Human immunodeficiency virus incidence in youth is disproportionately higher than the prevention research and services available to protect this vulnerable population. There are many ethical, legal, and regulatory considerations in ensuring that at-risk youth have unfettered access to safe and effective health-promoting interventions.
Keywords: HIV prevention, adolescents and young adults, preexposure prophylaxis, PrEP
Abstract
An alarming proportion of incident human immunodeficiency virus (HIV) infections worldwide occur in youth. In the United States, 69% of all new infections among youth occurred in young men who have sex with men (YMSM). Recent studies show the promise of preexposure prophylaxis (PrEP) for preventing HIV infection, but research efforts suffer from disproportionately low representation of the youth who are most at risk. Youth-focused research is critical and should include behavioral, community, and biomedical interventions to create a comprehensive HIV prevention package. The many ethical, legal, and regulatory considerations in conducting HIV research among, and in providing care services to, youth must be addressed so that those at high risk and most likely to benefit can have unfettered access to safe and effective health-promoting interventions. YMSM and minority youth are at substantial HIV risk and urgently need effective HIV prevention tools for which the short and long-term benefits and risks have been carefully considered.
(See the Editorial Commentary by Mayer and Zanoni on pages 1156–8.)
An alarming proportion of incident HIV infections worldwide occur in adolescents and young adults. The United Nations Joint Programme on HIV/AIDS (UNAIDS) estimates that 5 million youths are living with HIV worldwide. In 2008, there were approximately 920 000 new infections among youth, or 2500 new infections per day [1]. Although data show that young people now practice safer sex and experience a lower incidence of infection with human immunodeficiency virus (HIV), UNAIDS reports a global resurgence of the epidemic in men who have sex with men (MSM) [2].
In the United States, the epidemic disproportionately impacts racial and ethnic minorities. The HIV incidence rate is nearly 8 times higher in blacks and 3 times higher in Hispanics compared to that in whites [3]. Even more disparate are the epidemic's effects on vulnerable youth and minority MSM populations. Youth aged 13–29 years accounted for 39% of incident HIV infections in 2009 [3]. More than two-thirds (69%) of all new youth infections occurred in young MSM (YMSM) [3]. Among young black MSM (BMSM), new HIV infections increased 48% during 2006–2009 [3].
Typical adolescent risk-taking behaviors do not explain the disproportionate rates of HIV infection among BMSM [4–6]. BMSM are less likely to be aware of their HIV serostatus or to get tested, suggesting that disparities in access to and quality of care may also play a role [4–6].
Recent studies have shown promise for preventing HIV infections. Two studies in Africa, Partners PrEP and TDF2, enrolled HIV-serodiscordant couples and HIV-uninfected, sexually active women and men, respectively [7, 8]. Both studies demonstrated safety and efficacy in reducing HIV risk in both women and men who received preexposure prophylaxis (PrEP) as tenofovir disoproxil fumarate (TDF) or as coformulated TDF and emtricitabine (FTC), known as FTC/TDF and commercially as Truvada [7, 8].
The Iniciativa Profilaxis Pre-Exposición (iPrEx) study found that daily FTC/TDF as PrEP was an effective and well-tolerated intervention to prevent HIV infection in MSM and transgender women [9]. This phase III clinical trial also highlighted the importance of adherence: the protective efficacy was 44% overall but increased to 92% in those with detectable drug levels [9]. There are several limitations of the iPrEx study. The study population was not fully representative of the epidemic in the United States. The majority of the participants were from South America, with only 10% from the United States, making it difficult to determine the effects of the intervention at the US trial sites specifically [9]. The limited number of US minority participants, youth, and particularly minority youth participants in iPrEx did not proportionally reflect the epidemic in the United States (Figure 1A) [3, 9].
Figure 1.
