler echocardiographic measures
During visit 5, 2-dimensional and Doppler echocardiography were performed and stored in a DICOM digital format for analysis. Cardiac measurements were obtained according to the recommendations of the American Society of Echocardiography (ASE). A minimum of 3 cardiac cycles was measured using an offline analysis package installed on a dedicated workstation (Syngo Dynamics version 7; Siemens Medical Systems, Mountain View, CA) and then averaged. Cardiac structures, volumes, and LV function were assessed by 2-dimensional echocardiography. Interventricularseptal thickness during diastole (IVSd), posterior wall thickness during diastole (PWTd), LV internal diameter during diastole (LVIDd), LV internal diameter during systole (LVIDs), and left atrial antero-posterior diameter (LAD) were obtained from long axis parasternal views. Fractional shortening percentage (FS%) was calculated using the following formula: FS% = 100 × (LVIDd − LVIDs) / LVIDd. LVM was calculated using the corrected ASE method: 0.8 × (1.04 × [(IVSd + LVIDd + PWTd)3− LVIDd3]) + 0.6. LVM index (LVMI) was calculated by dividing LVM by estimated body surface area, calculated from height and weight. The presence of LVH was defined as LVMI ≥ 89g/m2 for women and ≥ 103g/m2 for men according to ASE guidelines.17 Relative wall thickness (RWT) was calculated using the following formula: RWT = (IVSd + PWTd) / LVIDd. LV end-diastole volume (LVEDV) and LV end-systole volume (LVESV) were obtained from apical 4-chamber and 2-chamber views using the modified Simpson rule. LV ejection fraction (LVEF) was calculated using the following formula: LVEF (%) = 100 × (LVEDV − LVESV) / LVEDV. Transmitral flow by pulse-wave Doppler was obtained from the apical 4-chamber view. The peak early (E-wave) and late (A-wave) diastolic filling velocities and E-wave deceleration time (E-DcT) were measured, and the E/A ratio was calculated. The mitral annular motion velocity by tissue Doppler imaging was obtained from the apical 4-chamber view. The averaged values at septal and lateral position were used for analysis. The peak early (E’-wave) and late (A’-wave) diastolic annular velocities were measured. The ratio of E to E’ (E/E’) was calculated to assess diastolic function. An impaired relaxation pattern occurs at early stage, which is characterized by lower E-wave, E/A ratio, and prolonged E-DcT.18 E’-wave of mitral annular velocity also decrease with impaired LV relaxation.19 E’-wave correlates with a variety of other invasively measured indexes such as tau, LV-dP/dt, and minimal LV pressure.19 E’/E ratio, the combination of mitral flow velocity and mitral annulus velocity, has been identified as the best parameter for diagnosis of diastolic dysfunction.20 It has shown to have a better correlation with the estimation of LV filling pressure when compared with other Doppler measures.21
Statistical analysis
Characteristics of the study population were calculated as mean ± SD or percentage. Echocardiographic measures were calculated for participants with NT, MHT, and SHT with differences across groups assessed using 1-way analysis of variance with pairwise comparisons (Tukey honestly significant difference method). Analysis of covariance with pairwise comparisons (Tukey honestly significant difference method) was used to evaluate differences in echocardiographic measures after adjusting for age, sex, race/ethnicity, and body mass index (BMI). BMI was calculated as body weight in kilograms divided by the square of the height in meters. To explore whether group differences in diastolic parameters were explained by LVMI, the analyses of diastolic function parameters were repeated after adding LVMI to an age-, sex-, race/ethnicity-, and BMI-adjusted model. Analyses of diastolic function parameters were also repeated after adding heart rate during the echocardiogram and LVEF to an age-, sex-, race/ethnicity-, and BMI-adjusted model. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
Study population
Figure 1 shows the distribution of participants by CBP and ABP categories. Among the final sample of 790 participants, 637 (80.6%) had NT, 116 (14.7%) had MHT, and 37 (4.7%) had SHT. Table 1 shows baseline characteristics of the entire sample (N = 790) and by group (NT, MHT, and SHT). The prevalence of LVH was 2.5% in the study sample (N = 790) and 1.7%, 4.3%, and 10.8% in participants with NT, MHT, and SHT respectively.
