Our analysis of population-based tuberculosis surveillance data shows that in California, baseline isoniazid or rifampicin drug resistance, human immunodeficiency virus infection, and cavitary lung lesions in the absence of directly observed therapy were independent predictors of acquired tuberculosis multidrug resistance.
Keywords: tuberculosis, isoniazid, rifampicin, multidrug resistance, acquired drug resistance
Abstract
Background. To inform efforts to prevent antituberculosis drug resistance acquired during treatment, particularly multidrug-resistant (MDR) tuberculosis, we analyzed surveillance records from the US state with the highest morbidity.
Methods. Surveillance data from the California tuberculosis registry of cases reported between 1994 and 2006 were examined retrospectively. Crude risks of acquired resistance were estimated. Multivariate logistic regression was used to estimate odds ratios of demographic, clinical, and case management characteristics associated with acquired drug resistance (ADR), and secular trends in the incidence of ADR were assessed.
Results. One in 688 patients acquired MDR tuberculosis, with crude risks varying greatly by initial drug susceptibility test results: 1 in 1909 if initially susceptible to isoniazid and rifampin, 1 in 113 if initially isoniazid resistant, and 1 in 23 if initially rifampicin resistant. Acquired isoniazid and rifampicin monoresistance occurred in 1 in 1018 and 1 in 1455 patients, respectively. Independent predictors of acquired MDR tuberculosis were initial isoniazid resistance (odds ratio [OR], 19.2; 95% confidence interval [CI], 8.25–44.7; P < .001), initial rifampicin resistance (OR, 35.9; 95% CI, 8.61–150; P < .001), human immunodeficiency virus (HIV) infection (OR, 5.07; 95% CI, 1.73–14.9; P = .003), and cavitary disease in the absence of directly observed therapy throughout therapy (OR, 2.65; 95% CI, 1.05–6.69; P = .04). The annual incidence of ADR declined over the study period.
Conclusions. Although ADR is rare and declining in California, its costly consequences warrant improvements in treatment practices. Our findings suggest that we ensure DOT throughout the course of therapy for patients with baseline drug resistance, cavitary disease, or HIV infection.
(See the Major Article by Franke et al on pages 770–6.)
Multidrug-resistant (MDR) tuberculosis cannot be effectively treated using the principal antituberculosis drugs isoniazid and rifampicin. In 2008, an estimated 440 000 new MDR tuberculosis cases were identified worldwide—approximately 3.6% of all tuberculosis cases [1]. In the United States, MDR tuberculosis represents a relatively low but persistent proportion of the total (1.2% of incident cases in 2009) [2], with California claiming 3 of 10 MDR tuberculosis cases in the country (33 cases in California in 2009 [3]).
Multidrug resistance poses significant challenges. Anti-MDR tuberculosis drug regimens, which include expensive, less well-tolerated, and less effective second- and third-line drugs, can take 2 years or more to complete [4]. Even under painstaking conditions, treatment can fail [5]. Patients with MDR tuberculosis are more likely than other tuberculosis patients to die during treatment [6]. Moreover, MDR tuberculosis is exorbitantly expensive: In New York City, the medical and public health costs to contain an epidemic in the late 1980s exceeded US$1 billion [7]. Hospitalization costs alone for a single episode of extensively drug-resistant tuberculosis can exceed US$600 000 [8].
There are several pathways to tuberculosis drug resistance. A patient with an initially drug-susceptible strain may acquire drug resistance during treatment because of inadequate drug regimens or poor treatment adherence [9]. Preexisting resistance may be amplified when a drug-resistant strain develops resistance to additional drugs. Resistant strains can also be transmitted from person to person, for instance in institutional settings [10] and in the community, despite the possibility of a fitness cost of resistance that could reduce transmissibility compared to susceptible strains [11].
Drug resistance acquired during treatment is not well characterized. In previous studies, particularly in regions where drug susceptibility tests (DSTs) are not standard practice, a history of previous treatment for tuberculosis disease has commonly served as a proxy for acquired drug resistance (ADR) [12]. Analyses of laboratory-confirmed acquired resistance based on consecutive isolates are rare. The few published studies have been limited to small samples and geographic regions or institutions with limited generalizability [13, 14]. We report the findings of the first large population-based analysis of the magnitude and predictors of ADR in California, the state with the largest population and tuberculosis burden in the United States.
METHODS
Setting, Study Population, and Data Sources
We analyzed all tuberculosis cases reported to the California Department of Public Health tuberculosis registry from January 1994 through December 2006. The case report contains patient demographic characteristics, social and behavioral risk factors, diagnostic and clinical factors, and case management information, including DST results. The case reports were matched to the California Office of AIDS registry of persons with human immunodeficiency virus (HIV) infection.
Definitions and Inclusion Criteria
In the United States, DST for all culture-positive cases became required in 1993, in response to a rising incidence of drug resistance [15]. Isolates from culture-positive cases are routinely tested at the beginning of treatment (“initial” DST), and a subset of cases with poor clinical response or delays in Mycobacterium tuberculosis culture conversion are candidates for a follow-up test (“final” DST). The final isolate is defined as the last isolate for which susceptibility testing is performed, and must be collected at least 30 days after the initial isolate. DST results were reported as resistant if any level of resistance was detected, including resistance at low drug concentrations.