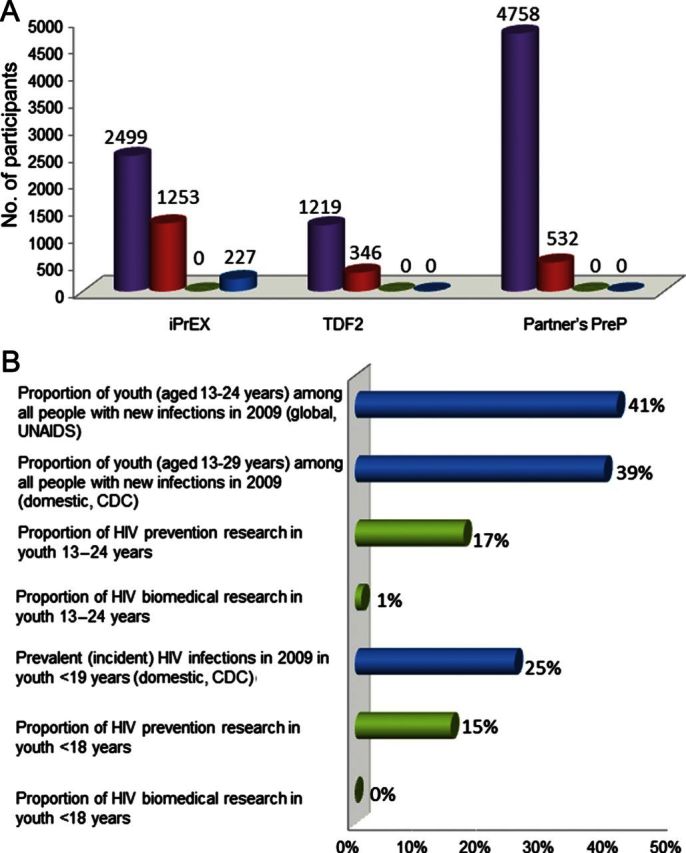
HIV prevention research infrastructure vs needs. A, Participant demographic characteristics in recent human immunodeficiency virus (HIV) preexposure prophylaxis (PrEP) studies. The purple bar shows the total participant sample for each study. The red bar shows the number of participants who were between 18 and 25 years of age. The green bar shows the number of participants who were younger than 18 years. The blue bar shows the number of US participants. In the iPrEX study, participants included men who have sex with men or transgender females who have sex with men; among the 28 US participants younger than 25 years, 5 were black/African American; 9% of the total participants (n = 214) were black, including African Americans, Afro Peruvians, Afro Brazilians, and black South Africans; sites were located in South America, South Africa, Thailand, and the United States. In the TDF2 study, participants included males and females; sites were located in Botswana. In the Partners PrEP study, participants included males and females; sites were located in Uganda and Kenya; total participant sample (purple bar) includes only the susceptible partner. B, HIV infections and HIV prevention research funding for youth. Age ranges for youth differ owing to different definitions of youth used by database sources (ie, Centers for Disease Control and Prevention [CDC], National Institutes of Health [NIH], World Health Organization, and Joint United Nations Programme on HIV/AIDS). The blue bars show the proportion of HIV infections among youth in a given age stratum during the year for which the report was published. The green bars show the proportion of HIV research funding from NIH during the year 2010, for HIV prevention research (includes biomedical) or only HIV biomedical prevention research, among youth in a given age stratum. Based on available data and statistical methods, CDC incident estimates do not allow for a stable HIV incidence estimate of youth younger than 18 years (third bar from bottom); given the age of this group and of expected sexual debut, it is likely that HIV prevalence data are a reasonable approximation of HIV incidence. Abbreviations: CDC, Centers for Disease Control and Prevention; HIV, human immunodeficiency virus.
Despite the promising results of studies of PrEP in several at-risk populations, including serodiscordant couples, HIV-uninfected and sexually active women and men, and MSM [7–9], none of these studies has included adequate representation of youth (Figure 1A).
Relative to their contribution to new HIV infections, youth are underrepresented in prevention research, particularly for biomedical intervention studies. This disparity is especially pronounced for youth under 18 years of age (Figure 1B). The Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN), sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), is addressing this gap through an ambitious domestic PrEP research and implementation agenda for youth at risk for HIV infection.
