Human immunodeficiency virus–positive children enrolled in China's national pediatric antiretroviral therapy program experience a mortality rate of 2.3 per 100 child-years, survival rate of 94%, and improved treatment outcomes including elevated CD4 percentage, hemoglobin, and weight-for-age and height-for-age z scores after 3 years of follow-up.
Keywords: HIV, mortality, pediatric, antiretroviral therapy, China
Abstract
Background. The aim of this study was to describe 3-year mortality rates, associated risk factors, and long-term clinical outcomes of children enrolled in China's national free pediatric antiretroviral therapy (ART) program.
Methods. Records were abstracted from the national human immunodeficiency virus (HIV)/AIDS case reporting and national pediatric ART databases for all HIV-positive children ≤15 years old who initiated ART prior to December 2010. Mortality risk factors over 3 years of follow-up were examined using Cox proportional hazards regression models. Life tables were used to determine survival rate over time. Longitudinal plots of CD4+ T-cell percentage (CD4%), hemoglobin level, weight-for-age z (WAZ) score, and height-for-age z (HAZ) score were created using generalized estimating equation models.
Results. Among the 1818 children included in our cohort, 93 deaths were recorded in 4022 child-years (CY) of observed time for an overall mortality rate of 2.31 per 100 CY (95% confidence interval [CI], 1.75–2.78). The strongest factor associated with mortality was baseline WAZ score <−2 (adjusted hazard ratio [HR] = 9.1; 95% CI, 2.5–33.2), followed by World Health Organization stage III or IV disease (adjusted HR = 2.4; 95% CI, 1.1–5.2), and hemoglobin <90 g/L (adjusted HR = 2.2; 95% CI, 1.2–3.9). CD4%, hemoglobin level, WAZ score, and HAZ score increased over time.
Conclusions. Our finding that 94% of children engaged in this program are still alive and of improved health after 3 years of treatment demonstrates that China's national pediatric ART program is effective. This program needs to be expanded to better meet treatment demands, and efforts to identify HIV-positive children earlier must be prioritized.
(See the Editorial Commentary by Sugandhi and Harwell on pages 745–6.)
As of 2010, an estimated 3.4 million children >15 years old were human immunodeficiency virus (HIV)–positive worldwide. Although the number of children receiving antiretroviral therapy (ART) increased to 456 000 in 2010, there is still a substantial gap in ART coverage for children globally [1]. Since the introduction of ART, pediatric HIV mortality in developed countries has fallen below 1 per 100 child-years (CY), with the majority of children now reaching adolescence and adulthood [2, 3]. In the past several years, many resource-limited countries have published outcomes of large-scale pediatric cohorts, with mortality rates of children on ART ranging from 2.2 to 11.6 per 100 CY in Africa [4–9], 1.9 to 5.2 per 100 CY in Asia [10–13], and 0.8 per 100 CY in South America [14], Typically, the majority of deaths among HIV-positive children on ART occur within the first 6 months of treatment [6]. Mortality risk factors include low baseline CD4+ T-cell percentage (CD4%), low weight-for-age z (WAZ) score, and World Health Organization (WHO) stage III or IV disease at treatment initiation [3, 5, 10, 15].
As of the end of 2011, the estimated number of people living with HIV/AIDS in China was 780 000, 1.1% of whom were infected through mother-to-child transmission [16]. More than 150 000 HIV-positive patients have thus far received treatment via China's national free antiretroviral treatment program [16]. As a result of this program, HIV-positive adults receiving ART now experience a dramatically reduced HIV-related mortality rate of approximately 5 deaths per 100 person-years [17, 18]. Due to the unavailability of pediatric ART formulations in the early years of China's national ART program, a small portion of HIV-positive children were treated using adult formulations by splitting pills. In 2005, the national pediatric ART program was launched in the 6 Chinese provinces with the highest HIV prevalence (Henan, Anhui, Hubei, Yunnan, Shanxi, and Guangxi) [19]. By 2010, the pediatric ART program had expanded to 28 provinces and autonomous regions [16].
Early in the pediatric ART program, treatment guidelines were developed and extensive clinical training was provided to local healthcare workers. Recommended first-line treatment regimen was zidovudine (AZT) or stavudine (D4T) plus lamivudine (3TC) plus nevirapine (NVP) or efavirenz (EFV). NVP was preferred for children <3 years or <10 kg. Free CD4+ T-cell count and CD4% testing was provided every 6 months for all pediatric ART patients and follow-up visits were conducted for all patients every 3 months. In 2008, ART eligibility criteria were expanded to include all children with (1) WHO stage III or IV disease, or (2) CD4+ T-cell counts <350 cells/µL or CD4% <15% for children >3 years old, CD4+ T-cell counts <750 cells/µL or CD4% <20% for children 1–3 years old, or virologically diagnosed children <1 year old regardless of CD4+ cell count or CD4%. Also starting in 2008, free annual plasma viral load (pVL) testing was provided to all pediatric ART patients [19].
Although 2 reports examining small cohorts of children in China's pediatric ART program have been published in the past 5 years [20, 21], no nationwide cohort study exists thus far describing outcomes for children on ART in China. It is in this context that we present the following descriptive study, aiming to examine mortality, its predictors, and treatment outcomes among HIV-positive children in China's national pediatric ART program over 3 years of follow-up.
METHODS
Study Design
This study was designed to be a nationwide, retrospective cohort study of HIV-positive children in China enrolled in China's national pediatric ART program from 1 July 2005 to 31 December 2010.
