In the last decade, timely initiation of antiretroviral therapy and resulting virologic suppression have greatly improved in North America concurrent with the development of better tolerated and more potent regimens, but significant barriers to treatment uptake remain.
Keywords: antiretroviral therapy, healthcare disparities, HIV, time factors, viral load
Abstract
Background. Since the mid-1990s, effective antiretroviral therapy (ART) regimens have improved in potency, tolerability, ease of use, and class diversity. We sought to examine trends in treatment initiation and resulting human immunodeficiency virus (HIV) virologic suppression in North America between 2001 and 2009, and demographic and geographic disparities in these outcomes.
Methods. We analyzed data on HIV-infected individuals newly clinically eligible for ART (ie, first reported CD4+ count <350 cells/µL or AIDS-defining illness, based on treatment guidelines during the study period) from 17 North American AIDS Cohort Collaboration on Research and Design cohorts. Outcomes included timely ART initiation (within 6 months of eligibility) and virologic suppression (≤500 copies/mL, within 1 year). We examined time trends and considered differences by geographic location, age, sex, transmission risk, race/ethnicity, CD4+ count, and viral load, and documented psychosocial barriers to ART initiation, including non–injection drug abuse, alcohol abuse, and mental illness.
Results. Among 10 692 HIV-infected individuals, the cumulative incidence of 6-month ART initiation increased from 51% in 2001 to 72% in 2009 (Ptrend < .001). The cumulative incidence of 1-year virologic suppression increased from 55% to 81%, and among ART initiators, from 84% to 93% (both Ptrend < .001). A greater number of psychosocial barriers were associated with decreased ART initiation, but not virologic suppression once ART was initiated. We found significant heterogeneity by state or province of residence (P < .001).
Conclusions. In the last decade, timely ART initiation and virologic suppression have greatly improved in North America concurrent with the development of better-tolerated and more potent regimens, but significant barriers to treatment uptake remain, both at the individual level and systemwide.
Since the mid-1990s, effective antiretroviral therapy (ART) regimens to treat human immunodeficiency virus (HIV) infection have improved in potency, tolerability, ease of use, and antiretroviral class diversity [1]. These have been linked to improvements in treatment adherence [2, 3] and clinical outcomes [4]. However, not all who are clinically eligible start treatment, a scenario sometimes associated with substance abuse and suboptimal insurance coverage, among other factors [5].
Monitoring trends in the successful initiation of ART is important, especially as population-based interventions such as expanded “test-and-treat” initiatives are introduced [6, 7] and as treatment guidelines recommend starting therapy at higher CD4+ counts [8, 9]. Collectively, these changes increase the identification of previously undiagnosed individuals and, consequently, the pool of persons newly eligible for treatment. Owing to their size and heterogeneity, collaborative observational studies are particularly useful to monitor trends [10]. They can help identify disparities among subpopulations, including those defined by geography, which may be informative as many health policies are instituted at the state or province level. Reducing HIV-related health disparities in vulnerable populations is a priority of both the US National HIV/AIDS Strategy and the Federal Initiative to Address HIV/AIDS in Canada [11, 12].
We examined ART initiation and virologic suppression among newly treatment-eligible individuals between 2001 and 2009 in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). To assess changes over time in this large cohort, we estimated the annual cumulative incidence of ART initiation and virologic suppression following treatment eligibility. We also examined factors associated with these outcomes, and explored heterogeneity by geographic location.
METHODS
Data Source
The NA-ACCORD is a collaboration of single- and multisite prospective cohort studies that includes >100 000 HIV-infected individuals from >100 sites in the United States and Canada, and is a regional contributor to the International Epidemiological Databases to Evaluate AIDS [13–15]. Each participating cohort submits data regarding enrolled participants’ demographic characteristics, prescribed medications, laboratory tests, clinical diagnoses, and vital status, which undergo quality control before being combined into standardized analysis files. Human subjects activities of the NA-ACCORD and each participating cohort are reviewed and approved by their respective institutional review boards.
Study Population
The study population included ART-naive HIV-infected adults (age ≥18 years) newly eligible to initiate ART between 2001 and 2009, from 17 NA-ACCORD cohorts. For consistency, clinical eligibility was based on US Department of Health and Human Services treatment guidelines during this period (ie, an incident AIDS-defining illness [ADI] or a recorded CD4+ count of <350 cells/µL) [16, 17]. Inclusion criteria were a known state or province of residence, at least 2 CD4+ counts in the study period (to ensure adequate follow-up), no prior CD4+ counts <350 cells/µL or documented ADIs, and no documented history of ART use.
Outcomes of Interest
The outcomes of interest were time to ART initiation and time to virologic suppression, using date of ART eligibility (ie, the first date that an incident ADI or a CD4+ count <350 cells/µL was recorded) as the time origin. Time to ART initiation was defined as the duration between the date of eligibility and the date ART was started. We used a standard definition for ART consistent with US guidelines [18]. On the basis of previous work using a 6-month window to capture successful initiation of treatment, [19] we censored time to ART initiation at 6 months after eligibility, focusing on timely initiation.
Virologic suppression was defined as any HIV type 1 viral load (VL) measurement ≤500 copies/mL within 1 year. This threshold was chosen to account for differences in detection limits of commercial assays over the study period [20, 21]. We censored suppression at 1 year after eligibility to focus on more timely virologic control. Seven percent of subjects did not have a second VL available by 1 year and were excluded from further analysis. A secondary outcome was time to virologic suppression, limited to those initiating ART within 6 months of eligibility. For this outcome, we used the date of ART initiation as the time origin instead of the date of ART eligibility.