Analysis was limited to isoniazid and rifampicin resistance; “MDR” is defined as resistance to at least isoniazid and rifampicin. Results susceptible to isoniazid and rifampicin are denoted “susceptible,” and those with resistance to one but not the other “monoresistant.” Cases with acquired monoresistance were those with susceptible initial DST results and monoresistance (but not MDR) on final DST. Acquired MDR was defined as the change from susceptible or monoresistant on initial DST to MDR on final DST. We analyzed cases if they had a positive M. tuberculosis culture, had DST results for at least isoniazid and rifampicin, and began antituberculosis treatment, including individuals who acquired isoniazid and/or rifampicin resistance compared to their initial DST results together with individuals who did not change their initial DST; we excluded cases that were MDR on initial DST.
Statistical Analysis
We determined the frequency, estimated the crude risks, identified epidemiologic predictors, and identified temporal clusters and trends of each ADR outcome. The 5 outcome variables for the crude risk calculations were acquired (1) isoniazid monoresistance, (2) rifampicin monoresistance, (3) MDR following a susceptible isolate, (4) MDR following isoniazid monoresistance, and (5) MDR following rifampicin monoresistance. For the univariate and multivariate analyses of predictors of ADR, and the trend analysis, we considered (1) any acquired isoniazid resistance (including both mono- and multidrug resistance), (2) any acquired rifampicin resistance, and (3) any acquired MDR (regardless of initial DST result). The crude risks of ADR were estimated by dividing the number of individuals who acquired resistance to the drug(s) by the number of individuals who were initially susceptible. We computed exact 95% confidence intervals (CIs) for all crude risks.
To identify risk factors for ADR, we first tested univariate associations between demographic, clinical, and tuberculosis risk variables and the outcome variables, and calculated Haldane-Gart-Zweifel estimates of odds ratios and their 95% CIs. To identify independent risk factors for acquired isoniazid monoresistance, rifampicin monoresistance, and MDR, we conducted multiple regression. We included demographic variables (age, sex, a binary US vs foreign birth variable), clinical and laboratory variables (history of previous tuberculosis disease, extrapulmonary disease, positive acid-fast bacilli [AFB] smear, HIV infection), and a health system variable (private sector tuberculosis care provider) in each model. We also included, a priori, an indicator variable for cavitary chest radiograph findings without directly observed therapy (DOT) throughout the course of treatment, as DOT is particularly important for patients with the high bacillary loads associated with cavitary pulmonary lesions (eg, potentially very infectious patients). Furthermore, we included initial rifampicin monoresistance when modeling acquired isoniazid resistance, initial isoniazid monoresistance when modeling acquired rifampicin resistance, and both when modeling acquired MDR. We excluded death during treatment from the multivariate model. Bias-reduced logistic regression was conducted whenever cells contained zero observations. We calculated P values using the Mantel-Haenszel χ2 method or Fisher exact test, and considered P values <.05 to be statistically significant. We tested for temporal clustering of ADR using the maximum excess events test [16] and tested for monotone trends over time using linear regression (using a time series bootstrap with a fixed window of 3 years). All analyses were done using R for Macintosh, V2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
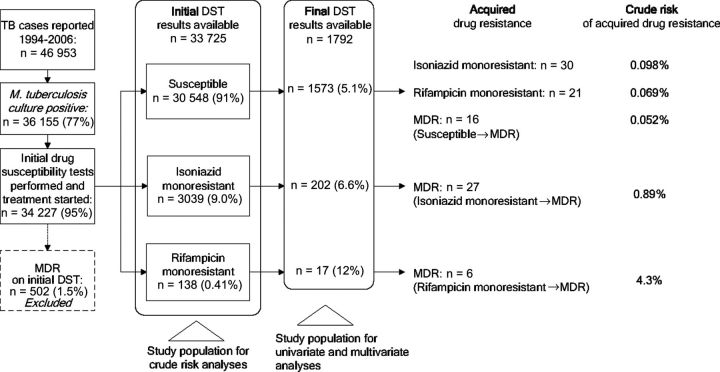
Between January 1994 and December 2006, 46 953 tuberculosis cases were reported to the California tuberculosis registry, of which 36 155 (77%) had a positive M. tuberculosis culture (Figure 1). Nearly all culture-positive cases had initial DST results reported for isoniazid and rifampicin, and began antituberculosis drug therapy (34 227 [95%]). Of these, 502 (1.5%) were already diagnosed with MDR on initial DST and were excluded from further analysis. The remaining 33 725 comprised the study population for our estimates of crude risks of ADR. Final DST results were subsequently reported for 1792 (5.3%) of these patients, who comprised the study population for the analyses of risk factors for drug resistance acquired during treatment. Table 1 compares patients whose surveillance record contained only initial DST results to patients for whom final results were also reported. Patients with final DST results were more likely to be male and US-born, and to have infectious pulmonary disease (positive AFB smear or cavitary chest radiograph), initial drug resistance, and risk factors for poor tuberculosis treatment outcomes (such as HIV infection and a history of substance abuse). Both groups were similar in the proportions of the outcomes of completion of and death during tuberculosis treatment.