Sources of Data
All cases of HIV in China are reported via the national HIV/AIDS case reporting system. Records in this system include identification and demographic information, HIV testing results, self-reported transmission route, and health status information. When these patients initiate ART, their treatment information is reported in either the pediatric ART system (all children <15 years old at time of ART initiation) or adult ART system (all individuals ≥15 years old when ART was initiated and children <15 years old who either initiated ART prior to 2005, were entered into the adult system mistakenly, or were treated at a clinic that did not have a pediatric ART program). In general, these systems retain information related to the clinical management of the patient and all ART-related information (ie, drugs and doses, CD4 and pVL test results) as well as standard identification and demographic information [22]. However, some of the information in the adult and pediatric ART databases is dissimilar. For example, CD4%, growth indicators and HIV testing method (HIV infection is determined by enzyme-linked immunosorbent assay and Western blot if the child is ≥18 months old or by DNA/RNA if the child is <18 months old), are only included in the pediatric ART system. Also, some patients in the pediatric ART system do not have true baseline (prior to ART initiation) data because they initiated ART before the pediatric ART program started and were only later followed in this system. Data were censored on 31 December 2010, and national HIV/AIDS case reporting system and pediatric ART system datasets were linked by identification number, treatment number, and name.
Study Participants
Inclusion criteria for this study were (1) having an HIV-positive serostatus, (2) being ≤15 years old when first identified as HIV positive, and (3) having a record in China's national HIV/AIDS case reporting system as of 31 December 2010. Patients were then excluded if any 1 of the following criteria applied: the patient (1) died before initiating ART, (2) was not being followed in the pediatric ART system but was instead being followed in the adult ART system, (3) had been lost to follow-up prior to initiating ART, (4) had not yet initiated ART as of 31 December 2010, or (5) had initiated ART before the pediatric ART system was created.
Active patients were defined as those with a follow-up record within 3 months of 31 December 2010. Data were stratified using 3 age groups at the time of ART initiation: ≤35 months, 36–59 months, and ≥60 months—for comparison of demographic and clinical characteristics and for analysis of mortality and factors associated with death. Further analysis of mortality rates, survival rates, and outcomes focused on the first 36 months after ART initiation.
Observed Time and Treatment Outcomes
Observed time was calculated in CY from ART initiation date until study end date or death, whichever occurred first. Immunological success was defined as CD4% ≥25% [23]. Weight-for-age z (WAZ) score and height-for-age z (HAZ) score was calculated according to the China National Growth Standards [24]. Viral suppression was defined as pVL <400 copies/mL. Because free pVL testing was not available until after 2008, only a subset of study participants had pVL test results in their records.
Statistical Analysis
Baseline characteristics were described using median and interquartile range (IQR) for continuous variables and number and percentage for categorical variables. Mortality rate was calculated by dividing the total number of subjects who died within each 6-month interval by the sum of observed time for subjects within the same interval. Life tables were used to determine survival over time with risk factors for mortality analyzed using Cox proportional hazards regression models. Univariate factors with P < .1 and factors predetermined to be clinically meaningful were included in the full multivariable regression models. Missing data were given no special treatment and multivariate regression analysis was performed only on complete cases. All P values are 2-sided, and P < .05 was considered to be statistically significant. Longitudinal plots of CD4%, hemoglobin, WAZ score, and HAZ score were created using median values every 6 months, with generalized estimating equation models used to compare clinical outcomes at different time intervals after ART initiation. All statistical analyses were performed using SAS software (version 9.1.3, SAS Institute).
Ethical Approval
This study was reviewed and approved by the Institutional Review Board of the National Center for AIDS/STD Control and Prevention, Chinese Center for Disease Control and Prevention (CDC). Parents or legal guardians signed informed consent forms before their children initiated ART. Because data used in this study were collected as a function of China's routine HIV/AIDS surveillance program and the regular administration of the national pediatric ART program, no additional informed consent for inclusion of these data in the study was required.
RESULTS
Characteristics of the Study Population
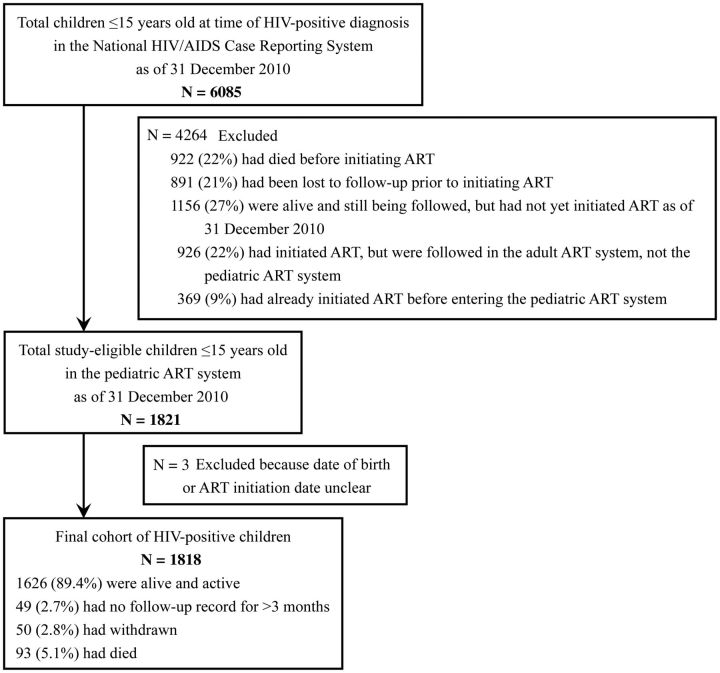
The development of our study cohort is described in Figure 1. As of 31 December 2010, a total of 6085 HIV-positive children ≤15 years old when they were first identified as HIV-positive had records in the national HIV/AIDS case reporting system, thus making them eligible for the study. However, after applying exclusion criteria, the final cohort was 1818 children, all of whom were included in our analysis. Among these 1818 children, 89.4% were alive and active in the pediatric ART program, 2.7% had no follow-up record for >3 months, 2.8% had withdrawn from the program, and 5.1% had died while receiving treatment via the pediatric ART program.