RESEARCH IN YOUTH POPULATIONS
Behavioral Interventions for HIV Risk Reduction Are Important Components of a Comprehensive Prevention Package for Youth
Combination HIV prevention is a blend of evidence-based behavioral, structural, and biomedical prevention strategies adapted for specific contexts [10, 11] and is recognized as one of the best hopes for reducing and eliminating HIV worldwide [12].
Behavioral HIV prevention interventions are effective in reducing risky behavior and decreasing acquisition of HIV and other sexually transmitted infections (STIs) among high-risk populations [13, 14]. Such interventions address several domains, including mental health, medical adherence, and HIV risk. The Centers for Disease Control and Prevention's (CDC) HIV/AIDS Prevention Research Synthesis project has recommended behavioral interventions that range from intensive one-on-one counseling to group sessions to standard risk-reduction counseling [15]. The identified interventions are ranked by tiers of evidence, creating a framework for classifying HIV behavioral interventions, from those with the most robust evidence to unevaluated interventions [16]. For research on the implementation of HIV biomedical prevention interventions, particularly in vulnerable youth, it is essential to include at least 1 established behavioral intervention to determine the most effective strategy to support participants. Larger trials may have the capacity to compare 2 or more behavioral approaches. Such research will identify psychosocially, developmentally, and culturally relevant behavioral interventions with demonstrated real-world feasibility among high-risk youth.
Community Prevention Infrastructure Is Critical to the Successful Conduct of HIV Prevention Interventions in Youth
There is a growing awareness among researchers that communities in large urban settings with access to minority at-risk youth provide an invaluable opportunity for HIV prevention interventions, such as those at the structural level [17–21]. NICHD has sponsored, through the ATN, a novel and promising community-level approach to studying HIV risk reduction interventions [17, 22]. Forming community coalitions, expanding existing community advisory structures, and educating parents and adolescents at a community level provide an infrastructure to implement HIV prevention interventions and to create positive structural change within communities to lower rates of HIV infection [17, 22]. Engaging communities in HIV prevention research can help scientists prioritize and tailor research and interventions to specific community needs and can increase effectiveness and dissemination of new HIV prevention strategies.
Communities may also pose barriers to prevention implementation, such as mistrust of research, a lack of perceived advantages to participating in clinical trials, and perceived stigma associated with participation [23]. On the other hand, communities also can help remove barriers to less accessible populations and can generate support for research and interventions [17, 23]. They should be educated on the importance, need, and limitations of current HIV prevention, care, and research services, particularly for adolescents and young adults, in advance of implementing interventions [17]. Relevant educational materials should focus on topics and concepts that are key to HIV research (eg, clinical research and ethics) and should be effective in communicating the intended messages readily to the target audience.
Interim CDC Guidance for Preexposure Prophylaxis in MSM
A comprehensive review of PrEP implementation guidelines is beyond the scope of this review. Based on the results of iPrEX study, the CDC released interim guidance on PrEP for the prevention of HIV infection in MSM [24].
This interim guidance does not address age, but the final implementation recommendations are likely to be limited to populations who have attained the age of majority and are to be consistent with the Food and Drug Administration (FDA) labeling of Truvada, approved by the FDA for an HIV prevention indication. In the future, as guidance for PrEP in YMSM and other youth is formulated, agencies should consider addressing relevant issues such as the ethical considerations around PrEP in youth and the unique struggles that YMSM and minority youth experience around identification, testing, linkage to care, and adherence. The prevention research community, including members with youth expertise, must work closely with healthcare implementation programs and regulators to develop guidance for use of PrEP in minors.