Variables of Interest
Individual-level variables of interest, assessed at ART eligibility, included age; race/ethnicity; sex; transmission risk (ie, male sex with men, history of injection drug use [IDU], or other risk); CD4+ count; VL; calendar year; jurisdiction of residence (ie, state or province); and psychosocial barriers to ART initiation, including documented histories of non–injection drug abuse, alcohol abuse, and mental illness. These barriers were dichotomized based on the presence of more specific diagnoses of drug or alcohol abuse/dependence or mental disorders as derived from electronic medical records and medical record reviews, and the variables were summed to form a single ordinal variable. State of residence was not available from 3 US cohorts, and for these the state of the clinic site was used as a proxy. To assess potential misclassification due to this decision, we calculated the percentage of individuals living outside the state of the clinic site from the remaining US clinical cohorts; only 1% lived outside the state of the clinic site.
To account for potential differences in ART initiation influenced by characteristics of the cohorts or clinics themselves, we used information regarding employment (eg, unemployed, disabled, working) and insurance status (eg, uninsured, public, private) collected by a standardized questionnaire administered to all US NA-ACCORD clinical cohorts on their study populations, categorizing each as follows: higher socioeconomic status US clinical cohort, lower socioeconomic US clinical cohort, US interval cohort, and Canadian cohort. Interval cohorts differ from clinical cohorts in both timing and data collection; individuals are followed at specified intervals (eg, every 6 months) that are unrelated to healthcare visits, and data are collected according to defined protocols [22]. We also included variables representing mechanisms undertaken by individual clinics to assist patients in gaining access to ART, such as AIDS Drug Assistance Program (ADAP) enrollment, and distinguished between those performed by clinic staff and those requiring referral to entities outside the clinic. For cohorts not surveyed, we used the median of the answers from participating cohorts.
Statistical Methods
For each calendar year, we estimated the cumulative incidence of ART initiation (at 6 months of eligibility) and virologic suppression (at 1 year of eligibility; and among ART initiators, at 1 year of ART initiation), adjusting for the aforementioned covariates. We used a published method that determines the cumulative incidence for a given exposure (eg, calendar year) by averaging the individual predicted survival curves of people with that exposure, based on a Cox model stratified by year [23]. For time to virologic suppression, we excluded the small number of individuals who already had a VL <500 copies/mL at eligibility.
We constructed multivariable Cox regression models to determine hazard ratios for factors associated with time to ART initiation and time to virologic suppression. We explored potential interactions between these factors and calendar time (ie, 2001–2005 and 2006–2009) in subgroup analyses by time period, based on observed time trends. Because not all cohorts could provide information on each of the psychosocial barriers examined, we performed sensitivity analysis among the 11 cohorts contributing data on all 3 (n = 9370). To account for potential selection bias in the analysis of virologic suppression among those who initiated treatment, we performed a sensitivity analysis using inverse probability of selection weighting as an alternative to simply limiting the analysis to ART initiators (Supplementary Appendix). We also replaced time-to-event outcomes with dichotomous outcomes as a further sensitivity analysis. Overall inferences did not change using these alternate approaches.
We tested for geographic heterogeneity in each outcome using likelihood ratio testing, with jurisdiction of residence modeled as a random effect. Because representativeness of estimates in NA-ACCORD to the general HIV-infected population may vary within each jurisdiction, we limited estimates to those with ≥100 eligible individuals and ≥1000 participants in the overall NA-ACCORD, and reported estimates by geographic region instead of the jurisdictions themselves.
Analyses were conducted using SAS software version 9.2 and the R package version 2.15.0. A P value < .05 guided statistical interpretation.
RESULTS
From 115 882 living HIV-infected individuals in NA-ACCORD between 2001 and 2009, 10 692 were ART-naive and became newly eligible for ART initiation during the study period. There were 9186 (86%) participants eligible for analyses of virologic suppression because they were not suppressed at eligibility and had at least 1 VL recorded within 1 year. Participant characteristics are listed in Table 1. Thirty-four percent were eligible for ART because of a CD4+ count < 200 cells/µL, and an additional 63% had a CD4+ count between 200 cells/µL and 350 cells/µL. Only 3.6% were eligible for ART based solely on an incident ADI. Among the participants, 0.3% had both a CD4+ count < 350 cells/µL and an incident ADI.
Table 1.
Characteristics of Newly Treatment-Eligible HIV-Infected Individuals, North American AIDS Cohort Collaboration on Research and Design, 2001–2009 (N = 10 692)
| Characteristic | No. | % |
|---|---|---|
| Age at eligibility, y, median (interquartile range) | 40 | (33–46) |
| 18–29 | 1848 | 17 |
| 30–39 | 3434 | 32 |
| 40–49 | 3618 | 34 |
| 50–59 | 1460 | 14 |
| ≥60 | 332 | 3 |
| Race/ethnicity | ||
| Black | 4414 | 41 |
| Hispanic, any race | 1804 | 17 |
| White | 3505 | 33 |
| Asian/Pacific Islander | 159 | 1.5 |
| Other race/ethnicitya | 810 | 8 |
| Sex and transmission risk | ||
| Men who have sex with men | 4586 | 43 |
| Male injection drug user | 1151 | 11 |
| Male, other risk | 2125 | 20 |
| Female injection drug user | 464 | 4 |
| Female, other risk | 2366 | 22 |
| Antiretroviral therapy eligibility criteria | ||
| CD4+ count 0–199 cells/µL | 3631 | 34 |
| CD4+ count 200–349 cells/µL | 6679 | 62 |
| Incident AIDS-defining illness | 382 | 4 |
| Viral load at eligibility | ||
| Undetectable | 774 | 7 |
| 501–9999 copies/mL | 1677 | 16 |
| 10 000–99 999 copies/mL | 4210 | 39 |
| ≥100 000 copies/mL | 2837 | 27 |
| Missing | 1194 | 11 |
| Documented history of psychosocial barriersb | ||
| Alcohol abuse | 684 | 7 |
| Non–injection drug abuse | 1303 | 14 |
| Mental illness | 2315 | 22 |
| Number of psychosocial barriersc | ||
| 0 | 7649 | 72 |
| 1 | 2033 | 19 |
| 2 | 757 | 7 |
| 3 | 253 | 2 |
| Calendar year of eligibility | ||
| 2001–2005 | 6035 | 56 |
| 2006–2009 | 4657 | 44 |
a Includes Native Americans, Aboriginal Canadians, and persons of other or unknown race/ethnicity.