Figure 1.
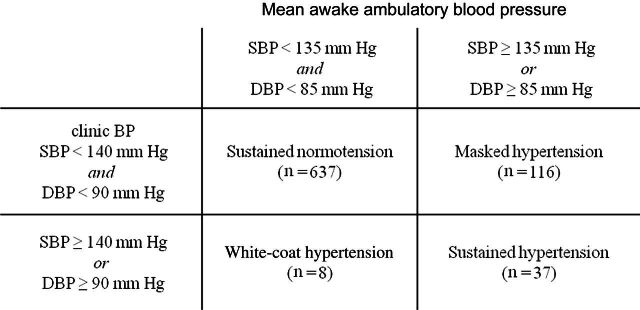
Participant categories defined by clinic and ambulatory blood pressure (BP). On the basis of clinic BP and ambulatory BP, the 798 participants were categorized into 4 groups: sustained normotension, masked hypertension, white-coat hypertension, and sustained hypertension. The participants (n = 8) with white-coat hypertension were excluded from the current analysis, which left a final sample size of 790.
Table 1.
Characteristics of the Masked Hypertensive Study participants included in this analysis
| Characteristics | Total (N = 790) | NT (n = 637) | MHT (n = 116) | SHT (n = 37) |
|---|---|---|---|---|
| Age, y | 45.5±10.3 | 44.8±10.3 | 48.0±9.6 | 49.6±1.0 |
| Sex, % male | 40.8 | 35.8 | 59.5 | 67.6 |
| Race/ethnicity | ||||
| % Black | 6.5 | 5.7 | 9.5 | 10.8 |
| % Hispanic | 10.8 | 11.5 | 6.9 | 10.8 |
| Body mass index, kg/m2 | 27.5±5.3 | 27.2±5.3 | 28.5±5.0 | 30.2±4.7 |
| History of high cholesterol, % | 26.7 | 25.6 | 25.9 | 48.6 |
| History of diabetes, % | 3.7 | 4.1 | 0.9 | 5.4 |
| Current smoking, % | 7.7 | 7.9 | 6.9 | 5.6 |
| Family history of hypertension, % | 66.3 | 66.4 | 65.5 | 66.7 |
| Clinic (mean of 9 readings, taken during 3 visits) | ||||
| Systolic blood pressure, mm Hg | 116±12 | 113±10 | 125±8 | 142±9 |
| Diastolic blood pressure, mm Hg | 75±8 | 73±6 | 81±5 | 91±6 |
| % Hypertensivea | 4.7 | 0 | 0 | 100.0 |
| Mean awake on 24-hour ABPM | ||||
| Systolic blood pressure, mm Hg | 123±10 | 120±8 | 135±7 | 143±8 |
| Diastolic blood pressure, mm Hg | 77±8 | 75±6 | 87±5 | 90±7 |
| % Hypertensiveb | 19.4 | 0 | 100.0 | 100.0 |
| Mean sleepc on 24-hour ABPM | ||||
| Systolic blood pressure, mm Hg | 106±11 | 104±9 | 116±10 | 123±10 |
| Diastolic blood pressure, mm Hg | 62±8 | 73±7 | 69±6 | 73±9 |
| % Hypertensived | 11.3 | 5.2 | 28.4 | 64.7 |
Data are mean ± SD unless otherwise noted.
Abbreviations: ABPM, ambulatory blood pressure monitoring; MHT, masked hypertension; NT, sustained normotension; SHT, sustained hypertension.
aDefined by systolic blood pressure ≥ 140mm Hg or diastolic blood pressure ≥ 90mm Hg.
bDefined by systolic blood pressure ≥ 135mm Hg or diastolic blood pressure ≥ 85mm Hg.
cMean sleep ABP was not calculated for 76 participants with < 4 sleep readings.
dDefined by systolic blood pressure ≥ 120mm Hg or diastolic blood pressure ≥ 80mm Hg.