ETHICAL CONSIDERATIONS IN RESEARCH WITH YOUTH
Regulations Protect Vulnerable Populations but Can Prevent Their Access to and Participation in Important Prevention and Treatment Research
Human subjects research ethical guidelines and regulations provide special protections for research participants who have not reached the age of majority, a concept that is well accepted [25]. An extension of this concept is the principle that ensuring access of minors to appropriate research is necessary to protect this group from widespread use of therapies that have never been adequately tested in them. It is likely that PrEP will be used widely in minors at high risk of HIV acquisition. Maximizing the safety and effectiveness of this intervention in minors will require ethically sound, scientifically rigorous studies in this group. Extrapolation from studies of PrEP in white, adult MSM may not be appropriate for predicting PrEP effectiveness in YMSM and BMSM. Sociocultural, hormonal, and many other differences require studies specifically in this population.
An average 17-year-old BMSM is clearly different from a middle-aged, white MSM but differs little from an average 19-year-old BMSM; however, under US human subjects research regulations, there is a great distinction between these 2 teenagers. A competent 19-year-old is allowed to consent for participation in research, whereas a 17-year-old routinely requires parental permission to do so [26]. There are 2 exceptions: (1) waiver of parental permission and (2) local laws that allow a minor to consent for certain protected conditions (eg, STI treatment) without parental permission [26]. One drawback of the waiver of parental permission approach is that different IRBs (in a multicenter trial) may reach discordant conclusions about whether requirements for the waiver are met. Another important consideration is that the FDA regulations do not allow waiver of parental permission, and therefore, FDA-monitored studies (Investigational New Drug studies) cannot include parental waiver of permission [27].
A more consistent approach to establishing the right of adolescents to consent to their participation in HIV prevention research should be considered. Efforts would be needed to work with local authorities at each clinical site to establish a more regular determination for criteria for adolescents to consent to participate in research within the parameters of local laws. In jurisdictions where local law precludes the right of adolescents to consent for participation, there is a need for policy level discussions within the local community.
In the US, local jurisdictions determine the criteria under which minors can consent to receive treatment for certain conditions. With the exception of contraception and some STI prevention, the ability of minors to consent for themselves to receive care for the prevention of a condition like HIV infection, whether for research or clinical practice, has been less well established. Implementation guidelines, based on the evidence of efficacy for PrEP among MSM, are not expected to include in their scope a policy focused on prevention for minors—a major group that would stand to benefit from such intervention. To ensure timely and safe access to PrEP for minors, it is essential that policy makers, providers, community members, and other opinion leaders and stakeholders begin addressing this tremendous gap in HIV prevention efforts. Similar engagement will be necessary with international policy makers, taking into consideration their regional regulatory landscape and perhaps an even greater epidemiologic burden of HIV infections among youth.
IMPLICATIONS FOR PrEP IN YOUTH
YMSM and Minority Youth Are at Substantial HIV Risk
The interim CDC guidance recommends that PrEP be considered in populations that are at substantial, ongoing, and high risk of HIV infection [24]. In the United States, the overwhelming majority of new adolescent HIV infections are due to sexual activity [3, 28]. Despite the recommendation for routine HIV testing, most youth, particularly YMSM and minority youth, who report risky behaviors do not get tested and many experience a multitude of social, behavioral, and economic challenges that impede their identification for services and research [29]. Even if at-risk youth can successfully be identified for services or research, adherence to medication may be difficult [30]. PrEP studies have illustrated the impact of adherence on effectiveness and shown that adherence to antiretrovirals (ARVs) for prevention or treatment is suboptimal among many populations, including MSM and youth [31, 32]. Given adolescent risk behaviors and domestic HIV epidemiology, researchers, policy makers, and community leaders should consider defining sexually active adolescents, including minors, as a population at substantial, ongoing, and high risk of HIV infection and should develop HIV prevention efforts that focus on youth, particularly among those most vulnerable racial and ethnic minorities and YMSM.
