Candida osteomyelitis most frequently presents subacutely with local symptoms but minimally elevated inflammatory biomarkers, hematogenous dissemination caused by C. albicans involving vertebrae in adults and femur/humerus in children, high relapse rate requiring extended therapy (median 3 months), and often surgical intervention.
Abstract
Background. The epidemiology, pathogenesis, clinical manifestations, management, and outcome of Candida osteomyelitis are not well understood.
Methods. Cases of Candida osteomyelitis from 1970 through 2011 were reviewed. Underlying conditions, microbiology, mechanisms of infection, clinical manifestations, antifungal therapy, and outcome were studied in 207 evaluable cases.
Results. Median age was 30 years (range, ≤ 1 month to 88 years) with a >2:1 male:female ratio. Most patients (90%) were not neutropenic. Localizing pain, tenderness, and/or edema were present in 90% of patients. Mechanisms of bone infection followed a pattern of hematogenous dissemination (67%), direct inoculation (25%), and contiguous infection (9%). Coinciding with hematogenous infection, most patients had ≥2 infected bones. When analyzed by age, the most common distribution of infected sites for adults was vertebra (odds ratio [OR], 0.09; 95% confidence interval [CI], .04–.25), rib, and sternum; for pediatric patients (≤18 years) the pattern was femur (OR, 20.6; 95% CI, 8.4–48.1), humerus, then vertebra/ribs. Non-albicans Candida species caused 35% of cases. Bacteria were recovered concomitantly from 12% of cases, underscoring the need for biopsy and/or culture. Candida septic arthritis occurred concomitantly in 21%. Combined surgery and antifungal therapy were used in 48% of cases. The overall complete response rate of Candida osteomyelitis of 32% reflects the difficulty in treating this infection. Relapsed infection, possibly related to inadequate duration of therapy, occurred among 32% who ultimately achieved complete response.
Conclusions. Candida osteomyelitis is being reported with increasing frequency. Localizing symptoms are usually present. Vertebrae are the most common sites in adults vs femora in children. Timely diagnosis of Candida osteomyelitis with extended courses of 6–12 months of antifungal therapy, and surgical intervention, when indicated, may improve outcome.
Candida osteomyelitis causes significant morbidity if not recognized early or treated effectively [1, 2]. Characterized by a chronic course from onset of symptoms, Candida osteomyelitis may persist for months [3, 4]. As most reports of Candida osteomyelitis are limited to individual case descriptions and relatively small case series, there is no comprehensive analysis that addresses the demographic, clinical, orthopedic, laboratory, diagnostic imaging, and therapeutic aspects of this infection. Moreover, there are numerous questions on Candida osteomyelitis that have not been adequately answered in the current literature.
Whether the frequency of Candida osteomyelitis has increased in parallel with reported cases of candidemia and other forms of invasive candidiasis is unknown. The possible mechanisms of infection that cause Candida osteomyelitis also are not known. Moreover, its osteoarticular distribution, clinical presentation, and microbiology are not well characterized. Potential differences in pediatric and adult populations with Candida osteomyelitis have not to our knowledge been systematically analyzed. To our knowledge there has been no extensive analysis of underlying mechanisms, clinical presentation, diagnostic imaging, medical and surgical treatment, or response to therapy.
We therefore conducted a systematic review of Candida osteomyelitis and analyzed 207 patients who fulfilled prespecified criteria for this infection. Our objective was to describe the demographics, possible mechanisms, clinical manifestations, osteoarticular features, diagnostic imaging, management, and outcome of Candida osteomyelitis, as well as to investigate and discuss potential differences between pediatric and adult populations.
PATIENTS AND METHODS
Patients
Patients included in this study consisted of 2 original cases and 205 cases of Candida osteomyelitis published in the English literature from 1970 through 2011. We initiated our search by reviewing the English references as published in PubMed (http://www.ncbi.nlm.nih.gov/pubmed) using the key words Candida, Torulopsis, candidiasis, osteomyelitis, and arthritis. We then carefully included only well-described references for single case reports or case series. After this initial series of reports was reviewed, individual references listed in each publication were again reviewed for ascertainment of additional case reports.
Criteria for Inclusion of Cases of Candida Osteomyelitis
Cases selected in the initial screen were then included in the final analysis if the following data were available: documentation of Candida osteomyelitis, anatomical location of infection, underlying condition, and therapeutic intervention.
Definitions
Proven osteomyelitis: (1) compatible clinical characteristics; (2) consistent radiographic features; and (3) isolation of Candida in culture and/or histology from samples of bone tissue or metal hardware obtained by open surgery or percutaneous biopsy.
Probable osteomyelitis: (1) evidence of positive culture of Candida and/or histology from other than bone tissue or metal hardware specimens, including disk, cartilage, adjacent abscess, blood, and synovial fluid with compatible clinical and radiological features.
Pediatric patients: patients ≤18 years of age.
Neutropenia: absolute neutrophil count of <500/µL.
Possible mechanisms of development of Candida osteomyelitis were classified as direct inoculation, contiguous spread, and hematogenous dissemination.
Direct inoculation: seeding of bone tissue by external trauma, open wound, ulcer, or surgical manipulation.
Contiguous infection: presence of an infectious Candida process in close proximity to subsequently infected bone.