Figure 1.
Flowchart showing patients included in the analysis. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus.
As shown in Table 1, the median age of participants was 74.5 months (IQR, 46.5–119.6) with 63.3% of children ≥60 months old when they initiated ART. Most children were male (57.4%), had acquired HIV via mother-to-child transmission (90.9%), and presented for treatment at WHO stage III or IV (57.4%). Median WAZ score was −1.4 (IQR, −2.1 to −0.8), median HAZ score was −2.2 (IQR, −3.3 to −1.1), median CD4% was 8.8% (IQR, 3.3–14.9), and median hemoglobin level was 111.0 g/L (IQR, 99.0–122.0). The proportion of children who presented in WHO stage III or IV disease, the median WAZ and HAZ scores, and hemoglobin levels were very similar across the 3 age categories, whereas median CD4% declined with increasing baseline age category.
Table 1.
Baseline Demographic Characteristics of Treatment-Naive Children at Time of Antiretroviral Therapy Initiation
| Characteristic | All (N = 1818) | Age ≤35 mo (n = 302, 16.6%) | Age 36–59 mo (n = 366, 20.1%) | Age ≥60 mo (n = 1150, 63.3%) |
|---|---|---|---|---|
| Age (mo) | ||||
| Median (IQR) | 74.5 (46.5–119.6) | 24.4 (17.1–30.0) | 48.6 (42.1–55.2) | 105.9 (77.5–138.1) |
| Sex | ||||
| Male | 1043 (57.4) | 166 (55.0) | 203 (55.5) | 674 (58.6) |
| Female | 775 (42.6) | 136 (45.0) | 163 (44.5) | 476 (41.4) |
| Infection route | ||||
| Blood transfusion | 156 (9.0) | 0 | 0 | 156 (14.4) |
| Injecting drugs | 1 (0.1) | 0 | 0 | 1 (0.1) |
| Sexual contact | 1 (0.1) | 0 | 0 | 1 (0.1) |
| Mother-to-child | 1584 (90.9) | 298 (100.0) | 358 (100.0) | 928 (85.5) |
| WHO clinical stage | ||||
| I or II | 769 (42.6) | 122 (40.5) | 172 (47.3) | 475 (41.6) |
| III or IV | 1037 (57.4) | 179 (59.5) | 192 (52.7) | 666 (58.4) |
| Hepatitis B serostatus | ||||
| Negative | 1306 (95.4) | 202 (96.2) | 260 (93.5) | 844 (95.8) |
| Positive | 63 (4.6) | 8 (3.8) | 18 (6.5) | 37 (4.2) |
| Hepatitis C serostatus | ||||
| Negative | 1137 (93.1) | 181 (93.8) | 238 (96.7) | 718 (91.8) |
| Positive | 84 (6.9) | 12 (6.2) | 8 (3.3) | 64 (8.2) |
| Weight-for-age z score | ||||
| Median (IQR) | −1.4 (−2.1, −0.8) | −1.5 (−2.5, −0.5) | −1.4 (−2.1, −0.5) | −1.4 (−2.1, −0.8) |
| ≥−1 | 618 (34.1) | 101 (33.4) | 136 (37.3) | 381 (33.2) |
| ≥−2, <−1 | 671 (37.0) | 89 (29.5) | 123 (33.7) | 459 (40.0) |
| <−2 | 525 (28.9) | 112 (37.1) | 106 (29.0) | 307 (26.8) |
| Height-for-age z score | ||||
| Median (IQR) | −2.2 (−3.3, −1.1) | −2.2 (−3.3, −0.8) | −2.2 (−3.3, −1.3) | −2.2 (−3.3, −1.1) |
| ≥−1 | 416 (23.7) | 84 (29.1) | 77 (22.1) | 255 (22.9) |
| ≥−2, <−1 | 397 (22.6) | 57 (19.7) | 84 (24.1) | 256 (23.0) |
| <−2 | 940 (53.6) | 148 (51.2) | 188 (53.9) | 604 (54.2) |
| CD4 cell percentage | ||||
| Median (IQR) | 8.8 (3.3–14.9) | 12.1 (6.1–17.1) | 9.2 (3.2–16.3) | 7.7 (2.7–14.1) |
| ≥15 | 386 (25.0) | 90 (33.8) | 93 (28.8) | 203 (21.2) |
| ≥10, <15 | 310 (20.1) | 71 (26.7) | 60 (18.6) | 179 (18.7) |
| <10 | 849 (55.0) | 105 (39.5) | 170 (52.6) | 574 (60.0) |
| Hemoglobin (g/L) | ||||
| Median (IQR) | 111.0 (99.0–122.0) | 104.0 (89.0–114.0) | 108.0 (95.0–120.0) | 114.0 (103.0–124.0) |
| ≥90 | 1484 (86.2) | 212 (73.9) | 289 (82.3) | 983 (90.7) |
| <90 | 238 (13.8) | 75 (26.1) | 62 (17.7) | 101 (9.3) |
| AZT/d4T regimen | ||||
| AZT + 3TC + EFV/NVP | 903 (59.3) | 180 (73.5) | 189 (61.6) | 534 (54.9) |
| d4T + 3TC + EFV/NVP | 621 (40.7) | 65 (26.5) | 118 (38.4) | 438 (45.1) |
| EFV/NVP regimen | ||||
| AZT/d4T + 3TC + EFV | 384 (25.2) | 20 (8.2) | 93 (30.3) | 271 (27.9) |
| AZT/d4T + 3TC + NVP | 1140 (74.8) | 225 (91.8) | 214 (69.7) | 701 (72.1) |
Data are No. (%) unless otherwise specified.