Figure 1.
Study selection and crude risks of drug resistance to isoniazid and/or rifampicin acquired during treatment. Abbreviations: DST, drug susceptibility test; MDR, multidrug resistant; TB, tuberculosis.
Table 1.
Comparison of Tuberculosis Cases With Final Drug Susceptibility Test (DST) Results to Cases With Only Initial DST Results, and With Any Acquired Drug Resistance (ADR) to Those Without ADR
| Characteristic | Cases With Final DST Results (n = 1792) | Cases With Initial DST Results Only (n = 31 933) | Cases With Any Acquired Drug Resistance (n = 100) | Cases With No Acquired Drug Resistance (n = 1692) |
|---|---|---|---|---|
| Demographic | ||||
| Mean age, y (SE) | 49 (18) | 48 (20) | 46 (17) | 49 (18) |
| Female | 530 (30) | 12 232 (38) | 30 (30) | 500 (30) |
| Foreign birth | 1187 (66) | 23 610 (74) | 68 (68) | 1117 (66) |
| Clinical/laboratory | ||||
| Extrapulmonary disease | 282 (16) | 8403 (26) | 29 (29) | 253 (15) |
| Positive AFB smear | 1330 (74) | 16 500 (52) | 67 (73) | 1184 (73) |
| Cavitations on chest radiograph | 514 (29) | 5998 (19) | 27 (29) | 487 (30) |
| Initial (baseline) drug resistance | ||||
| Isoniazid resistant | 202 (11) | 2837 (8.9) | 27 (27) | 175 (10) |
| Rifampicin resistant | 17 (0.95) | 121 (0.38) | 6 (6) | 11 (1) |
| Pyrazinamide resistant | 47 (2.6) | 956 (3.0) | 5 (7) | 42 (3) |
| Ethambutol resistant | 28 (1.6) | 318 (1.0) | 4 (4) | 24 (1) |
| Risk factors for tuberculosis | ||||
| HIV infection | 194 (11) | 2330 (7.3) | 35 (35) | 159 (9) |
| Alcohol or substance abuse | 474 (28) | 4927 (17) | 29 (29) | 554 (33) |
| Healthcare worker | 21 (1.2) | 734 (2.3) | 2 (2) | 19 (1) |
| Tuberculosis care and case management | ||||
| Private sector provider | 270 (15) | 11 028 (35) | 54 (54) | 825 (49) |
| DOT throughout therapy | 1158 (65) | 15 046 (47) | 58 (59) | 1100 (65) |
| Sputum culture conversion | 1500 (90) | 18 768 (79) | 88 (96) | 1457 (92) |
| Delayed (≥60 d) conversion | 1064 (64) | 7058 (31) | 70 (75) | 994 (63) |
| Treatment outcome | ||||
| Completed treatment | 1523 (85) | 26 387 (83) | 66 (67) | 1457 (86) |
| Died | 148 (8.3) | 2884 (9.0) | 26 (26) | 122 (7) |
Data are no. (%) unless otherwise specified.
Abbreviations: AFB, acid-fast bacilli; DOT, directly observed therapy; DST, drug susceptibility test; HIV, human immunodeficiency virus.
The initial DST results of the 33 725 cases yielded 30 548 (91%) susceptible isolates, 3039 (9.0%) isolates with isoniazid monoresistance, and 138 (0.41%) with rifampicin monoresistance.
Of the 33 725, a total of 49 (0.15%) acquired MDR during treatment. These consisted of 16 that were initially susceptible, 27 initially isoniazid monoresistant, and 6 initially rifampicin monoresistant (Figure 1). The crude risk of acquiring MDR tuberculosis ranged from 0.052% (95% CI, .030%–.085%) for patients with initial susceptible isolates, to 0.89% (95% CI, .59%–1.3%) for patients with initial isoniazid monoresistance, to 4.3% (95% CI, 1.6%–9.2%) for those with initial rifampicin monoresistance. Acquired isoniazid monoresistance occurred in 0.098% (95% CI, .067%–.14%) of cases with initial susceptible isolates, and rifampicin monoresistance in 0.069% (95% CI, .043%–.11%).
Univariate analysis revealed that patients with acquired isoniazid resistance were significantly more likely to have had initial rifampicin resistance (OR, 18.1; 95% CI, 6.42–51.1; P < .0001), HIV infection (OR, 3.05; 95% CI, 1.62–5.74; P < .001), and to die during treatment (OR, 2.35; 95% CI, 1.12–4.92; P = .024), compared to patients without acquired isoniazid resistance (Table 2). However, they were less likely to have received DOT throughout therapy (OR, 0.57; 95% CI, .32–.99; P = .046). Factors associated with acquired rifampicin resistance in the univariate analysis were death during treatment (OR, 14.3; 95% CI, 3.62–60.1; P < .0001), HIV infection (OR, 9.92; 95% CI, 5.91–16.7; P < .0001), initial resistance to isoniazid (OR, 6.68; 95% CI, 3.99–11.2; P < .0001), initial resistance to pyrazinamide (OR, 3.04; 95% CI, 1.04–8.89; P = .04), extrapulmonary disease (OR, 2.91; 95% CI, 1.71–4.94; P = .0001), Mexican origin (OR, 2.11; 95% CI, 1.27–3.50; P = .004) and younger age (OR, 0.97; 95% CI, .95–.99; P = .0002).