b Among participants with data available, 9865, 9515, and 10 648 had data on alcohol abuse, non–injection drug abuse, and mental illness, respectively.
c Includes a documented history of alcohol abuse, non–injection drug abuse, and mental illness.
The study population comprised 9619 individuals from 33 US states (plus the District of Columbia) and 1073 from 5 Canadian provinces. Fourteen states and 3 provinces had sufficient representation to estimate cumulative incidence at the jurisdictional level (Supplementary Appendix Table 1). The 3 most highly represented jurisdictions were California (19%), New York (18%), and Texas (11%).
Trends in ART Initiation and Virologic Suppression, 2001–2009
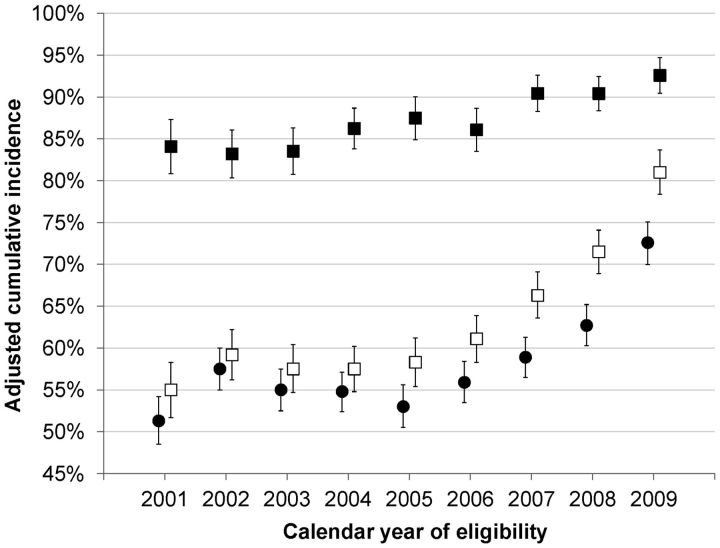
Figure 1 shows time trends in ART initiation and virologic suppression. The adjusted 6-month cumulative incidence of ART initiation was 57% overall, increasing from 51% in 2001 to 72% in 2009. The adjusted cumulative incidence of virologic suppression after 1 year of ART eligibility was 62%, increasing from 55% in 2001 to 81% in 2009. Among ART initiators, the cumulative incidence of virologic suppression after 1 year was 87%, increasing from 84% in 2001 to 93% in 2009. All trends were statistically significant (Ptrend < .001), and generally persisted within individual cohorts (data not shown).
Figure 1.
Trends in antiretroviral therapy (ART) initiation and virologic suppression among newly treatment-eligible individuals in North America, NA-ACCORD, 2001–2009. Solid circles: ART initiation 6 months after eligibility; hollow squares: virologic suppression 1 year after eligibility; solid squares: virologic suppression 1 year after ART initiation. Error bars represent 95% confidence intervals. Estimates were adjusted for age; race/ethnicity; sex; transmission risk; documented history of non–injection drug abuse, alcohol abuse, and mental illness; CD4+ count and viral load at eligibility; jurisdiction of residence; type of cohort; and clinic-specific mechanisms undertaken to assist with access to ART. Unadjusted trends (not shown) were similar.
Factors Associated With ART Initiation and Virologic Suppression
Table 2 shows factors associated with ART initiation and virologic suppression. Increasing age was associated with both more timely ART initiation and virologic suppression, among both the full study population (Ptrend < .001) and the subset who initiated ART (Ptrend = .001). Regarding race/ethnicity, those in the “other” category (comprising mostly Native Americans and Canadian aboriginals) were less likely to have timely ART initiation than those of white race (adjusted hazard ratio [HR], 0.84 [95% confidence interval {CI}, .72–.98]), but this association disappeared when examining virologic suppression.
Table 2.