Abbreviations: 3TC, lamivudine; AZT, zidovudine; d4T, stavudine; EFV, efavirenz; IQR, interquartile range; NVP, nevirapine; WHO, World Health Organization.
Observed Time, Mortality Rate, and Predictors of Death
Median observed time was 24.4 months (IQR, 10.0–40.9, data not shown). As shown in Table 2, a total of 93 deaths were observed in 4022 CY of observed time, for an overall mortality rate of 2.31 per 100 CY (95% confidence interval [CI], 1.75–2.78). Patients with hemoglobin levels <90 g/L had the highest mortality rate at 6.07 per 100 CY. Results from multivariate analysis identified having a baseline WAZ score <−2 as the strongest factor associated with death (adjusted hazard ratio [HR] = 9.1; 95% CI, 2.5–33.2), followed by WHO stage III or IV disease (adjusted HR = 2.4; 95% CI, 1.1–5.2), and hemoglobin <90 g/L (adjusted HR = 2.2; 95% CI, 1.2–3.9). No other factors were found to have statistically significantly associations with mortality.
Table 2.
Deaths, Observed Time, Mortality, and Factors Associated With Death as Determined by Multivariate Cox Proportional Hazards Model Analysis
| Characteristic | All Cases |
Complete Cases Only |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases, No. | Deaths, No. | Observed Time, CY | Mortality Rate per 100 CY (95% CI) | Unadjusted HR (95% CI) | P Value | Cases, No. | Mortality Rate per 100 CY (95% CI) | Adjusted HR (95% CI) | P Value | |
| Age (mo) | ||||||||||
| ≤35 | 302 | 14 | 622 | 2.25 (1.23–3.78) | 1.0 | |||||
| 36–59 | 366 | 12 | 875 | 1.37 (.71–2.40) | .7 (.3–1.4) | .28 | ||||
| ≥60 | 1150 | 67 | 2525 | 2.65 (2.02–3.29) | 1.2 (.7–2.2) | .51 | ||||
| Sex | ||||||||||
| Male | 1043 | 55 | 2351 | 2.34 (1.72–2.96) | 1.0 | |||||
| Female | 775 | 38 | 1672 | 2.27 (1.55–3.00) | 1.0 (.6–1.4) | .81 | ||||
| Infection route | ||||||||||
| Blood transfusion | 156 | 10 | 444 | 2.25 (1.08–4.14) | 1.0 | |||||
| Mother-to-child | 1584 | 78 | 3343 | 2.29 (1.78–2.80) | .9 (.5–1.7) | .72 | ||||
| WHO clinical stage | ||||||||||
| I or II | 769 | 17 | 1583 | 1.07 (.63–1.72) | 1.0 | 511 | .75 (.32–1.47) | 1.0 | ||
| III or IV | 1037 | 74 | 2414 | 3.07 (2.37–3.76) | 3.0 (1.8–5.1) | <.01 | 717 | 2.70 (1.92–3.49) | 2.4 (1.1–5.2) | .02 |
| Hepatitis B serostatus | ||||||||||
| Negative | 1306 | 56 | 3182 | 1.76 (1.30–2.22) | 1.0 | |||||
| Positive | 63 | 4 | 150 | 2.67 (.73–6.83) | 1.5 (.5–4.2) | .42 | ||||
| Hepatitis C serostatus | ||||||||||
| Negative | 1137 | 47 | 2739 | 1.72 (1.23–2.21) | 1.0 | |||||
| Positive | 84 | 4 | 252 | 1.59 (.43–4.06) | 1.0 (.4–2.8) | .96 | ||||