Toxicity Potential of ARVs in Youth May Have Implications for Feasibility
The potential immediate and longer-term complications of ARVs must be deliberated when considering PrEP intervention. The immediate adverse effects of TDF-FTC, such as nausea, vomiting, and headaches, have been better characterized in adults than in adolescents but are expected to be relatively mild, reversible, and not to lead to discontinuation [7–9]. Renal toxicity, related to the TDF component of TDF-FTC, though uncommon, can be detected through routine monitoring of serum creatinine and urinalysis, and is generally reversible upon discontinuation in adults receiving treatment for HIV infection or PrEP [33]; data are more limited about renal safety of TDF in youth, especially in those youth using TDF as part of PrEP.
An area of special concern for use of TDF-FTC as PrEP in youth is the potential for adverse effects on bone. Bone mineral density (BMD) loss and potential increased fracture risk have been associated with antiretroviral therapy (ART; especially TDF) use in HIV-infected adults in some, but not all, studies [34–36]. More limited data raise concerns that TDF may have greater adverse effects on still-developing bones of HIV-infected children and adolescents [37, 38]. The contributions of HIV infection itself, and other factors, make it difficult to extrapolate findings in HIV-infected populations to potential TDF effects on bones of HIV-uninfected PrEP recipients. Studies show that adult MSM participating in PrEP trials have exhibited a small but statistically significant decline in BMD [39]. Youth may be more vulnerable to greater adverse bone effects of TDF-containing PrEP because BMD normally continues to increase into the third decade of life. To better inform guidance on implementation of PrEP among younger populations, studies should be grounded on an acceptable level of risk vs benefit by taking into careful consideration toxicities of ARVs, and should evaluate appropriate immediate and longer-term safety signals in carefully designed licensure bridging studies that ideally also determine the appropriate indications for initiation and duration of PrEP among youth.
Additional Considerations: Postexposure Prophylaxis vs PrEP
The CDC interim guidance does not address the use of postexposure prophylaxis (PEP) in coordination with a PrEP regimen. In practice, ARVs prescribed as PrEP may be taken by users only after a high-risk exposure (similar to PEP), particularly by youth who may least recognize the difference between these prevention strategies. More data are required to understand the need for and implications of PEP in someone who is already prescribed PrEP. Accordingly, for adolescents who may be participating in PrEP trials, the ethics and scientific implications of adolescents using PrEP ARVs for PEP should also be carefully considered in protocol design. The extent of risk compensation (behavioral disinhibition) that may emerge among youth who will be receiving PrEP remains to be determined in ongoing research by iPrEx investigators. Real-world demonstration projects will likely offer the most reliable estimates of this phenomenon. Researchers need to examine the current PrEP platform and identify areas of concern for participants to best understand how individuals will take these agents in the real world so that only the most promising research strategies are advanced into these clinical trials [40].
Priorities for PrEP Studies in YMSM and Minority Youth
There are several priorities for upcoming PrEP studies in YMSM and minority youth. First, bridging studies that explore tolerability and longer-term safety related to potential side effects and long-term consequences of PrEP should be conducted. Second, demonstration projects in urban centers with large YMSM and minority youth populations will be integral in determining how best to identify and recruit this hard-to-reach group, and how to optimally implement PrEP. This includes feasibility studies that look at the most effective models and places to implement PrEP for youth. Third, studies on supporting adherence in youth, with a focus on the potential for risk compensation, should be prioritized.
Cost-effectiveness Varies by Geographic HIV Incidence Density
Studies of cost-effectiveness of PrEP using mathematical models and various assumptions [41–44] have shown that PrEP is most cost-effective when it is targeted to populations with a higher baseline incidence, where there are more infections to avert, and where ART coverage is low [42–44]. In South Africa, PrEP was the most cost-effective when targeted to 25- to 35-year-old women. The study assumed a baseline incidence of 0.8% per year with a low ART coverage rate [44]. In this particular population, the cost per infection averted using PrEP was estimated to be as low as US$12 000 [44]. Importantly, as ART coverage expands throughout the population, the cost per infection averted with PrEP dramatically increases and its cost-effectiveness as an intervention decreases [44]. Modeling shows that PrEP appears to have a window of opportunity to be most cost-effective during the time when the incidence is high and the ART coverage remains low, making PrEP ideal for low-resource settings [44].