Factors Associated With Antiretroviral Therapy Initiation and Virologic Suppression Among Newly Treatment-Eligible HIV-Infected Individuals (CD4+ Count <350 Cells/µL or AIDS-Defining Illness), 2001–2009
| ART Initiation (at 6 mo of Eligibility) |
Viral Load Suppression (at 1 y of Eligibility)a |
Viral Load Suppression (1 y After ART Initiation)b |
||||
|---|---|---|---|---|---|---|
| No. | Adjusted HR (95% CI) | No. | Adjusted HR (95% CI) | No. | Adjusted HR (95% CI) | |
| Total | 10 692 | 9186 | 5329 | |||
| Age at eligibility | ||||||
| 18–29 | 1848 | Ref. | 1639 | Ref. | 910 | Ref. |
| 30–39 | 3434 | 1.10 (1.02, 1.19) | 2987 | 1.06 (.97, 1.14) | 1728 | 1.08 (.99, 1.19) |
| 40–49 | 3618 | 1.14 (1.06, 1.24) | 3056 | 1.11 (1.02, 1.21) | 1777 | 1.11 (1.01, 1.21) |
| 50–59 | 1460 | 1.23 (1.12, 1.36) | 1236 | 1.28 (1.16, 1.42) | 750 | 1.24 (1.11, 1.38) |
| ≥60 | 332 | 1.13 (.97, 1.33) | 268 | 1.29 (1.10, 1.52) | 164 | 1.12 (.94, 1.34) |
| Race/ethnicity | ||||||
| White | 3505 | Ref. | 3074 | Ref. | 1764 | Ref. |
| Asian/Pacific Islander | 159 | 1.18 (.95, 1.47) | 140 | 1.17 (.94, 1.46) | 74 | 1.24 (.97, 1.58) |
| Black | 4414 | 0.93 (.87, 1.00) | 3720 | 0.95 (.88, 1.02) | 2139 | 0.98 (.90, 1.06) |
| Hispanic (any race) | 1804 | 1.01 (.93, 1.10) | 1529 | 1.04 (.96, 1.14) | 944 | 0.99 (.90, 1.09) |
| Other race/ethnicityc | 810 | 0.84 (.72, 0.98) | 723 | 0.99 (.85, 1.15) | 408 | 1.01 (.85, 1.19) |
| Sex and transmission risk | ||||||
| Men who have sex with men | 4586 | Ref. | 4036 | Ref. | 2411 | Ref. |
| Female injection drug user | 464 | 0.64 (.54, .75) | 365 | 0.73 (.62, .86) | 135 | 0.85 (.69, 1.04) |
| Female, other risk | 2366 | 0.87 (.81, .94) | 2028 | 0.99 (.92, 1.06) | 1139 | 0.99 (.91, 1.08) |
| Male injection drug user | 1151 | 0.81 (.74, .90) | 924 | 0.80 (.73, .89) | 462 | 0.85 (.76, .96) |
| Male, other risk | 2125 | 1.00 (.93, 1.08) | 1833 | 0.94 (.87, 1.02) | 1182 | 0.91 (.84, .99) |
| ART eligibility criteria | ||||||
| CD4+ count 0–199 cells/µL | 3631 | 2.29 (2.16, 2.42) | 3257 | 1.75 (1.64, 1.85) | 2597 | 0.99 (.93, 1.05) |
| CD4+ count 200–349 cells/µL | 6679 | Ref. | 5641 | Ref. | 2623 | Ref. |
| Incident AIDS-defining illness | 382 | 0.66 (.55, .79) | 288 | 0.85 (.71, 1.01) | 109 | 0.93 (.76, 1.15) |
| Viral load at eligibility | ||||||
| Undetectable | 774 | Ref. | N/A | N/A | N/A | N/A |
| 501–9999 copies/mL | 1677 | 0.51 (.45, .58) | 1539 | Ref. | 580 | Ref. |
| 10 000–99 999 copies/mL | 4210 | 0.79 (.71, .88) | 3888 | 1.24 (1.14, 1.35) | 2130 | 0.77 (.70, .85) |
| ≥100 000 copies/mL | 2837 | 1.04 (.94, 1.16) | 2675 | 1.33 (1.21, 1.45) | 2002 | 0.58 (.52, .64) |
| Missing | 1194 | 0.69 (.61, .78) | 1084 | 1.35 (1.21, 1.50) | 617 | 0.65 (.57, .74) |
| No. of psychosocial barriersd | ||||||
| 0 | 7649 | Ref. | 6573 | Ref. | 4042 | Ref. |
| 1 | 2033 | 0.83 (.77, .89) | 1757 | 0.89 (.83, .96) | 916 | 0.99 (.91, 1.07) |
| 2 | 757 | 0.70 (.62, .79) | 642 | 0.84 (.75, .94) | 289 | 1.02 (.90, 1.17) |
| 3 | 253 | 0.58 (.47, .72) | 214 | 0.66 (.54, .82) | 82 | 0.85 (.66, 1.08) |
| Calendar year of eligibility | 1.05 (1.04, 1.06) | 1.07 (1.06, 1.09) | 1.03 (1.02, 1.04) | |||
Cox regression, adjusted for all other factors, plus state or province of residence, type of cohort.
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio; N/A, not applicable.
a Excludes those with undetectable viral load at eligibility and those with no viral load within 1 year.
b Also excludes those who did not initiate ART within 6 months.
c Includes Native Americans, Aboriginal Canadians, and persons of other or unknown race/ethnicity.
d Includes a documented history of alcohol abuse, non–injection drug abuse, and mental illness.
Compared to those with a CD4+ count 200–349 cells/µL, those with a CD4+ count <200 cells/µL were more likely to have timely ART initiation (HR, 2.29 [95% CI, 2.16–2.42]) and virologic control (HR, 1.75 [95% CI, 1.64–1.85]) after eligibility. However, among ART initiators, those with a CD4+ count <200 cells/µL were no more or less likely to become suppressed (HR, 0.99 [95% CI, .93–1.05]). Individuals clinically eligible for treatment solely due to an ADI were less likely to initiate ART (HR, 0.66 [95% CI, .55–.79]) or achieve suppression (HR, 0.85 [95% CI, .71–1.01]) than those with a CD4+ count 200–349 cells/µL, but once initiating ART, they also reached similar suppression (HR, 0.93 [95% CI, .76–1.15]).
A history of IDU was associated with less timely ART initiation and virologic suppression, particularly in female users, but after taking into account ART initiation, the association with virologic suppression was diminished (Table 2). We identified a significant dose–response effect on the basis of an increasing number of psychosocial barriers (including history of non–injection drug use, alcohol abuse, and mental illness) when assessing the hazard of ART initiation (HR decreased from 0.83 for 1 barrier to 0.58 for 3 barriers, Ptrend < .001) and suppression (HR decreased from 0.89 to 0.66, Ptrend < .001). However, limiting the analysis to those who initiated ART mitigated this association (Ptrend = .53).