Cardiac structure, volume, and function in MHT, SHT, and NT
Table 2 shows the mean levels of cardiac structures, volumes, LV function, and diastolic function for participants with NHT, MHT, and SHT in unadjusted (top panel) and adjusted (lower panel) models. In unadjusted models, LVIDs was significantly smaller, and FS%, LVEDV, LVM, LVMI, and RWT were significantly greater in participants with SHT than in participants with NT. LVM, LVMI, and RWT were significantly greater in participants with MHT than in participants with NT. Further, LVIDs was significantly greater and FS%, LVM, LVMI, and RWT were significantly lower in participants with MHT compared with participants with SHT. There were no significant differences in LVIDd, LVESV, and LVEF among the 3 groups.
Table 2.
Unadjusted and age-, sex-, race/ethnicity-, and body mass index–adjusted analyses comparing cardiac structures, volumes, and left ventricular function parameters between participants with masked hypertension (MHT), sustained hypertension (SHT), and sustained normotension (NT)
| Parameters | NT (n = 637) | MHT (n = 116) | SHT (n = 37) | NT vs. MHT | NT vs. SHT | MHT vs. SHT |
|---|---|---|---|---|---|---|
| Mean (95% CI) | P values | |||||
| Unadjusted (ANOVA) | ||||||
| LVIDd, cm | 4.54 (4.50–4.58) | 4.63 (4.53–4.73) | 4.44 (4.26–4.61) | 0.23 | 0.48 | 0.13 |
| LVIDs, cm | 3.05 (3.01–3.09) | 3.09 (3.00–3.18) | 2.83 (2.66–2.99) | 0.72 | <0.05 | <0.05 |
| FS, % | 32.9 (32.3–33.4) | 33.4 (32.1–34.8) | 35.8 (33.4–38.3) | 0.72 | <0.005 | <0.05 |
| LVEDV, ml | 91 (89–93) | 94 (90–99) | 101 (94–109) | 0.36 | <0.05 | 0.26 |
| LVESV, ml | 32 (31–33) | 34 (32–36) | 37 (33–40) | 0.32 | 0.07 | 0.43 |
| LVEF, % | 64.9 (64.4–65.3) | 64.4 (63.3–65.5) | 64.3 (62.4–66.3) | 0.70 | 0.87 | 0.99 |
| LVM, g | 115 (112–117) | 132 (125–139) | 155 (143–167) | <0.0001 | <0.0001 | <0.005 |
| LVMI, g/m2 | 61.2 (60.0–62.3) | 66.9 (64.2–69.7) | 75.0 (70.1–79.8) | <0.001 | <0.0001 | <0.01 |
| RWT, % | 35.1 (34.2–35.8) | 37.7 (35.8–39.6) | 49.2 (45.7–52.6) | <0.05 | <0.0001 | <0.0001 |
| Age-, sex-, race/ethnicity-, and body mass index–adjusted analyses (ANCOVA) | ||||||
| LVIDd, cm | 4.56 (4.52–4.60) | 4.57 (4.48–4.66) | 4.38 (4.22–4.54) | 0.95 | 0.09 | 0.09 |
| LVIDs, cm | 3.06 (3.03–3.10) | 3.05 (2.96–3.14) | 2.76 (2.60–2.91) | 0.95 | <0.001 | <0.005 |
| FS, % | 32.8 (32.2–33.4) | 33.4 (32.1–34.8) | 37 (34.6–39.4) | 0.63 | <0.005 | <0.05 |
| LVEDV, ml | 92 (90–93) | 90 (86–94) | 91 (90–93) | 0.62 | 0.97 | 0.95 |
| LVESV, ml | 33 (32–33) | 32 (30–34) | 33 (29–36) | 0.93 | 0.99 | 0.97 |
| LVEF, % | 64.9 (64.4–65.4) | 64.6 (63.5–65.7) | 64.6 (62.6–66.7) | 0.89 | 0.98 | 0.99 |
| LVM, g | 116 (114–119) | 122 (116–127) | 134 (125–143) | 0.18 | <0.005 | 0.06 |
| LVMI, g/m2 | 61.8 (60.7–62.8) | 64.0 (61.5–66.6) | 69.6 (65.1–74.1) | 0.24 | <0.005 | 0.08 |
| RWT, % | 35.3 (34.6–36) | 36.2 (34.6–37.9) | 43 (40–46) | 0.55 | <0.005 | <0.0005 |
Bolded P values identify statistically significant differences between groups.