In the United States, a recent cost-effectiveness study of daily PrEP among the MSM population predicted a cost of US$298 000 per year of life gained [42, 43]. This estimate was based on the current treatment cost of Truvada and estimates of PrEP efficacy [42, 43], with a background HIV incidence of 1.6 events per 100 person-years. The estimate also assumes the cost of treating incident HIV to be between US$1139 and $3338 per month, depending on the ARV regimen [43]. The study concludes that the high cost per year of life gained would make PrEP a less cost-effective intervention option for the United States [42, 43]; however, should drug costs diminish or if the targeted population were at higher risk, the intervention would be made more cost-effective [42]. Epidemiological data suggest that MSM, YMSM, and BMSM populations are at an increasing risk for infection [3, 4, 6], making them an appropriate population for targeted PrEP. In this group, the most cost-effective strategy is to initiate PrEP among the highest-risk MSM [41, 42]. Provision of PrEP for all high-risk MSM (average of 5 partners per year) would cost approximately US$50 000 per quality-adjusted life-years (QALYs) gained, compared to US$172 091 per QALY gained if only 20% of all MSM (any risk) receive PrEP and US$216 480 per QALY if all MSM receive PrEP [41]. Using PrEP as an intervention in the populations highest at risk is the most cost-effective and is more appropriate for resource-sufficient settings. Given the expense of PrEP and the high-risk youth populations most likely to benefit, youth will need to rely on access via both public and private insurance programs; however, careful coordination with insurance companies at the local level will be important to ensure that confidentiality is managed in accordance to the patient's wishes [45].
CONCLUSIONS
Although the HIV/AIDS epidemic is stabilizing for many groups, adolescents, YMSM, blacks, and other minority populations continue to be at high risk. PrEP studies, despite their limited generalizability to US minority youth, have shown exciting promise in the prevention of new HIV infections among MSM. PrEP should continue to be explored in combination biomedical and behavioral clinical trials that focus on vulnerable populations. Such trials should account for appropriate psychosocial and developmental factors that affect the levels of adherence necessary for effectiveness. A prevention platform grounded in community education and engagement will best enable research interventions on PrEP for adolescents and young adults. Ethical issues of informed consent for adolescents under the age of 18 and the ability for adolescents to make informed decisions should be explored and a dialogue begun with regulators to address policy-level changes needed to ensure that these most vulnerable youths have access to effective HIV prevention research and care as they emerge. PrEP has important implications for YMSM and minority youth in the United States because of their high risk of HIV acquisition. These implications include the much-needed benefit of preventing new infections, weighed carefully against the potential for harm from issues such as risk of ARV toxicity among healthy individuals, downstream disinhibition of risk behaviors, surreptitious and/or inappropriate use of PrEP, and barriers to proper access of what may likely become a popular HIV prevention method. These considerations and the need for newer, less expensive, safer, and more efficacious PrEP regimens notwithstanding, an ounce of combination prevention is quite definitely worth a pound of cure. The current alternative possibility of acquiring lifelong HIV infection is unacceptable.
Notes
Acknowledgments. We thank Dr Lynne Mofenson for her thoughtful review and valuable guidance in helping produce this manuscript.