Consistent with the aforementioned time trends, there was a statistically significant increase in ART initiation and virologic suppression with each successive calendar year: 5% and 7% per year, respectively. We explored interactions between all factors and calendar period (2001–2005, and 2006–2009), but did not observe any substantial differences in effect estimates for these factors except for calendar year itself (ie, no annual change between 2001–2005 for ART initiation [Ptrend = .11], but a significant increase between 2006–2009 [Ptrend < .001]).
Estimation of Treatment Outcome Rates, by Jurisdiction of Residence
Among the 14 states and 3 provinces examined, the cumulative incidence of ART initiation after 6 months ranged from 35% to 94% after adjusting for individual- and cohort-level characteristics (Figure 2A), a 2.7-fold relative difference. We found a statistically significant effect of jurisdiction of residence on ART initiation (P < .001), suggesting heterogeneity exceeding what would be expected by chance.
Figure 2.
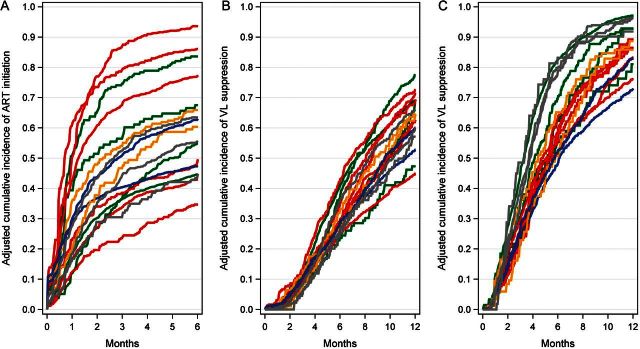
Adjusted cumulative incidence of antiretroviral therapy (ART) initiation and virologic suppression, by jurisdiction, 2001–2009. A, ART initiation, through 6 months after eligibility. B, Virologic suppression, through 1 year after eligibility. C, Virologic suppression among ART initiators, through 1 year after initiation. Estimates were adjusted for age; race/ethnicity; sex; transmission risk; documented history of non–injection drug abuse, alcohol abuse, and mental illness; CD4+ count and viral load at eligibility; calendar year; type of cohort; and clinic-specific mechanisms undertaken to assist with access to ART. Colors used to identify geographic regions: blue = Northeast United States; green = Western United States; gray = Canada; orange = Midwest United States; red = Southern United States. Abbreviations: ART, antiretroviral therapy; VL, viral load.
The adjusted 1-year virologic suppression incidence by jurisdiction ranged from 45% to 78% (Figure 2B), with a similarly significant, though smaller, jurisdiction effect (P < .001). The effect remained statistically significant (P < .001) among those who initiated ART (Figure 2C). Among states and provinces, the cumulative incidence of ART initiation was only weakly correlated with virologic suppression (r = 0.14).
DISCUSSION
In this heterogeneous population of HIV-infected individuals newly eligible to begin ART, we documented between 2001 and 2009 a substantial improvement in timely ART initiation and resulting virologic suppression, with sustained increases since 2006. Several temporal changes occurred during this period, including the use of better tolerated and more convenient formulations, [4] and increasing evidence that starting therapy earlier results in better outcomes [19, 24–26]. Documenting treatment patterns in this large subset of the North American HIV-infected population is important as newer interventions are developed to further improve clinical outcomes, both at the individual level (eg, newer formulations) and at the population level (eg, “test and treat” strategies). As US guidelines now recommend starting ART regardless of CD4+ cell count, [9] it will be even more important to understand temporal trends in ART initiation and resulting clinical outcomes.
We confirmed previously noted barriers to timely initiation of ART, such as younger age and higher CD4+ counts [27, 28]. Less timely ART initiation among those solely eligible due to an incident ADI may have been a consequence of concerns about immune reconstitution inflammatory syndrome, as data supporting the safety of initiating ART in such individuals were not available until recently, [29] or of physicians wanting patients to demonstrate adherence to opportunistic infection treatment before prescribing ART [30].
We also identified potential barriers to ART initiation that, once overcome, may play less of a role in achieving virologic suppression, which is an immediate goal of ART. Persons with a history of IDU were less likely to initiate ART or achieve virologic suppression compared to other risk groups, but these differences were moderated when suppression was considered among injection drug users who initiated ART. The effects of other factors often considered stumbling blocks for patients—such as a history of mental illness, abuse of other drugs and alcohol, and the co-occurrence of these [31]—were also mitigated when examining their impact on achieving virologic suppression. These findings are consistent with some studies that have found that if people have adequate support systems when they initiate treatment, they can greatly improve their chances of virologic success [32–34]. Additional studies that account for duration, severity, and specific diagnoses of mental illness and substance use are warranted, to better understand the nuances of their influences on treatment goals.
After we controlled for individual-level factors, disparities in the timely initiation of therapy by state or province of residence remained, suggesting that system-level factors likely contribute to differential ART access. Disparities by state or province are relevant because there may be policy-related factors that could be modified to reduce these differences. For example, an estimated two-thirds of HIV-infected Americans obtain ART through public programs like Medicaid and the Ryan White Part B ADAP, [35] where state-specific differences in funding and eligibility may play a role in observed disparities [36]. Other recent studies have identified geographic disparities in HIV-related outcomes in the United States [37, 38] and even in Canada, despite universal healthcare [39]. We are conducting further studies in NA-ACCORD to assess how differences in US state-specific ADAP benefits may factor into geographic disparities.