Abbreviations: ANCOVA, analysis of covariance; ANOVA, analysis of variance; CI, confidence interval; FS, fractional shortening; LVEDV, left ventricular end-diastole volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVIDd, left ventricular internal diameter during end diastole; LVIDs, left ventricular internal diameter during end systole; LVM, left ventricular mass; LVMI, left ventricular mass index; RWT, relative wall thickness.
In age-, sex-, race/ethnicity-, and BMI-adjusted models, LVIDs was significantly smaller and FS%, LVM, LVMIm and RWT were significantly greater in participants with SHT than in participants with NT. There were no significant adjusted differences in these echocardiographic parameters between participants with MHT and participants with NT. LVIDs was significant higher and FS% and RWT were significantly lower in participants with MHT compared with participants with SHT. None of the other measures, including LVIDd, LVEDV, LVESV, and LVEF, differed significantly across the 3 groups in adjusted models.
Diastolic function in MHT, SHT, and NT
Table 3 shows diastolic function parameters for those with NHT, MHT, and SHT in unadjusted (top panel) and adjusted (lower panel) models. In unadjusted models, participants with SHT had significantly greater LAD, A-wave, and E/E’ ratio and significantly lower E/A ratio and E’-wave compared with participants with NT. Participants with MHT had significantly greater LAD, A-wave, and E/E’ ratio and significantly lower E/A ratio and E’-wave than participants with NT. None of the diastolic function parameters differed significantly between participants with MHT and participants with SHT. Finally, there were no differences among the 3 groups in 3 of the diastolic function parameters: E-wave, E-DcT, and A’-wave.
Table 3.
Unadjusted and age-, sex-, race/ethnicity-, and body mass index–adjusted analyses comparing diastolic function parameters between participants with masked hypertension (MHT), sustained hypertension (SHT), and sustained normotension (NT)
| Parameters | NT (n = 637) | MHT (n = 116) | SHT (n = 37) | NT vs. MHT | NT vs. SHT | MHT vs. SHT |
|---|---|---|---|---|---|---|
| Mean (95% CI) | P values | |||||
| Unadjusted (ANOVA) | ||||||
| LAD, cm | 3.41 (3.38–3.44) | 3.58 (3.50–3.66) | 3.58 (3.44–3.72) | <0.001 | <0.05 | 0.99 |
| E-velocity, m/s | 0.71 (0.70–0.72) | 0.68 (0.65–0.71) | 0.68 (0.63–0.73) | 0.18 | 0.49 | 0.99 |
| A-velocity, m/s | 0.54 (0.53–0.55) | 0.58 (0.55–0.61) | 0.63 (0.57–0.68) | <0.05 | <0.005 | 0.28 |
| E/A ratio | 1.41 (1.37–1.44) | 1.25 (1.17–1.33) | 1.15 (1.01–1.29) | 0.001 | <0.005 | 0.48 |
| E-DcT, ms | 194 (191–197) | 193 (186–199) | 205 (193–216) | 0.95 | 0.16 | 0.18 |
| E’ velocity, m/s | 0.133 (0.130–0.136) | 0.117 (0.111–0.124) | 0.110 (0.098–0.122) | <0.0005 | 0.001 | 0.54 |
| A’ velocity, m/s | 0.112 (0.11–0.115) | 0.120 (0.114–0.126) | 0.124 (0.114–0.135) | 0.05 | 0.08 | 0.76 |
| E/E’ ratio | 5.69 (5.55–5.83) | 6.23 (5.90–6.57) | 6.72 (6.13–7.31) | <0.01 | <0.005 | 0.33 |
| Age-, sex-, race/ethnicity-, and body mass index–adjusted analyses (ANCOVA) | ||||||