| Weight-for-age z score | ||||||||||
| ≥−1 | 618 | 6 | 1406 | .43 (.16–.93) | 1.0 | 398 | .34 (.07–.98) | 1.0 | ||
| ≥−2, <−1 | 671 | 25 | 1535 | 1.63 (1.05–2.40) | 3.8 (1.6–9.3) | <.01 | 477 | 1.26 (.69–2.11) | 3.1 (.8–11.6) | .09 |
| <−2 | 525 | 62 | 1069 | 5.80 (4.35–7.24) | 12.9 (5.6–29.7) | <.01 | 353 | 4.82 (3.27–6.37) | 9.1 (2.5–33.2) | <.01 |
| Height-for-age z score | ||||||||||
| ≥−1 | 416 | 12 | 909 | 1.32 (.68–2.30) | 1.0 | 281 | .65 (.18–1.65) | 1.0 | ||
| ≥−2, <−1 | 397 | 15 | 907 | 1.65 (.93–2.73) | 1.3 (0.6–2.7) | .53 | 282 | 1.19 (.51–2.34) | 1.0 (.3–3.6) | .96 |
| <−2 | 940 | 63 | 2023 | 3.11 (2.34–3.88) | 2.4 (1.3–4.4) | <.01 | 665 | 2.83 (1.98–3.69) | 1.1 (.4–3.5) | .84 |
| CD4 cell percentage (%) | ||||||||||
| ≥15 | 386 | 6 | 762 | .79 (.29–1.71) | 1.0 | 302 | .82 (.27–1.92) | 1.0 | ||
| ≥10, <15 | 310 | 3 | 723 | .41 (.09–1.21) | .6 (.1–2.3) | .44 | 251 | .51 (.11–1.50) | .6 (.1–2.4) | .46 |
| <10 | 849 | 56 | 1938 | 2.89 (2.13–3.65) | 4.0 (1.7–9.4) | <.01 | 675 | 2.90 (2.06–3.74) | 2.4 (.9–6.0) | .07 |
| Hemoglobin (g/L) | ||||||||||
| ≥90 | 1484 | 54 | 3377 | 1.60 (1.17–2.03) | 1.0 | 1067 | 1.48 (1.00–1.97) | 1.0 | ||
| <90 | 238 | 30 | 494 | 6.07 (4.10–8.67) | 3.7 (2.4–5.8) | <.01 | 161 | 5.23 (3.10–8.27) | 2.2 (1.2–3.9) | .01 |
| AZT/d4T regimen | ||||||||||
| AZT + 3TC + EFV/NVP | 903 | 37 | 1914 | 1.93 (1.31–2.56) | 1.0 | 769 | 1.47 (.94–2.18) | 1.0 | ||
| d4T + 3TC + EFV/NVP | 621 | 42 | 1545 | 2.72 (1.90–3.54) | 1.6 (1.0–2.4) | .05 | 459 | 2.63 (1.78–3.76) | 1.3 (.7–2.3) | .35 |
| EFV/NVP regimen | ||||||||||
| AZT/d4T + 3TC + EFV | 384 | 14 | 667 | 2.10 (1.15–3.52) | 1.0 | |||||
| AZT/d4T + 3TC + NVP | 1140 | 65 | 2792 | 2.33 (1.76–2.89) | 1.4 (0.8–2.4) | .30 | ||||
| Overall | 1818 | 93 | 4022 | 2.31 (1.75–2.78) | … | … | 1228 | … | … | … |
Abbreviations: 3TC, lamivudine; AZT, zidovudine; CI, confidence interval; CY, child-years; d4T, stavudine; EFV, efavirenz; HR, hazard ratio; NVP, nevirapine; WHO, World Health Organization.
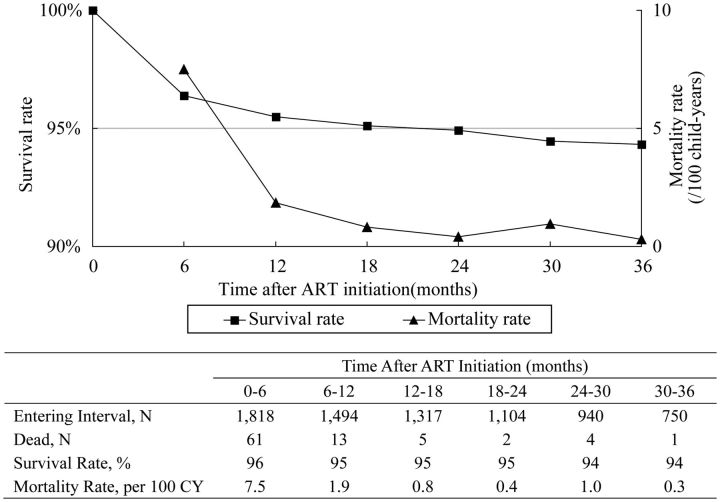
As shown in Figure 2, upon closer examination of the first 36 months after treatment initiation, we found that overall mortality declined from 7.5 per 100 CY during the initial 6 months of treatment to 1.9 per 100 CY from 6 to 12 months, and then remained approximately 1.0 per 100 CY from 12 to 36 months. More than 94% of patients on treatment were still alive at 3 years.
Figure 2.
Mortality and survival rates at time intervals up to 36 months after antiretroviral therapy initiation. Abbreviation: ART, antiretroviral therapy.