Table 2.
Univariate Analysis of Characteristics Associated With Isoniazid Resistance and Rifampicin Resistance Acquired During Treatment
| Category | Characteristic | Acquired Isoniazid Resistance (n = 1590), ORa (95% CI) | P Value | Acquired rifampicin resistance (n = 1775), ORa (95% CI) | P Value |
|---|---|---|---|---|---|
| Demographic | Age, per y | 1.01 (.996–1.03) | .16 | 0.97 (.95–.99) | .0002 |
| Hispanic ethnicity | 0.91 (.51–1.86) | .96 | 1.96 (1.19–3.24) | .008 | |
| Mexican-born | 0.98 (.52–1.86) | .17 | 2.11 (1.27–3.50) | .004 | |
| Clinical/laboratory | Extrapulmonary disease | 1.75 (.92–3.33) | .088 | 2.91 (1.71–4.94) | .0001 |
| Cavitary chest radiograph | 1.45 (.68–3.09) | .17 | 0.49 (.11–1.60) | .14 | |
| HIV infection | 3.05 (1.62–5.74) | <.001 | 9.92 (5.91–16.7) | <.0001 | |
| Initial resistance to isoniazid | N/A | 6.68 (3.99–11.2) | <.0001 | ||
| Initial resistance to rifampicin | 18.1 (6.42–51.1) | <.0001 | N/A | ||
| Initial resistance to pyrazinamide | 1.03 (.14–7.8) | .98 | 3.04 (1.04–8.89) | .04 | |
| Initial resistance to ethambutol | 1.71 (.08–35.7)b | .73 | 4.59 (1.55–13.6) | .006 | |
| Case management | Directly observed therapy throughout treatment | 0.57 (.32–.99) | .046 | 0.92 (.55–1.53) | .74 |
| Treatment outcome | Died during treatment | 2.35 (1.12–4.92) | .024 | 14.3 (3.62–60.1) | <.0001 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; N/A, not applicable; OR, odds ratio.
a Unadjusted odds ratio.
b Bias-reduced logistic regression used due to cells containing zero. Haldane-Gart-Zweifel estimate of the OR is reported.
Table 3 summarizes univariate analysis of acquired MDR, by initial resistance pattern. Among individuals with initially susceptible isolates, HIV infection (OR, 8.44; 95% CI, 3.12–22.8; P < .0001), death during treatment (OR, 3.79; 95% CI, 1.2–11.9; P = .02), extrapulmonary disease (OR, 3.15; 95% CI, 1.14–8.75; P = .03), and Mexican origin (OR, 3.0; 95% CI, 1.12–8.04; P = .03) were significantly associated with acquired MDR. In the group with initial isoniazid monoresistance, patients with acquired MDR were significantly more likely to die during treatment (OR, 14.3; 95% CI, 4.25–48.2; P < .0001) and to have HIV coinfection (OR, 4.19; 95% CI, 1.29–13.6; P = .017). Owing to small cell sizes, we did not estimate associations with acquired MDR for patients with initial rifampicin monoresistance. Any acquired MDR tuberculosis (regardless of initial DST result) was associated with death during treatment (OR, 5.34; 95% CI, 2.84–10.1; P < .0001) and HIV infection (OR, 4.26; 95% CI, 2.30–7.90; P < .0001).
Table 3.
Univariate Analysis of Multidrug Resistance Acquired During Treatment Among All Case Patients With Initial Drug Susceptibility Test Results for Isoniazid and Rifampicin
| Characteristic | Acquired MDR Tuberculosis Following Susceptible Initial Result (n = 1573), ORa (95% CI) | P Value | Acquired MDR Tuberculosis Following Isoniazid Monoresistance (n = 202), ORa (95% CI) | P Value | Any Acquired MDR Tuberculosis (n = 1792), ORa (95% CI) | P Value |
|---|---|---|---|---|---|---|
| No. of cases | 16 | 27 | 49 | |||
| Age, per y | 0.99 (.96–1.02) | .38 | 0.99 (.96–1.01) | .23 | 0.98 (.97–1.00) | .07 |
| Mexican-born | 3.0 (1.12–8.04) | .03 | 0.64 (.24–1.68) | .36 | 1.28 (.73–2.26) | .39 |
| Extrapulmonary disease | 3.15 (1.14–8.75) | .03 | 1.43 (.45–4.57) | .55 | 1.77 (.91–3.44) | .09 |
| Cavitary chest radiograph | 0.36 (.039–1.60) | .083 | 1.68 (.71–3.94) | .12 | 0.91 (.47–1.74) | .39 |
| HIV infection | 8.44 (3.12–22.8) | <.0001 | 4.19 (1.29–13.6) | .017 | 4.26 (2.30–7.90) | <.0001 |
| Death during treatment | 3.79 (1.2–11.9) | .02 | 14.3 (4.25–48.2) | <.0001 | 5.34 (2.84–10.1) | <.0001 |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; MDR, multidrug-resistant; OR, odds ratio.
a Unadjusted odds ratio.