| LAD, cm | 3.44 (3.41–3.46) | 3.49 (3.42–3.55) | 3.39 (3.27–3.46) | 0.33 | 0.73 | 0.31 |
| E-velocity, m/s | 0.71 (0.69–0.72) | 0.70 (0.67–0.73) | 0.71 (0.69–0.72) | 0.94 | 0.99 | 0.99 |
| A-velocity, m/s | 0.54 (0.53–0.55) | 0.57 (0.54–0.59) | 0.59 (0.55–0.64) | 0.09 | 0.06 | 0.60 |
| E/A ratio | 1.39 (1.36–1.42) | 1.31 (1.24–1.38) | 1.28 (1.16–1.41) | 0.12 | 0.21 | 0.89 |
| E-DcT, ms | 194 (192–197) | 190 (184–197) | 201 (189–212) | 0.51 | 0.55 | 0.27 |
| E’ velocity, m/s | 0.131 (0.128–0.134) | 0.121 (0.114 –0.127) | 0.116 (0.105–0.127) | <0.01 | <0.05 | 0.76 |
| A’ velocity, m/s | 0.113 (0.111–0.115) | 0.116 (0.111–0.122) | 0.118 (0.108–0.129) | 0.57 | 0.60 | 0.94 |
| E/E’ ratio | 5.71 (5.58–5.84) | 6.20 (5.88–6.51) | 6.57 (6.01–7.14) | <0.05 | <0.05 | 0.47 |
Bolded P values identify statistically significant differences between groups.
Abbreviations: ANCOVA, analysis of covariance; ANOVA, analysis of variance; A-wave, peak late diastolic filling velocity of mitral inflow; A’-wave, peak late diastolic mitral annular velocity; CI = confidence interval; E-DcT, E-wave deceleration time; E-wave, peak early diastolic filling velocity of mitral inflow; E’-wave, peak early diastolic mitral annular velocity; LAD, left atrial diameter.
In age-, sex-, race/ethnicity-, and BMI-adjusted models, participants with SHT and MHT had lower E’-wave and higher E/E’ ratio than participants with NT. These parameters were not statistically different between participants with MHT and participants with SHT. Further, participants with SHT had greater A-wave than participants with NT, but this difference was not statistically significant (P = 0.06). None of the other measures of diastolic function—LAD, E-wave, A-wave, E/A ratio, E-DcT, and A’-wave—differed significantly across the 3 groups. The associations of age, sex, race/ethnicity, and BMI with each of the diastolic function parameters in an adjusted model are shown in Supplementary Table S1.
To explore whether group differences in diastolic parameters were explained by LVMI, the analyses of diastolic function parameters were repeated after adding LVMI to an age-, sex-, race/ethnicity-, and BMI-adjusted model. After further adjustment for LVMI, the results were similar to the age-, sex-, race/ethnicity-, and BMI-adjusted model. In participants with SHT, E’-wave was significantly lower (P = 0.01) and E/E’ ratio and A-wave were significantly higher (P = 0.006 and P = 0.048, respectively) than in participants with NT. In participants with MHT, E’-wave was significantly lower (P = 0.003) and E/E’ ratio was significantly higher (P = 0.008) than in participants with NT. There were no significant differences in A-wave, E’-wave, and E/E’ ratio between participants with MHT and participants with SHT. None of the other measures of diastolic function—LAD, E-wave, E/A ratio, E-DcT, and A’-wave—differed significantly across the 3 groups. Finally, after adding heart rate during the echocardiogram and LVEF to an age-, sex-, race/ethnicity-, and BMI-adjusted model, the results also did not change (not shown).