Our analysis has several limitations. First, we cannot determine the extent to which psychosocial barriers delay treatment, either due to a patient's inability to remain engaged in care or a provider being less likely to initiate treatment in patients with disrupted lives [40]. However, our findings suggest that once individuals do initiate ART, many achieve virologic suppression despite these barriers. Furthermore, assessment of psychosocial factors was broad in that we did not distinguish between recent and past history or level of severity, which may influence the timeliness of treatment initiation and positive outcomes in different ways. Other potential psychosocial or structural barriers that we did not have information on include lack of social support, perceived stigma, incarceration, or unstable housing [41–45] In addition, 1 cohort could not supply data on mental illness, and 5 did not supply data on alcohol abuse or non–injection drug abuse. Excluding these cohorts did not change our findings appreciably (data not shown), but more complete measurement of these factors will be valuable in future studies.
Although treatment guidelines aim for successful initiation of ART and virologic suppression, our study cannot distinguish nonuse of ART owing to barriers to treatment vs by choice, and similarly, virologic failure due to nonadherence versus metabolic effects. Nonetheless, our analysis adjusts for many patient- and system-level factors, including mechanisms that clinics have to assist patients in obtaining medications.
Our jurisdiction-level inferences may not be generalizable to all HIV-infected persons living in a particular state or province, because of the variable extent to which patients attending individual clinics represent the underlying HIV population. Therefore, we did not rank states and provinces in terms of treatment outcomes. However, NA-ACCORD is the largest longitudinal cohort of HIV-infected individuals with detailed histories in North America, and its size and diversity play a major role in being able to monitor the larger population [18].
We followed people through 6 months of eligibility to assess “timely” ART initiation. However, other HIV-care quality measures are being developed as performance standards, such as being prescribed potent ART within a 1-year period [46]. Other relevant outcomes that could be examined include longer-term virologic control and retention in care, both of which are important to fully realize the benefits of ART [47, 48].
The importance of timely ART initiation is now well established [9]. Unfortunately many barriers, both at the individual level and systemwide, complicate access to and successful use of treatment for people with HIV infection, even in resource-rich countries. Continued monitoring will be crucial in addressing these disparities, including those related to geography, especially as HIV testing practices change, and, in the United States, as the Affordable Care Act is implemented [49].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Cohorts and representatives of NA-ACCORD. AIDS Link to the IntraVenous Experience: Gregory D. Kirk. Adult AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson, Ronald J. Bosch, and Ann C. Collier. Fenway Health HIV Cohort: Stephen Boswell, Chris Grasso, and Ken Mayer. HAART Observational Medical Evaluation and Research: Robert S. Hogg, Richard Harrigan, Julio Montaner, and Angela Cescon. HIV Outpatient Study: John T. Brooks and Kate Buchacz. HIV Research Network: Kelly A. Gebo. Johns Hopkins HIV Clinical Cohort: Richard D. Moore. John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Benigno Rodriguez. Kaiser Permanente Mid-Atlantic States: Michael A. Horberg. Kaiser Permanente Northern California: Michael A. Horberg and Michael J. Silverberg. Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne. Multicenter Hemophilia Cohort Study–II: James J. Goedert. Multicenter AIDS Cohort Study: Lisa P. Jacobson. Montreal Chest Institute Immunodeficiency Service Cohort: Marina B. Klein. Ontario HIV Treatment Network Cohort Study: Sean B. Rourke, Ann Burchell, and Anita R. Rachlis. Retrovirus Research Center, Puerto Rico: Robert F. Hunter-Mellado and Angel M. Mayor. Southern Alberta Clinic Cohort: M. John Gill. Studies of the Consequences of the Protease Inhibitor Era: Steven G. Deeks and Jeffrey N. Martin. University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and James Willig. University of North Carolina, Chapel Hill, HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik. University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane. Veterans Aging Cohort Study: Amy C. Justice, Robert Dubrow, and David Fiellin. Vanderbilt-Meharry Centers for AIDS Research Cohort: Timothy R. Sterling, David Haas, Sally Bebawy, and Megan Turner. Women's Interagency HIV Study: Stephen J. Gange and Kathryn Anastos. NA-ACCORD Executive Committee: Richard D. Moore, Michael S. Saag, Stephen J. Gange, Mari M. Kitahata, Rosemary G. McKaig, Amy C. Justice, and Aimee M. Freeman. NA-ACCORD Administrative Core: Richard D. Moore, Aimee M. Freeman, Carol Lent and Aaron Platt. NA-ACCORD Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Eric Webster, Liz Morton, and Brenda Simon. NA-ACCORD Epidemiology and Biostatistics Core: Stephen J. Gange, Alison G. Abraham, Bryan Lau, Keri N. Althoff, Jinbing Zhang, Jerry Jing, Elizabeth Golub, Shari Modur, David B. Hanna, Peter Rebeiro, Cherise Wong, and Adell Mendes.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support. This work was supported by the National Institutes of Health (grant numbers F31-DA30254, U01-AI069918, U01-AI31834, U01-AI34989, U01-AI34993, U01-AI34994, U01-AI35004, U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, U01-AI35043, U01-AI37613, U01-AI37984, U01-AI42590, U01-HD32632, UL1-RR024131, UL1-RR024131, M01-RR-00052, M01-RR00071, M01-RR00079, M01-RR00083, M01-RR00722, M01-RR025747, P30-AI27757, P30-AI27767, P30-AI27763, P30-AI50410, P30-AI54999, P30-AI036219, R01-DA04334, R01-DA12568, R01-DA11602, R01-AA16893, R24-AI067039, AHQ290-01-0012, N02-CP55504, K01-AI071725, K01-AI071754, K01-AI093197, K23-AI610320, K24-DA00432, and K24-AI1065298); the Centers for Disease Control and Prevention (contract CDC200-2006-18797); the Canadian Institutes of Health Research (grant numbers TGF-96118, HCP-97105, CBR-86906, CBR-94036, KRS-86251, and 169621); the Canadian HIV Trials Network (project 24); and the government of British Columbia.