In the multivariate analysis, independent predictors of acquired isoniazid resistance were initial rifampicin resistance (OR, 10.3; 95% CI, 2.82–37.7; P < .001) and HIV infection (OR, 3.36; 95% CI, 1.33–8.50; P = .01) (Table 4). For acquired rifampicin resistance, we found significant associations with initial resistance to isoniazid (OR, 11.2; 95% CI, 5.2–24.4; P < .001), HIV infection (OR, 9.39; 95% CI, 3.76–23.4; P < .001), and cavitary disease in the absence of DOT throughout the treatment regimen (OR, 2.65; 95% CI, 1.09–6.46; P = .03). We also found an inverse relationship between age and acquired rifampicin resistance (OR, 0.96; 95% CI, .94–.98; P = .002). Acquired MDR was independently predicted by initial rifampicin resistance (OR, 35.9; 95% CI, 8.61–150; P < .001), initial isoniazid resistance (OR, 19.2; 95% CI, 8.25–44.7; P < .001), HIV infection (OR, 5.07; 95% CI, 1.73–14.9; P = .03), and cavitary disease in the absence of DOT throughout treatment (OR, 2.65; 95% CI, 1.05–6.69; P = .04).
Table 4.
Multivariate Analysis of Independent Predictors of Drug Resistance Acquired During Treatment Among All California Tuberculosis Patients With Initial Drug Susceptibility Test Results for Isoniazid and Rifampicin
| Characteristic | Acquired Isoniazid Resistance (n = 1590), OR (95% CI) | P Value | Acquired Rifampicin Resistance (n = 1775), OR (95% CI) | P Value | Acquired Multidrug Resistance (n = 1792), OR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age, per y | 1.02 (.997–1.03) | .09 | 0.96 (.94–.98) | .002 | 0.98 (.96–1.0) | .06 |
| Female sex | 1.14 (.56–2.35) | .72 | 0.99 (.46–2.15) | .98 | 1.51 (.71–3.23) | .28 |
| Foreign birth | 0.56 (.29–1.07) | .08 | 1.39 (.63–3.06) | .42 | 1.14 (.54–2.43) | .72 |
| Clinical/laboratory | ||||||
| History of previous tuberculosis | 1.77 (.65–4.81) | .26 | 2.14 (.77–5.95) | .15 | 2.49 (.87–7.16) | .09 |
| Positive AFB smear | 0.62 (.31–1.23) | .17 | 0.86 (.40–1.84) | .69 | 1.30 (.51–3.33) | .59 |
| Extrapulmonary TB disease | 0.67 (.24–1.89) | .45 | 1.46 (.57–3.73) | .42 | 1.15 (.39–3.36) | .80 |
| Initial isoniazid resistance | N/A | … | 11.2 (5.2–24.4) | <.001 | 19.2 (8.25–44.7) | <.001 |
| Initial rifampicin resistance | 10.3 (2.82–37.7) | <.001 | N/A | … | 35.9 (8.61–150) | <.001 |
| Initial pyrazinamide resistance | 2.45 (.43–13.9) | .31 | 1.36 (.37–5.08) | .64 | 0.20 (.01–3.1) | .25 |
| Initial ethambutol resistance | 2.78 (.11–69.6) | .53 | 0.05 (.001–1.83) | .10 | 0.11 (.003–3.3) | .20 |
| HIV infection | 3.36 (1.33–8.50) | .01 | 9.39 (3.76–23.4) | <.001 | 5.07 (1.73–14.9) | .003 |
| Case management | ||||||
| Cavitary disease without DOT throughout treatment | 0.97 (.31–3.03) | .96 | 2.65 (1.09–6.46) | .03 | 2.65 (1.05–6.69) | .04 |
| Initial use of rifampicin in treatment | 0.996 (.18–5.56) | >.99 | 0.38 (.10–1.35) | .13 | 0.47 (.08–2.63) | .39 |
| Initial use of isoniazid in treatment | 0.45 (.02–8.3) | .59 | 0.33 (.06–1.66) | .18 | 0.29 (.05–1.55) | .15 |
| Health system | ||||||
| Private sector TB care provider | 0.85 (.44–1.63) | .62 | 1.07 (.54–2.12) | .85 | 0.99 (.46–2.11) | .98 |
Abbreviations: AFB, acid-fast bacilli; DOT, directly observed therapy; CI, confidence interval; HIV, human immunodeficiency virus; N/A, not applicable; OR, odds ratio; TB, tuberculosis.
The mean annual proportions of tuberculosis cases with ADR were 0.19% for rifampicin and 0.17% for isoniazid, compared to 0.15% for MDR tuberculosis. All 3 outcomes decreased significantly over the course of the 13-year study period (isoniazid: P = .009; rifampicin: P = .011; MDR: P = .014).