DISCUSSION
The results of our study indicate that tissue Doppler-derived diastolic function parameters, specifically E’-wave and E/E’ ratio, are impaired in individuals with MHT compared with individuals with NT and were similar for individuals with MHT and individuals with SHT. These findings suggest that alterations in diastolic function are observed in asymptomatic individuals with MHT at a level that is similar to individuals with SHT.
The findings of altered diastolic function in our study, which included generally healthy individuals with a wide range of cardiovascular disease risk, are consistent with a small clinical study of diabetic outpatients by Marchesi et al.13 which showed that although LVMI increased progressively from NT to MHT to SHT, diastolic function defined by E/A ratio, E’/A’ ratio, and E-DcT was found to be similarly impaired in MHT and SHT compared with NT. As mentioned previously, parameters driven from mitral inflow, such as E/A ratio and E-DcT, reflect abnormal LV relaxation, but they may be affected by left atrial pressure, heart rate, and mitral valve disease.22 E’/A’ ratio driven from tissue Doppler imaging is minimally affected by preload, and a previous study demonstrated that E’/A’ ratio correlates with LV relaxation.18 However, it is not commonly used and currently not recommended by the ASE.23 In contrast, E’-wave and E/E’ ratio, which were altered in participants with MHT in our study, are recommended by the ASE for the assessment of diastolic function.23 E/E’ reflects the degree of LV filling pressure and is presently recognized as the gold-standard index to detect diastolic dysfunction.20
In our study, the association of MHT with diastolic function was also independent of LVMI, suggesting that MHT-associated changes in diastolic function are not explained by increases in LVMI. Several studies of patients with HT have shown that diastolic dysfunction may occur in the absence of LVH.11, 24 A clinical study of Nigerians with newly diagnosed HT11 demonstrated that impaired diastolic function occurs in approximately 60% of hypertensive individuals with LVH and 40% of hypertensive individuals without LVH. Similarly, a study by Aeschbacher et al.12 demonstrated that impaired diastolic function was detected in the young, normotensive male offspring of hypertensive parents before the development of LVH. A study by Mineeva et al. 25 demonstrated that altered transmitral blood flow is observed at the prehypertension stage that precedes heart remodeling. Consistent with these previous findings in HT patients, our results suggest that impaired diastolic function may be an early subclinical alteration seen in individuals with MHT before the development of LV structural changes. The mechanisms underlying the impairment in diastolic function independent of changes in LVMI associated with MHT are unknown. Although LVH induced by chronic pressure or volume overload may contribute to diastolic dysfunction,26 other factors besides LVH are associated with impairment in diastolic function; these include contractile alterations in myocytes due to impaired sarcoplasmic reticulum calcium uptake, extracellular and perivascular fibrosis, and myocardial ischemia.27–30 These mechanisms may have played a role in the association between MHT and impaired diastolic function in our study.
Diastolic heart failure as a consequence of impaired diastolic function in the general population is associated with a high mortality rate that is comparable with that of systolic heart failure mortality rates.31–33 Given the association between diastolic dysfunction and the subsequent development of heart failure,27 our findings suggest screening for diastolic dysfunction among individuals with MHT may be useful for the identification of an early phase of cardiac dysfunction. The follow-up of participants in the ongoing Masked Hypertension Study may eventually help determine whether or not E’-wave and E/E’ ratio have prognostic value for individuals with MHT.
Several limitations of this study should be acknowledged. First, our sample consisted of employed adults who were generally healthy. Whether our results are generalizable to other population-based or clinic-based samples remains unknown. Second, because awake ABP was estimated from one 24-hour monitoring period, we cannot exclude the possibility that the study results would have differed with the inclusion of additional 24-hour periods to define MHT, SHT, and NT. Finally, because this is a cross-sectional observational study, causality cannot be determined in our study.