Potential conflicts of interest. K. A. G. has served as a consultant to Tibotec and Bristol-Myers Squibb (BMS) and has received research funding from Tibotec. M. A. H. has received research funding from Merck and Pfizer. L. P. J. has served as a consultant to BMS. G. D. K. has served as a consultant to GlaxoSmithKline (GSK) and Merck, and has received payment for educational presentations from GSK. R. D. M. has served as a consultant to Gilead. M. J. S. has received research funding from Merck and Pfizer. T. R. S. has received research funding from Pfizer and BMS and has served on an advisory board for Otsuka. J. H. W. has served as a consultant to BMS, Definicare, and Gilead, and has received research funding from BMS, Definicare, Gilead, Pfizer, and Tibotec. H. M. C. has received payment for educational presentations from Medscape. A. C. C. has received research funding from Schering-Plough and Merck, has served on an advisory board for Merck, and has past stock options from Abbott, BMS, Johnson & Johnson, and Pfizer. J. E. T. has served on an advisory board for Allergan and has served as a consultant to Santen and XOMA. M. J. G. has served on advisory boards for Abbott, ViiV, Gilead, Janssen, and Merck. M. B. K. has served as a consultant to ViiV, has received research funding from Merck, and has received payment for lectures or educational presentations from BMS, Gilead, and ViiV. B. R. has served as a consultant to Gilead. S. B. R. has served as a consultant to and has received payment for lectures and educational presentations from Abbott. S. J. G. has served on an advisory board for Merck.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Nachega JB, Mugavero MJ, Zeier M, Vitoria M, Gallant JE. Treatment simplification in HIV-infected adults as a strategy to prevent toxicity, improve adherence, quality of life and decrease healthcare costs. Patient Prefer Adherence. 2011;5:357–67. doi: 10.2147/PPA.S22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willig JH, Abroms S, Westfall AO, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22:1951–60. doi: 10.1097/QAD.0b013e32830efd79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann B, Milloy MJ, Kerr T, Zhang R, Montaner J, Wood E. Improved adherence to modern antiretroviral therapy among HIV-infected injecting drug users. HIV Med. 2012;13:596–601. doi: 10.1111/j.1468-1293.2012.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyd MA. Improvements in antiretroviral therapy outcomes over calendar time. Curr Opin HIV AIDS. 2009;4:194–9. doi: 10.1097/COH.0b013e328329fc8d. [DOI] [PubMed] [Google Scholar]
- 5.Lillie-Blanton M, Stone VE, Snow Jones A, et al. Association of race, substance abuse, and health insurance coverage with use of highly active antiretroviral therapy among HIV-infected women, 2005. Am J Public Health. 2010;100:1493–9. doi: 10.2105/AJPH.2008.158949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Results of the Expanded HIV Testing Initiative—25 jurisdictions, United States, 2007-2010. MMWR Morb Mortal Wkly Rep. 2011;60:805–10. [PubMed] [Google Scholar]
- 7.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; 2009. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL002111.pdf . Accessed 22 December 2009. [Google Scholar]
- 9.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; 2012. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. Accessed 24 April 2012. [Google Scholar]
- 10.Committee to Review Data Systems for Monitoring HIV Care, Board on Population Health and Public Health Practice. Monitoring HIV care in the United States: indicators and data systems. Washington, DC: National Academies Press; 2012. [PubMed] [Google Scholar]
- 11.Office of National AIDS Policy. National HIV/AIDS strategy for the United States. 2010. Available at: http://www.whitehouse.gov/administration/eop/onap/nhas . Accessed 15 July 2010. [Google Scholar]
- 12.Public Health Agency of Canada. Federal initiative to address HIV/AIDS in Canada. 2004. Available at: http://www.phac-aspc.gc.ca/aids-sida/fi-if/fa-if/pdf/fed_init_e.pdf . Accessed 13 May 2012. [Google Scholar]
- 13.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) Int J Epidemiol. 2007;36:294–301. doi: 10.1093/ije/dyl286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health. Welcome to IeDEA—International Epidemiologic Databases to Evaluate AIDS. Available at: http://www.iedea.org/ . Accessed 24 April 2012. [Google Scholar]
- 15.North American AIDS Cohort Collaboration on Research and Design. Welcome to NA-ACCORD. Available at: http://statepiaps.jhsph.edu/naaccord/ . Accessed 27 November 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Panel on Clinical Practices for Treatment of HIV Infection. Department of Health and Human Services; 2001. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL02052001009.pdf. Accessed 15 August 2011. [Google Scholar]
- 17.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 18.Althoff KN, Buchacz K, Hall IH, et al. U.S. trends in antiretroviral therapy use, HIV RNA plasma viral loads, and CD4 T-lymphocyte cell counts among HIV-infected persons, 2000 to 2008. Ann Intern Med. 2012;157:325–35. doi: 10.7326/0003-4819-157-5-201209040-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–26. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relucio K, Holodniy M. HIV-1 RNA and viral load. Clin Lab Med. 2002;22:593–610. doi: 10.1016/s0272-2712(02)00008-2. [DOI] [PubMed] [Google Scholar]