DISCUSSION
We present the results of this large population-based study of the risk of ADR using consecutive drug susceptibility test results during the same tuberculosis treatment course. Our main finding is that isoniazid or rifampicin drug resistance at tuberculosis diagnosis, HIV infection, and cavitary tuberculosis disease in the absence of complete DOT were independent predictors of laboratory-confirmed acquired MDR. Patients who acquired MDR were also significantly more likely than other tuberculosis patients to die during treatment.
In a previous study of all MDR tuberculosis in California spanning a similar period, we reported that baseline resistance at the time of diagnosis was independently associated with a history of previous tuberculosis disease, positive AFB smear, Asian or Pacific Islander origin, recent immigration to the United States, and death during treatment [6]. Unlike in the present study of acquired resistance, HIV infection was not found to predict baseline MDR tuberculosis, suggesting that risk factors for acquired resistance are not necessarily the same risk factors for baseline resistance [17].
Our finding that isoniazid resistance was a risk factor for subsequently acquiring rifampicin resistance corroborates evidence that the risk of acquiring further drug resistance in the presence of initial drug resistance (eg, amplification of isoniazid monoresistance to MDR) is higher than the risk of acquiring resistance in susceptible strains [18–21]. Previous studies have also reported a significant association between HIV infection and antituberculosis drug resistance—attributed to malabsorption of rifampicin or to drug interactions with antiretroviral medications—as was demonstrated here [14, 22, 23].
There was no statistically significant association between any demographic characteristic and ADR in the multivariate analysis. However, we identified a univariate association between Mexican origin and acquired rifampicin monoresistance, and MDR (when the initial DST was susceptible). While the link between pyrazinamide resistance characteristic of Mycobacterium bovis infection and Mexican origin of tuberculosis patients has been well characterized in the United States [24], associations with other forms of drug resistance are less recognized. In previous analyses, the Asian population had disproportionately high rates of baseline isoniazid and/or rifampicin resistance, but Mexican origin has not been associated with elevated rates of resistance to these principal drugs [6, 15]. The majority of the Mexican-born patients in the current study with ADR also had HIV infection (data not shown), warranting further investigation of this vulnerable demographic group with tuberculosis-HIV coinfection.
Our study contributes new information on ADR regarding the intersection of cavitary disease and DOT. Even when there is an adequate drug regimen, the high bacillary loads in cavitary disease theoretically predispose a tuberculosis patient to the development of drug resistance because more drug resistant phenotypes exist in the bacillary population. Previous studies have found cross-sectional associations between cavitary disease and baseline multidrug resistance [25, 26]. Because the application of DOT in an observational study is influenced by knowledge of risk factors and public health practice patterns the impact of DOT on ADR should be assessed using a sufficiently powered randomized controlled trial—a prohibitively expensive endeavor because ADR is rare. Our data did not support the findings of a population-based study in San Francisco [27] that reported the same rate of ADR among patients on DOT compared to those on self-administered treatment. In contrast, in a population-based study of rifampicin monoresistance in New York in the early 1990s, infrequent application of DOT was found to be an independent risk factor for acquired rifampicin resistance [28]. Our findings provide new, large population-based evidence to support recommendations that DOT be prioritized for tuberculosis patients with cavitary disease.
The statistically significant decline in ADR in California is encouraging, in light of a previous study in San Francisco that had detected increases during 1990–1994, compared to 1985–1989 [14]. It is likely that the decline identified in the current study is in large part driven by the decline in tuberculosis cases with HIV coinfection seen in California since the late 1990s [3]. However, the decline in ADR has occurred in the context of a relatively constant proportion of baseline MDR tuberculosis [6] and the observation that an increasing portion of MDR tuberculosis in California is nearly extensively drug resistant [8]. Since the study period ended, decreasing public health resources may have compromised the application of DOT and increased ADR, warranting ongoing measurement.
Because acquisition of drug resistance during therapy is rare in a setting of low tuberculosis incidence, the large sample size of our study provides an important advantage. However, there were several limitations. Because crude risks ignore risk factors, they may not be generalizable to other populations with different risk factor profiles. Furthermore, because DSTs require a positive culture, we excluded culture-negative cases (23% of all cases); any acquired resistance that may have occurred in culture-negative cases was not ascertained. Similarly, some culture-positive cases without a reported final DST may have had undetected ADR, rendering our estimates artificially low. However, we note that proportions of treatment completion and of death during treatment in the group without final DST results were statistically similar to those in the group with final DST results, lessening concerns about the substantial impact of unrecognized ADR. Additionally, repeat specimens for DST were indicated only when treatment complications such as delayed culture conversion were encountered. The vast majority of patients had no follow-up DST, because they were responding well to therapy and ADR was unlikely to have occurred. Overestimation of the associations between predictor and outcome variables are a possibility, if patients with certain characteristics (eg, HIV infection) had follow-up DSTs at higher proportions than others (ie, if the study group was biased toward persons with conditions predisposing them to having a follow-up DST). However, the strong association between HIV infection and ADR may not be completely explained by an intermediate relationship between HIV infection and having a repeat DST. Due to the incompleteness of genotyping data spanning the study period, we could not assess the strain concordance of consecutive isolates, and therefore could not measure the contribution of reinfection during treatment. However, findings in high-tuberculosis-prevalence regions that serial isolates from the same patient tend to be genotypically concordant [29, 30], suggest that we observed true acquisition of resistance and not reinfection.