Clinical Treatment Outcomes
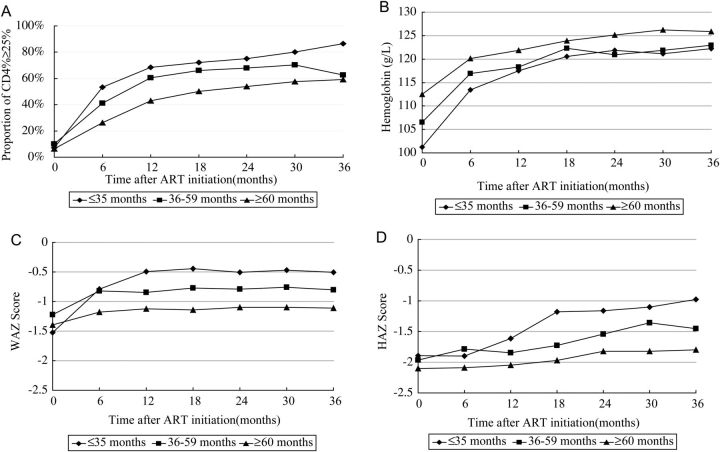
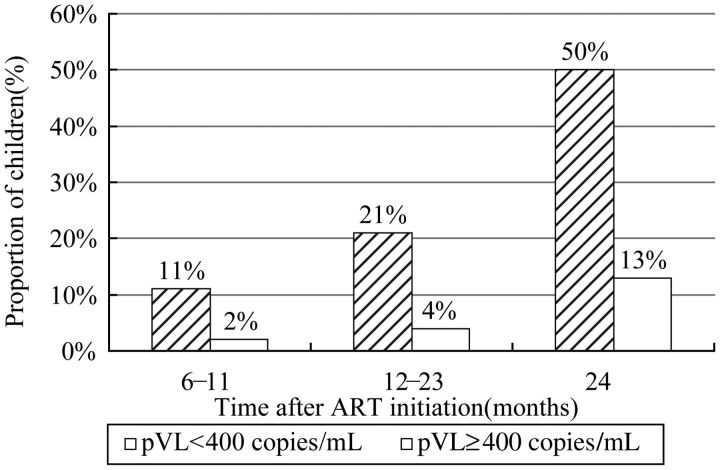
As shown in Figure 3, the proportions of children who achieved immunologic success (CD4% ≥25%) increased over the entire 3-year follow-up period, but most dramatically during the first year after ART initiation for all age groups. CD4% recovery in children ≤35 months of age at ART initiation was more rapid than in the 2 other age groups. Following treatment initiation, CD4%, hemoglobin level, WAZ score, and HAZ score all increased significantly over time for all 3 age groups compared to baseline (P < .01). The one exception was HAZ scores for children ≥60 months old at time of ART initiation. The proportions of children who achieved virological success (pVL < 400 copies/mL) are presented in Figure 4. A total of 1149 of 1818 children (63%) received HIV pVL testing in 2010. Among these children, approximately 82% (940 of 1149) had achieved viral suppression, most of whom had already been on ART for ≥24 months.
Figure 3.
Graphical representation of clinical outcomes of all children and specific age groups at time intervals up to 36 months after ART initiation. A, Proportion of patients with CD4+ T-cell percentage ≥25%. B, Hemoglobin levels. C, Weight-for-age z scores. D, height-for-age z scores. Abbreviations: ART, antiretroviral therapy; CD4%, CD4+ T-cell percentage; HAZ, height-for-age z score; WAZ, weight-for-age z score.
Figure 4.
Graphical representation of proportions of patients who achieved viral suppression (defined as viral loads <400 copies/mL) among 1149 children of the total 1818 children in the cohort (63%) who received plasma viral load testing in 2010. Abbreviations: ART, antiretroviral therapy; pVL, plasma viral load.
DISCUSSION
The aim of this study was to describe mortality, survival, risk factors, and treatment outcomes among a nationwide cohort over 3 years of follow-up. Our main finding is that mortality rates for this cohort are 2.31 per 100 CY with 94% of children surviving after 3 years of ART. Effective ART is well known to reduce HIV morbidity and mortality among children [25], with immunologic and virologic responses comparable in both well-resourced and resource-limited settings [26, 27], We find that mortality is greatest within the first 6 months of treatment (7.5 per 100 CY), but that by 12 months it has stabilized to a new level (approximately 1.0 per 100 CY). This pattern has been observed in previous studies [6, 10, 13, 27–29], though the new stabilized mortality rate varies (<1 per 100 CY in developed countries [3, 30] and 3 to 8 per 100 CY in developing countries [9, 30]). The findings presented here represent the first from such a cohort in China, with only 2 smaller studies having been performed within the context of this program previously—one that examined treatment outcomes among only 83 children in 2005 [20], and the other that examined drug resistance among 124 children in virologic failure in 2008 [21].
The effectiveness of this program is further demonstrated by the significantly improved CD4%, hemoglobin levels, WAZ scores, HAZ scores, and pVL levels observed in our cohort once ART began. These data provide evidence that immune reconstitution was occurring in these children and that they were beginning to rebound from significantly impaired growth and development common in HIV-positive children [31]. Similar to previous reports [32, 33], WAZ scores rebounded to a greater degree and more quickly than HAZ scores, with HAZ scores in older children not responding as well as in younger children. The immunologic and growth responses of our cohort were similar or better than have been reported in sub-Saharan Africa [27] and in other countries in Asia [12]. In terms of pVL, it is clear that the ART medicines the children in our cohort received were capable of suppressing viral load in the majority of cases. Although we could not observe changes in pVL over time or even at a single time point in every child, we were able to examine pVL test results from the majority of children in the cohort. Among these, 82% had achieved viral suppression, somewhat better than the 70% pooled proportion reported in a recent systematic review of children on ART 12 months in developing countries [27].
Despite the fact that >90% of the children in our cohort were infected via mother-to-child transmission, the median age at treatment initiation was 6.2 years. Not surprisingly, these children were seriously immunosuppressed as a consequence of their delayed treatment [5, 9, 28, 32, 34]. With significant mortality occurring in the first year of life without treatment [35, 36]. it is likely that a majority of the children infected with HIV at birth had already died by the age of 6. There are 2 major barriers to the early diagnosis of children in China. First, there is a lack of widespread early infant diagnostic capability for infants born to HIV-positive mothers. Second, a large proportion of those estimated to be HIV-positive have not yet been identified. China's national HIV/AIDS program is currently making a strong push to diagnose and treat people earlier to reduce mortality [18, 37].
Our findings related to risk factors associated with mortality, including WAZ score <−2, WHO stage III or IV disease, and hemoglobin level <90 g/L, were not unexpected. Severe immunosuppression and low WAZ scores at time of treatment initiation have consistently been identified in the literature as risk factors for death in HIV-positive children [3, 5, 10, 15]. It was somewhat surprising that we did not find a statistically significant association between CD4% and mortality. Although it is not clear why this is the case, we speculate that it may be related to the fact that approximately 15% of the children in our cohort did not have baseline CD4% data.