Disclaimer. The comments and views of the authors do not necessarily represent the views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Financial support. This work was completed as part of official federal government duties of all authors.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Joint United Nations Programme on HIV/AIDS. UNAIDS annual report 2009: uniting the world against AIDS, 2009. Geneva, Switzerland: UNAIDS; 2009. [Google Scholar]
- 2.Joint United Nations Programme on HIV/AIDS. Global report: UNAIDS report on the global AIDS epidemic, 2010. Geneva, Switzerland: UNAIDS; 2010. [Google Scholar]
- 3.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006-2009. PLoS One. 2011;6:e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flores SA, Bakeman R, Millett GA, Peterson JL. HIV risk among bisexually and homosexually active racially diverse young men. Sex Transm Dis. 2009;36:325–9. doi: 10.1097/OLQ.0b013e3181924201. [DOI] [PubMed] [Google Scholar]
- 5.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS. 2007;21:2083–91. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 6.Millett GA, Peterson JL, Wolitski RJ, Stall R. Greater risk for HIV infection of black men who have sex with men: a critical literature review. Am J Public Health. 2006;96:1007–19. doi: 10.2105/AJPH.2005.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baeten J, Celum C. Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women in Botswana: the Partners PrEP study. Rome, Italy: IAS; 2011. [Google Scholar]
- 8.Thigpen MC, Kebaabetswe PM, Smith DK, et al. Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study. Rome, Italy: IAS; 2011. [Google Scholar]
- 9.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet. 2008;372:669–84. doi: 10.1016/S0140-6736(08)60886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzger DS, Navaline H. HIV prevention among injection drug users: the need for integrated models. J Urban Health. 2003;80(4 suppl 3):iii59–66. doi: 10.1093/jurban/jtg083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall B, Evan W. Toward a comprehensive approach to HIV prevention for people who use drugs. J Acquir Immune Defic Syndr. 2010;55:S23–6. doi: 10.1097/QAI.0b013e3181f9c203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20:143–57. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 14.Semaan S, Kay L, Strouse D, et al. A profile of U.S.-based trials of behavioral and social interventions for HIV risk reduction. J Acquir Immune Defic Syndr. 2002;30(suppl 1):S30–50. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Diffusion of evidence based interventions. Available at: http://www.effectiveinterventions.org/en/interventions.aspx. Accessed 12 December 2012. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Tiers of evidence: a framework for classifying HIV behavioral interventions. Available at: http://www.cdc.gov/hiv/topics/research/prs/tiers-of-evidence.htm. Accessed 12 December 2012. [Google Scholar]
- 17.Ellen JM, Wallace M, Sawe FK, Fisher K. Community engagement and investment in biomedical HIV prevention research for youth: rationale, challenges, and approaches. J Acquir Immune Defic Syndr. 2010;54(suppl 1):S7–11. doi: 10.1097/QAI.0b013e3181e25779. [DOI] [PubMed] [Google Scholar]
- 18.Jaspan HB, Cunningham CK, Tucker TJ, et al. Inclusion of adolescents in preventive HIV vaccine trials: public health policy and research design at a crossroads. J Acquir Immune Defic Syndr. 2008;47:86–92. doi: 10.1097/QAI.0b013e31815d2f27. [DOI] [PubMed] [Google Scholar]
- 19.Kapogiannis BG, Handelsman E, Ruiz MS, Lee S. Introduction: paving the way for biomedical HIV prevention interventions in youth. J Acquir Immune Defic Syndr. 2010;54(suppl 1):S1–4. doi: 10.1097/QAI.0b013e3181e2cf8f. [DOI] [PubMed] [Google Scholar]
- 20.MacQueen KM, Karim QA. Practice brief: adolescents and HIV clinical trials: ethics, culture, and context. J Assoc Nurses AIDS Care. 2007;18:78–82. doi: 10.1016/j.jana.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClure CA, Gray G, Rybczyk GK, Wright PF. Challenges to conducting HIV preventative vaccine trials with adolescents. J Acquir Immune Defic Syndr. 2004;36:726–33. doi: 10.1097/00126334-200406010-00010. [DOI] [PubMed] [Google Scholar]