Acquired tuberculosis MDR persists at low levels in California. Although the crude risk of developing MDR tuberculosis disease when initially susceptible represents a small risk for an individual (0.052%), the increased cost and worse clinical outcomes render the prevention of acquired MDR a continuing priority. At a minimum, prevention of ADR may prevent outbreaks attributed to transmission of resistant strains [31]. A commitment to DOT throughout therapy for patients with cavitary disease—in addition to those for whom DOT is already recommended (eg, children and adolescents, residents of institutional settings, substance and excess alcohol users, those with a history of previous tuberculosis disease, and those with a drug-resistant isolate)—may help prevent the costly and dangerous acquisition of drug resistance. Potential savings from the prevention of ADR in these risk groups may be substantial, as an MDR case can on average cost >4 times as much as a drug-susceptible case [32, 33].
Notes
Acknowledgments. We acknowledge local health jurisdiction tuberculosis programs in California for their efforts to combat drug-resistant tuberculosis. We thank Janice Westenhouse, Jennifer Allen, and William Elms for assistance with providing surveillance data; Chihori Lietman for assistance with translation; and Julia Hill and Allison Kelley for editorial assistance.
Author contributions. All authors participated substantially in the implementation of the study and the drafting of the manuscript. T. C. P. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial support. The study was supported by the California Department of Public Health and by Cooperative Agreement funds provided by the CDC Division of Tuberculosis Elimination (U52/CCU900515–23). T. C. P. acknowledges support in part by the National Institutes of Health, National Institute of General Medical Sciences (U01-GM08778; Modeling Infectious Disease Agent Study). No external source of funding played a role in the study design, implementation, analysis, manuscript preparation, or decision to submit the manuscript for publication.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. Geneva, Switzerland: World Health Organization; 2010. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. Reported tuberculosis in the United States, 2009. [Google Scholar]
- 3.Westenhouse J, Allen J, Johnson L, Kanowitz S, Waldow K. California Department of Public Health. Report on tuberculosis in California, 2009. 2010 [Google Scholar]
- 4.Francis J. Curry National Tuberculosis Center. Drug-resistant tuberculosis: a survival guide for clinicians. 2nd ed. San Francisco, CA: Francis J. Curry National Tuberculosis Center and California Department of Public Health; 2008. [Google Scholar]
- 5.Goble M, Iseman MD, Madsen LA, Waite D, Ackerson L, Horsburgh CR. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–32. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 6.Granich RM, Oh P, Lewis B, Porco TC, Flood J. Multidrug resistance among persons with tuberculosis in California, 1994–2003. JAMA. 2005;293:2732–9. doi: 10.1001/jama.293.22.2732. [DOI] [PubMed] [Google Scholar]
- 7.Gerberding J. Extensively drug-resistant TB: CDC's public health response. Committee on Foreign Affairs, Subcommittee on Africa and Global Health; 2007. http://www.hhs.gov/asl/testify/2007/03/t20070321c.html . Accessed 18 December 2012. [Google Scholar]
- 8.Banerjee R, Allen J, Westenhouse J, et al. Extensively drug-resistant tuberculosis in California, 1993–2006. Clin Infect Dis. 2008;47:450–7. doi: 10.1086/590009. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie SH. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrob Agents Chemother. 2002;46:267–74. doi: 10.1128/AAC.46.2.267-274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small PM, Shafer RW, Hopewell PC, et al. Exogenous reinfection with multidrug-resistant Mycobacterium tuberculosis in patients with advanced HIV infection. New Engl J Med. 1993;328:1137–44. doi: 10.1056/NEJM199304223281601. [DOI] [PubMed] [Google Scholar]
- 11.Cohen T, Sommers B, Murray M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. Lancet Infect Dis. 2003;3:13–21. doi: 10.1016/s1473-3099(03)00483-3. [DOI] [PubMed] [Google Scholar]
- 12.Van Rie A, Warren R, Richardson M, et al. Classification of drug-resistant tuberculosis in an epidemic area. Lancet. 2000;356:22–5. doi: 10.1016/S0140-6736(00)02429-6. [DOI] [PubMed] [Google Scholar]