The primary strengths of our study were the large cohort size, long follow-up time, and very limited loss to follow-up, with only 5.4% of patients lost at 3 years. Retention in care is a significant problem for ART programs around the world [10, 29, 38, 39]. The major reasons for this high retention rate are China's household registration system and the strict management system in place for tracking patients on ART. Both of these systems facilitate tracking and follow-up of patients by public health personnel as well as capturing deaths. This is especially true for children, who generally stay at their registered address to be cared for by relatives even if one or both parents become migrant workers. Unfortunately, retention of HIV-positive patients who have not yet engaged in the ART program is currently much less effective and is known to be an area in urgent need of improvement [29].
There are limitations to this analysis. First, data were obtained from an observational cohort database and thus may contain biases of which we are not aware. Second, our study does have survivor selection bias associated with enrolling median 6-year-old children, the majority of whom were infected at birth. Because without treatment approximately 50% of vertically infected children die by the age of 2, and 75% by age 5 [36], many are neither diagnosed, nor treated in time. The mortality rate in this analysis is therefore not generalizable to all HIV-positive children in China. Third, our cohort only included children who initiated ART. Inclusion of HIV-positive children who have not yet initiated ART would result in a more complete assessment of China's HIV/AIDS program and should be the subject of future research [29]. Fourth, CD4% and pVL testing have not always been available at all clinics, resulting in missing values for some children, and ART eligibility guidelines have changed over time, resulting in some children being eligible earlier than others. These issues may have introduced biases that are difficult to quantify.
Results of the present study suggest that China's pediatric ART program effectively improves patient outcomes. The lessons learned included herein can benefit other resource-limited countries in their attempts to start or scale up similar programs. As China's program continues to expand and improve, earlier diagnosis and treatment of infants and children should be made a major priority.
Notes
Acknowledgments. The authors thank Mingjie Zhang of the National Center for AIDS/STD Control and Prevention, Chinese CDC, for assistance with data checking of the manuscript preparation. The authors also thank all pediatric antiretroviral treatment center and provincial CDC staff members and all international partners at the Clinton Foundation HIV/AIDS Initiative and the China offices of the United Nations Children's Fund, the US Centers for Disease Control and Prevention Global AIDS Program, and the World Health Organization.
Financial support. This work was supported by the Chinese government; the Important National Science and Technology Specific Projects (2008ZX10001-007); and the Fogarty International Center and the National Institute on Drug Abuse of the US National Institutes of Health (5U2RTW006918-07 to Y. Z.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global HIV/AIDS response. Epidemic update and health sector progress towards universal access—progress report. 2011 Available at: http://www.who.int/hiv/pub/progress_report2011/en/ . Accessed 30 November 2011. [Google Scholar]
- 2.Judd A, Doerholt K, Tookey PA, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996–2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–24. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 3.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53:86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabue MM, Buck WC, Wanless SR, et al. Mortality and clinical outcomes in HIV-infected children on antiretroviral therapy in Malawi, Lesotho, and Swaziland. Pediatrics. 2012;130:E591–9. doi: 10.1542/peds.2011-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 6.Callens SF, Shabani N, Lusiama J, et al. Mortality and associated factors after initiation of pediatric antiretroviral treatment in the Democratic Republic of the Congo. Pediatr Infect Dis J. 2009;28:35–40. doi: 10.1097/INF.0b013e318184eeb9. [DOI] [PubMed] [Google Scholar]
- 7.Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J. 2011;30:974–9. doi: 10.1097/INF.0b013e31822539f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanoni BC, Phungula T, Zanoni HM, et al. Risk factors associated with increased mortality among HIV infected children initiating antiretroviral therapy (ART) in South Africa. PLoS One. 2011;6:e22706. doi: 10.1371/journal.pone.0022706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmonds A, Yotebieng M, Lusiama J, et al. The effect of highly active antiretroviral therapy on the survival of HIV-infected children in a resource-deprived setting: a cohort study. PLoS Med. 2011;8:e1001044. doi: 10.1371/journal.pmed.1001044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConnell MS, Chasombat S, Siangphoe U, et al. National program scale-up and patient outcomes in a pediatric antiretroviral treatment program, Thailand, 2000–2007. J Acquir Immune Defic Syndr. 2010;54:423–9. doi: 10.1097/QAI.0b013e3181dc5eb0. [DOI] [PubMed] [Google Scholar]
- 11.Janssens B, Raleigh B, Soeung S, et al. Effectiveness of highly active antiretroviral therapy in HIV-positive children: evaluation at 12 months in a routine program in Cambodia. Pediatrics. 2007;120:e1134–40. doi: 10.1542/peds.2006-3503. [DOI] [PubMed] [Google Scholar]