Strengths of the study include a large sample size, the careful assessment of CBP over 3 visits, the inclusion of a large proportion of participants with normal CBP levels who otherwise would be classified as lower risk based on CBP, the exclusion of participants on antihypertensive medications, and the assessment of cardiac structures, volumes, and systolic and diastolic function using validated 2-dimensional and Doppler echocardiographic methods.
In conclusion, we demonstrated that diastolic function represented by E’-wave and E/E’ ratio was impaired in MHT compared with NT, even after controlling for LVMI. These data suggest MHT is associated with impaired diastolic function in the absence of LV structural changes. The mechanisms underlying the association between MHT and impaired diastolic function and whether or not these subclinical alterations have prognostic value for subsequent diastolic heart failure in MHT are unknown. Future studies are needed to evaluate the prognostic value of impaired diastolic function in MHT as well as promising interventions targeting MHT-associated impaired diastolic function.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at American Journal of Hypertension (http://ajh.oxfordjournals.org).
DISCLOSURE
The authors declared no conflict of interest.
ACKNOWLEDGMENTS
We are indebted to the study participants and research staff of the Masked Hypertension Study, without whose cooperation and dedication this study would not have been possible. This work was directly supported by P01-HL047540 (PI: J.E. Schwartz) from the National Heart, Lung, and Blood Institute at the National Institutes of Health (NIH). The research was also supported by T32-HL007854-15, General Clinical Research Center grant MO1-RR10710 (to Stony Brook University), and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000040, formerly the National Center for Research Resources, grant number UL1 RR024156. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
REFERENCES
- 1. Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension 2002; 40:795–796 [DOI] [PubMed] [Google Scholar]
- 2. Selenta C, Hogan BE, Linden W. How often do office blood pressure measurements fail to identify true hypertension? An exploration of white-coat normotension. Arch Fam Med 2000; 9:533–540 [DOI] [PubMed] [Google Scholar]
- 3. Bobrie G, Clerson P, Menard J, Postel-Vinay N, Chatellier G, Plouin PF. Masked hypertension: a systematic review. J Hypertens 2008; 26:1715–1725 [DOI] [PubMed] [Google Scholar]
- 4. Bjorklund K, Lind L, Zethelius B, Andren B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation 2003; 107:1297–1302 [DOI] [PubMed] [Google Scholar]
- 5. Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension 2006; 47:846–853 [DOI] [PubMed] [Google Scholar]
- 6. Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure and mortality: a population-based study. Hypertension 2005; 45:499–504 [DOI] [PubMed] [Google Scholar]
- 7. Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, Valagussa F, Bombelli M, Giannattasio C, Zanchetti A, Mancia G. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study). Circulation 2001; 104:1385–1392 [DOI] [PubMed] [Google Scholar]
- 8. Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med 1999; 131:564–572 [DOI] [PubMed] [Google Scholar]
- 9. Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C, Santucci A, Santucci C, Reboldi G, Porcellati C. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol 1995; 25:871–878 [DOI] [PubMed] [Google Scholar]
- 10. Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 1995; 26:1565–1574 [DOI] [PubMed] [Google Scholar]
- 11. Adamu GU, Katibi AI, Opadijo GO, Omotoso AB, Araoye AM. Prevalence of left ventricular diastolic dysfunction in newly diagnosed Nigerians with systemic hypertension: a pulsed wave Doppler echocardiographic study. Afr Health Sci 2010 10:177–182 [PMC free article] [PubMed] [Google Scholar]
- 12. Aeschbacher BC, Hutter D, Fuhrer J, Weidmann P, Delacretaz E, Allemann Y. Diastolic dysfunction precedes myocardial hypertrophy in the development of hypertension. Am J Hypertens 2001; 14:106–113 [DOI] [PubMed] [Google Scholar]