- 21.Cobb BR, Vaks JE, Do T, Vilchez RA. Evolution in the sensitivity of quantitative HIV-1 viral load tests. J Clin Virol. 2011;52(suppl 1):S77–82. doi: 10.1016/j.jcv.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 22.Lau B, Gange SJ, Moore RD. Interval and clinical cohort studies: epidemiological issues. AIDS Res Hum Retroviruses. 2007;23:769–76. doi: 10.1089/aid.2006.0171. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JA, May M, et al. When To Start Consortium. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373:1352–63. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Writing Committee for the CASCADE Collaboration. Timing of HAART initiation and clinical outcomes in human immunodeficiency virus type 1 seroconverters. Arch Intern Med. 2011;171:1560–9. doi: 10.1001/archinternmed.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cain LE, Logan R, et al. HIV-CAUSAL Collaboration. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154:509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fleishman JA, Yehia BR, Moore RD, Gebo KA, Agwu AL. Disparities in receipt of antiretroviral therapy among HIV-infected adults (2002-2008) Med Care. 2012;50:419–27. doi: 10.1097/MLR.0b013e31824e3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadland SE, Milloy MJ, Kerr T, et al. Young age predicts poor antiretroviral adherence and viral load suppression among injection drug users. AIDS Patient Care STDS. 2012;26:274–80. doi: 10.1089/apc.2011.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4:e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58(RR-4):1–207. [PubMed] [Google Scholar]
- 31.Chander G, Himelhoch S, Fleishman JA, et al. HAART receipt and viral suppression among HIV-infected patients with co-occurring mental illness and illicit drug use. AIDS Care. 2009;21:655–63. doi: 10.1080/09540120802459762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spire B, Lucas GM, Carrieri MP. Adherence to HIV treatment among IDUs and the role of opioid substitution treatment (OST) Int J Drug Policy. 2007;18:262–70. doi: 10.1016/j.drugpo.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Tsai AC, Weiser SD, Petersen ML, Ragland K, Kushel MB, Bangsberg DR. A marginal structural model to estimate the causal effect of antidepressant medication treatment on viral suppression among homeless and marginally housed persons with HIV. Arch Gen Psychiatry. 2010;67:1282–90. doi: 10.1001/archgenpsychiatry.2010.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Himelhoch S, Brown CH, Walkup J, et al. HIV patients with psychiatric disorders are less likely to discontinue HAART. AIDS. 2009;23:1735–42. doi: 10.1097/QAD.0b013e32832b428f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blair JM, McNaghten AD, Frazier EL, Skarbinski J, Huang P, Heffelfinger JD. Clinical and behavioral characteristics of adults receiving medical care for HIV infection—Medical Monitoring Project, United States, 2007. MMWR Surveill Summ. 2011;60:1–20. [PubMed] [Google Scholar]
- 36.Mansergh G, Valdiserri RO, Yakovchenko V, Koh H. Aligning resources to fight HIV/AIDS in the United States: funding to states through the US Department of Health and Human Services. J Acquir Immune Defic Syndr. 2012;59:516–22. doi: 10.1097/QAI.0b013e318245cc05. [DOI] [PubMed] [Google Scholar]
- 37.Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis. 2011;203:442–51. doi: 10.1093/infdis/jiq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanna DB, Selik RM, Tang T, Gange SJ. Disparities among US states in HIV-related mortality in persons with HIV infection, 2001-2007. AIDS. 2012;26:95–103. doi: 10.1097/QAD.0b013e32834dcf87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raboud JM, Loutfy MR, Su D, et al. Regional differences in rates of HIV-1 viral load monitoring in Canada: insights and implications for antiretroviral care in high income countries. BMC Infect Dis. 2010;10:40. doi: 10.1186/1471-2334-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westergaard RP, Ambrose BK, Mehta SH, Kirk GD. Provider and clinic-level correlates of deferring antiretroviral therapy for people who inject drugs: a survey of North American HIV providers. J Int AIDS Soc. 2012;15:10. doi: 10.1186/1758-2652-15-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Waddell EN, Messeri PA. Social support, disclosure, and use of antiretroviral therapy. AIDS Behav. 2006;10:263–72. doi: 10.1007/s10461-005-9042-x. [DOI] [PubMed] [Google Scholar]
- 42.Sayles JN, Wong MD, Kinsler JJ, Martins D, Cunningham WE. The association of stigma with self-reported access to medical care and antiretroviral therapy adherence in persons living with HIV/AIDS. J Gen Intern Med. 2009;24:1101–8. doi: 10.1007/s11606-009-1068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palepu A, Tyndall MW, Chan K, Wood E, Montaner JS, Hogg RS. Initiating highly active antiretroviral therapy and continuity of HIV care: the impact of incarceration and prison release on adherence and HIV treatment outcomes. Antivir Ther. 2004;9:713–9. [PubMed] [Google Scholar]
- 44.Westergaard RP, Kirk GD, Richesson DR, Galai N, Mehta SH. Incarceration predicts virologic failure for HIV-infected injection drug users receiving antiretroviral therapy. Clin Infect Dis. 2011;53:725–31. doi: 10.1093/cid/cir491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Douaihy AB, Stowell KR, Bui T, Daley D, Salloum I. HIV/AIDS and homelessness, part 2: treatment issues. AIDS Read. 2005;15:604–6. 11-3, 18. [PubMed] [Google Scholar]
- 46.Horberg MA, Aberg JA, Cheever LW, Renner P, O'Brien Kaleba E, Asch SM. Development of national and multiagency HIV care quality measures. Clin Infect Dis. 2010;51:732–8. doi: 10.1086/655893. [DOI] [PubMed] [Google Scholar]
- 47.Mugavero MJ, Amico KR, Westfall AO, et al. Early retention in HIV care and viral load suppression: implications for a test and treat approach to HIV prevention. J Acquir Immune Defic Syndr. 2012;59:86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mugavero MJ, Westfall AO, Zinski A, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61:574–80. doi: 10.1097/QAI.0b013e318273762f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin EG, Schackman BR. What does US health reform mean for HIV clinical care? J Acquir Immune Defic Syndr. 2012;60:72–6. doi: 10.1097/QAI.0b013e31824c0dd4. [DOI] [PMC free article] [PubMed] [Google Scholar]