- 22.Ziff MA, Willard N, Harper GW, Bangi AK, Johnson J, Ellen JM. Connect to protect researcher-community partnerships: assessing change in successful collaboration factors over time. Glob J Community Psychol Pract. 2010;1:32–9. doi: 10.7728/0101201004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiClemente RJ, Ruiz MS, Sales JM. Barriers to adolescents’ participation in HIV biomedical prevention research. J Acquir Immune Defic Syndr. 2010;54(suppl 1):S12–7. doi: 10.1097/QAI.0b013e3181e1e2c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Interim guidance: preexposure prophylaxis for the prevention of HIV infection in men who have sex with men. MMWR Morb Mortal Wkly Rep. 2011;60:65–8. [PubMed] [Google Scholar]
- 25.45 CFR 46. Human subjects protection. Washington, D.C.: Department of Health and Human Services; 2009. [Google Scholar]
- 26.45 CFR 46 Subpart D. Additional protections for children involved as subjects in research. Washington, D.C.: Department of Health and Human Services; 2009. [PubMed] [Google Scholar]
- 27.21 CFR 50. Protection of human subjects. Washington, D.C.: Food and Drug Administration; 2012. [Google Scholar]
- 28.Eaton DK, Kann L, Kinchen S, et al. Youth risk behavior surveillance—United States, 2009. MMWR Surveill Summ. 2010;59:1–142. [PubMed] [Google Scholar]
- 29.Wilcox RD. Identification of HIV-infected youth and linkage to care can be major challenges. HIV Clin. 2012;24:1–2. [PubMed] [Google Scholar]
- 30.Woods JL, Shew ML, Tu W, Ofner S, Ott MA, Fortenberry JD. Patterns of oral contraceptive pill-taking and condom use among adolescent contraceptive pill users. J Adolesc Health. 2006;39:381–7. doi: 10.1016/j.jadohealth.2005.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahana SY, Rohan J, Allison S, Frazier TW, Drotar D. A meta-analysis of adherence to antiretroviral therapy and virologic responses in HIV-infected children, adolescents, and young adults. AIDS Behav. 2012 doi: 10.1007/s10461-012-0159-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Mayer KH. Antiretrovirals and HIV prevention: new insights, challenges, and new directions. Curr Opin HIV AIDS. 2012;7:495–7. doi: 10.1097/COH.0b013e328358e4d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer SM, Eron JJ, Jr., Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society–USA panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 34.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–61. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 35.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–46. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 37.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med. 2010;61:169–85. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 38.Jacobson DL, Lindsey JC, Gordon CM, et al. Total body and spinal bone mineral density across Tanner stage in perinatally HIV-infected and uninfected children and youth in PACTG 1045. AIDS. 2010;24:687–96. doi: 10.1097/QAD.0b013e328336095d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulligan K, Glidden D, Gonzales P, et al. Effects of FTC/TDF on bone mineral density in seronegative men from 4 continents: DEXA results of the global iPrEx study. Conference on Retroviruses and Opportunistic Infections; 27 February–2 March 2011; Boston, MA. [Google Scholar]
- 40.Galea JT, Kinsler JJ, Salazar X, et al. Acceptability of pre-exposure prophylaxis as an HIV prevention strategy: barriers and facilitators to pre-exposure prophylaxis uptake among at-risk Peruvian populations. Int J STD AIDS. 2011;22:256–62. doi: 10.1258/ijsa.2009.009255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juusola JL, Brandeau ML, Owens DK, Bendavid E. The cost-effectiveness of preexposure prophylaxis for HIV prevention in the United States in men who have sex with men. Ann Intern Med. 2012;156:541–50. doi: 10.1059/0003-4819-156-8-201204170-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matthews LT, Baeten JM, Celum C, Bangsberg DR. Periconception pre-exposure prophylaxis to prevent HIV transmission: benefits, risks, and challenges to implementation. AIDS. 2010;24:1975–82. doi: 10.1097/QAD.0b013e32833bedeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paltiel AD, Freedberg KA, Scott CA, et al. HIV preexposure prophylaxis in the United States: impact on lifetime infection risk, clinical outcomes, and cost-effectiveness. Clin Infect Dis. 2009;48:806–15. doi: 10.1086/597095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pretorius C, Stover J, Bollinger L, Bacaer N, Williams B. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One. 2010;5:e13646. doi: 10.1371/journal.pone.0013646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cruz H. Dear colleague letter. Albany, NY: New York Department of Health; 2012. [Google Scholar]