- 12.Hansudewechakul R, Sirisanthana V, Kurniati N, et al. Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. J Acquir Immune Defic Syndr. 2010;55:503–9. doi: 10.1097/QAI.0b013e3181f5379a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumbiganon P, Kariminia A, Aurpibul L, et al. Survival of HIV-infected children: a cohort study from the Asia-Pacific region. J Acquir Immune Defic Syndr. 2011;56:365–71. doi: 10.1097/QAI.0b013e318207a55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cardoso CA, Pinto JA, Candiani TM, Carvalho IR, Linhares RM, Goulart EM. The impact of highly active antiretroviral therapy on the survival of vertically HIV-infected children and adolescents in Belo Horizonte, Brazil. Mem Inst Oswaldo Cruz. 2012;107:532–8. doi: 10.1590/s0074-02762012000400014. [DOI] [PubMed] [Google Scholar]
- 15.George E, Noel F, Bois G, et al. Antiretroviral therapy for HIV-1-infected children in Haiti. J Infect Dis. 2007;195:1411–8. doi: 10.1086/514823. [DOI] [PubMed] [Google Scholar]
- 16.Ministry of Health of the People's Republic of China. 2012 China AIDS response progress report. Available at http://wwwunaidsorg/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_CN_Narrative_Report[1]pdf2012 . Accessed 31 March 2012. [Google Scholar]
- 17.Zhang F, Dou Z, Ma Y, et al. Five-year outcomes of the China national free antiretroviral treatment program. Ann Intern Med. 2009;151:241–51. doi: 10.7326/0003-4819-151-4-200908180-00006. W-52. [DOI] [PubMed] [Google Scholar]
- 18.Zhang F, Dou Z, Ma Y, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11:516–24. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Sun X, He Y, et al. Progress of the national pediatric free antiretroviral therapy program in China. AIDS Care. 2010;22:1182–8. doi: 10.1080/09540121003615129. [DOI] [PubMed] [Google Scholar]
- 20.Zhang F, Haberer JE, Zhao Y, et al. Chinese pediatric highly active antiretroviral therapy observational cohort: a 1-year analysis of clinical, immunologic, and virologic outcomes. J Acquir Immune Defic Syndr. 2007;46:594–8. doi: 10.1097/QAI.0b013e318158c08e. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Y, Mu W, Harwell J, et al. Drug resistance profiles among HIV-1 infected children experiencing delayed switch and 12-months efficacy after using second-line antiretroviral therapy: an observational cohort study in rural China. J Acquir Immune Defic Syndr. 2011;58:47–53. doi: 10.1097/QAI.0b013e318229f2a2. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Zhang F, Zhao Y, et al. Cohort profile: the Chinese national free antiretroviral treatment cohort. Int J Epidemiol. 2010;39:973–9. doi: 10.1093/ije/dyp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soh CH, Oleske JM, Brady MT, et al. Long-term effects of protease inhibitor-based combination therapy on CD4 T-cell recovery in HIV-1-infected children and adolescents. Lancet. 2003;362:2045–51. doi: 10.1016/s0140-6736(03)15098-2. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Ji C-Y, Zong X-N, Zhang Y-Q. Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years. Chin J Pediatr. 2009;47:487–92. [PubMed] [Google Scholar]
- 25.Resino S, Resino R, Maria Bellon J, et al. Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clin Infect Dis. 2006;43:243–52. doi: 10.1086/505213. [DOI] [PubMed] [Google Scholar]
- 26.Kekitiinwa A, Lee KJ, Walker AS, et al. Differences in factors associated with initial growth, CD4, and viral load responses to ART in HIV-infected children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008;49:384–92. doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- 27.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis. 2009;49:1915–27. doi: 10.1086/648079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KIDS-ART-LINC Collaboration. Low risk of death, but substantial program attrition, in pediatric HIV treatment cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008;49:523–31. doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- 29.Raguenaud M-E, Isaakidis P, Zachariah R, et al. Excellent outcomes among HIV+ children on ART, but unacceptably high pre-ART mortality and losses to follow-up: a cohort study from Cambodia. BMC Pediatrics. 2009;9:54. doi: 10.1186/1471-2431-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peacock-Villada E, Richardson BA, John-Stewart GC. Post-HAART outcomes in pediatric populations: comparison of resource-limited and developed countries. Pediatrics. 2011;127:e423–41. doi: 10.1542/peds.2009-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin DK, Jr, Miller WC, Benjamin DK, et al. A comparison of height and weight velocity as a part of the composite endpoint in pediatric HIV. AIDS. 2003;17:2331–6. doi: 10.1097/00002030-200311070-00007. [DOI] [PubMed] [Google Scholar]
- 32.Sutcliffe CG, van Dijk JH, Munsanje B, et al. Weight and height z-scores improve after initiating ART among HIV-infected children in rural Zambia: a cohort study. BMC Infect Dis. 2011;11:54. doi: 10.1186/1471-2334-11-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kabue MM, Kekitiinwa A, Maganda A, Risser JM, Chan W, Kline MW. Growth in HIV-infected children receiving antiretroviral therapy at a pediatric infectious diseases clinic in Uganda. AIDS Patient Care STDS. 2008;22:245–51. doi: 10.1089/apc.2007.0049. [DOI] [PubMed] [Google Scholar]
- 34.Ekouevi DK, Azondekon A, Dicko F, et al. 12-month mortality and loss-to-program in antiretroviral-treated children: the IeDEA pediatric West African database to evaluate AIDS (pWADA), 2000–2008. BMC Public Health. 2011;11:519. doi: 10.1186/1471-2458-11-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Poundstone KE, Montaner J, Zun-you W. New policies and strategies to tackle HIV/AIDS in China. Chinese Med J (Engl) 2012;125:1331–7. [PubMed] [Google Scholar]
- 38.Geoffrey F, Bock P, MRCGP , Eley B, Mothibi E, Grimwood A. Temporal trends in baseline characteristics and treatment outcomes of children starting antiretroviral treatment: an analysis in four provinces in South Africa, 2004–2009. J Acquir Immune Defic Syndr. 2011;58:e60–7. doi: 10.1097/QAI.0b013e3182303c7e. [DOI] [PubMed] [Google Scholar]
- 39.Sutcliffe CG, van Dijk JH, Bolton C, et al. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89 doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]