- 13. Marchesi C, Maresca AM, Solbiati F, Franzetti I, Laurita E, Nicolini E, Gianni M, Guasti L, Marnini P, Venco A, Grandi AM. Masked hypertension in type 2 diabetes mellitus. Relationship with left-ventricular structure and function. Am J Hypertens 2007; 20:1079–1084 [DOI] [PubMed] [Google Scholar]
- 14. Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Polo Friz H, Grassi G, Sega R. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension 2009; 54:226–232 [DOI] [PubMed] [Google Scholar]
- 15. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Erdine S, Kiowski W, Agabiti-Rosei E, Ambrosioni E, Lindholm LH, Viigimaa M, Adamopoulos S, Agabiti-Rosei E, Ambrosioni E, Bertomeu V, Clement D, Erdine S, Farsang C, Gaita D, Lip G, Mallion JM, Manolis AJ, Nilsson PM, O’Brien E, Ponikowski P, Redon J, Ruschitzka F, Tamargo J, van Zwieten P, Waeber B, Williams B. Management of Arterial Hypertension of the European Society of Hypertension European Society of Cardiology 2007 guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2007; 25:1105–1187 [DOI] [PubMed] [Google Scholar]
- 16. The Japanese Circulation Society (JCS) Joint Working Group Guidelines for the clinical use of 24 hour ambulatory blood pressure monitoring (ABPM) (JCS 2010): digest version. Circ J 2012 76:508–519 [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Chamber Quantification Writing Group American Society of Echocardiography’s Guidelines and Standards Committee European Association of Echocardiography Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005; 18:1440–1463 [DOI] [PubMed] [Google Scholar]
- 18. Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997; 30:474–480 [DOI] [PubMed] [Google Scholar]
- 19. Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol 2001; 37:278–285 [DOI] [PubMed] [Google Scholar]
- 20. Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschöpe C. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 2007; 116:637–647 [DOI] [PubMed] [Google Scholar]
- 21. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 2000; 102:1788–1794 [DOI] [PubMed] [Google Scholar]
- 22. Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 1988; 12:426–440 [DOI] [PubMed] [Google Scholar]
- 23. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10:165–193 [DOI] [PubMed] [Google Scholar]
- 24. De Marchi SF, Allemann Y, Seiler C. Relaxation in hypertrophic cardiomyopathy and hypertensive heart disease: relations between hypertrophy and diastolic function. Heart 2000; 83:678–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mineeva EE, Gvozdenko TA, Antoniuk MV. [Diastolic dysfunction—predictor of cardiac remodeling in arterial hypertension in young males]. Klin Med (Mosk) 2008; 86:23–25 [PubMed] [Google Scholar]
- 26. Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis 2006; 48:326–341 [DOI] [PubMed] [Google Scholar]
- 27. Mandinov L, Eberli FR, Seiler C, Hess OM. Diastolic heart failure. Cardiovasc Res 2000; 45:813–825 [DOI] [PubMed] [Google Scholar]
- 28. Frohlich ED. State of the art lecture. Risk mechanisms in hypertensive heart disease. Hypertension 1999; 34:782–789 [DOI] [PubMed] [Google Scholar]
- 29. Slama M, Susic D, Varagic J, Frohlich ED. Diastolic dysfunction in hypertension. Curr Opin Cardiol 2002; 17:368–373 [DOI] [PubMed] [Google Scholar]
- 30. Dupont S, Maizel J, Mentaverri R, Chillon JM, Six I, Giummelly P, Brazier M, Choukroun G, Tribouilloy C, Massy ZA, Slama M. The onset of left ventricular diastolic dysfunction in SHR rats is not related to hypertrophy or hypertension. Am J Physiol Heart Circ Physiol 2012; 302:H1524–H1532 [DOI] [PubMed] [Google Scholar]
- 31. Chopra HK. Diastolic heart failure: a clinical challenge early recognition & timely intervention is the need of the hour. Indian Heart J 2009; 61:138–145 [PubMed] [Google Scholar]
- 32. Schillaci G, Pasqualini L, Verdecchia P, Vaudo G, Marchesi S, Porcellati C, de Simone G, Mannarino E. Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol 2002; 39:2005–2011 [DOI] [PubMed] [Google Scholar]
- 33. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289:194–202 [DOI] [PubMed] [Google Scholar]