- 13.El Sahly HM, Teeter LD, Pawlak RR, Musser JM, Graviss EA. Drug-resistant tuberculosis: a disease of target populations in Houston, Texas. J Infect. 2006;53:5–11. doi: 10.1016/j.jinf.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Bradford WZ, Martin JN, Reingold AL, Schecter GF, Hopewell PC, Small PM. The changing epidemiology of acquired drug-resistant tuberculosis in San Francisco, USA. Lancet. 1996;348:928–31. doi: 10.1016/S0140-6736(96)03027-9. [DOI] [PubMed] [Google Scholar]
- 15.Moore M, Onorato IM, McCray E, Castro KG. Trends in drug-resistant tuberculosis in the United States, 1993–1996. JAMA. 1997;278:833–7. [PubMed] [Google Scholar]
- 16.Tango T. A test for spatial disease clustering adjusted for multiple testing. Stat Med. 2000;19:191–204. doi: 10.1002/(sici)1097-0258(20000130)19:2<191::aid-sim281>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Conaty SJ, Hayward AC, Story A, Glynn JR, Drobniewski FA, Watson JM. Explaining risk factors for drug-resistant tuberculosis in England and Wales: contribution of primary and secondary drug resistance. Epidemiol Infect. 2004;132:1099–108. doi: 10.1017/s0950268804002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lew W, Pai M, Oxlade O, Martin D, Menzies D. Initial drug resistance and tuberculosis treatment outcomes: systematic review and meta-analysis. Ann Intern Med. 2008;149:123–34. doi: 10.7326/0003-4819-149-2-200807150-00008. [DOI] [PubMed] [Google Scholar]
- 19.Mitchison DA. How drug resistance emerges as a result of poor compliance during short course chemotherapy for tuberculosis. Int J Tuberc Lung Dis. 1998;2:10–5. [PubMed] [Google Scholar]
- 20.Quy HT, Lan NT, Borgdorff MW, et al. Drug resistance among failure and relapse cases of tuberculosis: is the standard re-treatment regimen adequate? Int J Tuberc Lung Dis. 2003;7:631–6. [PubMed] [Google Scholar]
- 21.Yoshiyama T, Yanai H, Rhiengtong D, et al. Development of acquired drug resistance in recurrent tuberculosis patients with various previous treatment outcomes. Int J Tuberc Lung Dis. 2004;8:31–8. [PubMed] [Google Scholar]
- 22.Lutfey M, Della-Latta P, Kapur V, et al. Independent origin of mono-rifampin-resistant Mycobacterium tuberculosis in patients with AIDS. Am J Respir Crit Care Med. 1996;153:837–40. doi: 10.1164/ajrccm.153.2.8564140. [DOI] [PubMed] [Google Scholar]
- 23.Ridzon R, Whitney CG, McKenna MT, et al. Risk factors for rifampin monoresistant tuberculosis. Am J Respir Crit Care Med. 1998;157:1881–4. doi: 10.1164/ajrccm.157.6.9712009. [DOI] [PubMed] [Google Scholar]
- 24.Hlavsa MC, Moonan PK, Cowan LS, et al. Human tuberculosis due to Mycobacterium bovis in the United States, 1995–2005. Clin Infect Dis. 2008;47:168–75. doi: 10.1086/589240. [DOI] [PubMed] [Google Scholar]
- 25.Liaw YS, Hsueh PR, Yu CJ, Wang SK, Yang PC, Luh KT. Drug resistance pattern of Mycobacterium tuberculosis in a university hospital in Taiwan, 1998–2002. J Formos Med Assoc. 2004;103:671–7. [PubMed] [Google Scholar]
- 26.Fujino T, Hasegawa N, Satou R, Komatsu H, Kawada K. Attributable factors to the emergence of multidrug-resistant Mycobacterium tuberculosis based on the observation of consecutive drug resistance test results. Kekkaku. 1998;73:471–6. [PubMed] [Google Scholar]
- 27.Jasmer RM, Seaman CB, Gonzalez LC, Kawamura LM, Osmond DH, Daley CL. Tuberculosis treatment outcomes: directly observed therapy compared with self-administered therapy. Am J Respir Crit Care Med. 2004;170:561–6. doi: 10.1164/rccm.200401-095OC. [DOI] [PubMed] [Google Scholar]
- 28.Munsiff SS, Joseph S, Ebrahimzadeh A, Frieden TR. Rifampin-monoresistant tuberculosis in New York City, 1993–1994. Clin Infect Dis. 1997;25:1465–7. doi: 10.1086/516146. [DOI] [PubMed] [Google Scholar]
- 29.Han LL, Sloutsky A, Canales R, et al. Acquisition of drug resistance in multidrug-resistant Mycobacterium tuberculosis during directly observed empiric retreatment with standardized regimens. Int J Tuberc Lung Dis. 2005;9:818–21. [PubMed] [Google Scholar]
- 30.Sonnenberg P, Murray J, Shearer S, Glynn JR, Kambashi B, Godfrey-Faussett P. Tuberculosis treatment failure and drug resistance: same strain or reinfection? Trans R Soc Trop Med Hyg. 2000;94:603–7. doi: 10.1016/s0035-9203(00)90205-0. [DOI] [PubMed] [Google Scholar]
- 31.Heifets LB, Cangelosi GA. Drug susceptibility testing of Mycobacterium tuberculosis: a neglected problem at the turn of the century. Int J Tuberc Lung Dis. 1999;3:564–81. [PubMed] [Google Scholar]
- 32.Marks SM, Armstrong L, Flood J, et al. Treatment practices, outcomes, and cost of multidrug-resistant and extensively drug resistant tuberculosis in the United States: preliminary results [abstract] Am J Respir Crit Care Med. 2012;185:A3308. [Google Scholar]
- 33.Holmquist L, Russo CA, Elixhauser A. Rockville, MD: Agency for Healthcare Research and Quality; Tuberculosis stays in U.S. hospitals, 2006. HCUP Statistical Brief #60, 2008. [PubMed] [Google Scholar]