Hematogenous infection: seeding of bone tissue by bloodstream route in the absence of contiguous or direct inoculation.
De novo Candida osteomyelitis: patients who were not receiving systemic antifungal therapy when the episode of Candida osteomyelitis occurred.
Breakthrough Candida osteomyelitis: patients who were simultaneously receiving systemic antifungal agents before or at onset of Candida osteoarticular infection.
Response to antifungal therapy with or without surgery: complete response, partial response, or failure.
Complete response: complete resolution of clinical and radiological findings of Candida osteomyelitis.
Partial response: incomplete resolution of clinical and/or radiological findings of osteomyelitis, or partial clinical improvement without availability of follow-up radiological data.
Relapse: recurrence of infection after complete or partial response.
Failure: death or lack of complete or partial response despite completion of antifungal therapy.
Data Collection and Analysis
Data regarding epidemiology, clinical and radiological features, demographic characteristics, microbiology, management, and outcome of patients were collected and analyzed with descriptive statistics using Instat GraphPad (GraphPad Software, San Diego, California). Continuous variables were summarized using median and range and categorical variables were summarized using frequencies and percentages. Odds ratio (ORs) and 95% confidence intervals (CIs) for analysis of differences between pediatric and adult cases were determined for prespecified variables.
RESULTS
From 1970 through 2011, a total of 205 published cases [1–133] of Candida osteomyelitis and 2 original cases from Weill Cornell Medical Center of Cornell University (New York Presbyterian Hospital and the Hospital for Special Surgery) fulfilled predefined criteria for evaluability. The number of cases increased approximately 2-fold during the study period (Figure 1).
Figure 1.
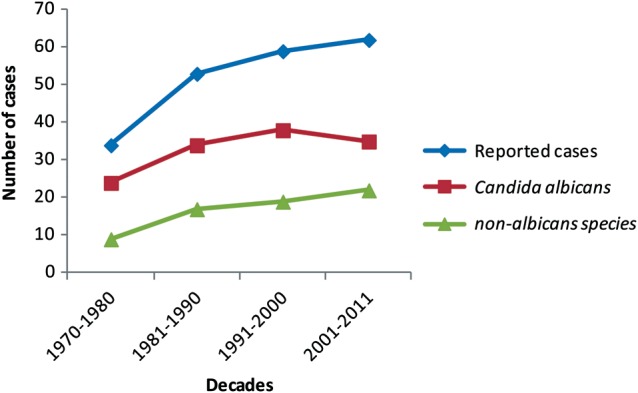
Number of reported cases of Candida osteomyelitis per decade, 1970–2011.
Demographic Characteristics and Underlying Conditions
Among a total of 207 cases of Candida osteomyelitis, median age was 30 years (range, ≤1 month to 88 years) with a predominance of males (Table 1). The majority of patients were not heavily immunosuppressed (ie, underlying hematology malignancy, transplantation, or solid tumor). Only a minority of patients (10%) had trauma or open wounds (Table 1).
Table 1.
Demographic Characteristics in 207 Cases of Candida Osteomyelitis
| Characteristic | No. (%) |
|---|---|
| Median age (neonates–88 years)a | 30 years |
| Adults (≥19 years) | 164 (79) |
| Pediatric population | 37 (18) |
| Neonates (<1 months) | 11 (5) |
| Infants (<12 months) | 15 (7) |
| Children (1 year–18 years) | 11 (5) |
| Sex | |
| Females | 58 (28) |
| Males | 146 (71) |
| Unknown | 3 (1) |
| Underlying conditions | |
| Solid tumors | 19 (9) |
| Hematologic malignancy | 17 (8) |
| Solid organ transplantation | 5 (2) |
| Bone marrow transplantation | 5 (2) |
| Surgery | |
| Facial/neck | 6 (3) |
| Thoracicb | 31 (15) |
| Abdominalc | 47 (23) |
| Orthopedicd | 23 (11) |
| Prior broad-spectrum antibiotics | 115 (56) |
| Prior antifungal agents | 59 (29) |
| Central catheter | 78 (38) |
| Open fracture | 3 (1) |
| Trauma/open wound | 20 (10) |
| Neutropenia | 21 (10) |
| Corticosteroids | 28 (14) |
| Pharmacological immunosuppression other than corticosteroids | 24 (12) |
| Total parenteral nutrition | 39 (19) |
| Intravenous drug use | 29 (14) |
| Intensive care unit | 27 (13) |
| Alcohol abuse | 12 (6) |
| Metal hardware/prosthesis | 11 (5) |
| Human immunodeficiency virus | 7 (3) |
| Hemodialysis | 7 (3) |
| Previous bacterial osteomyelitis | 4 (2) |
| Osteomyelitis as first proven site of candidiasis | 100 (48) |
| Preceding Candida infection | 107 (52) |
| Candidemia | 57 (28) |
| Cutaneous and subcutaneous infection | 61 (29) |
| Central catheter | 35 (17) |
| Endocarditis | 3 (1) |
| Candiduria | 22 (11) |
| Eye | 12 (6) |
| Abdominal cavity | 11 (5) |
| Othere | 22 (11) |
a Six cases (3%) had lack of the age data point.
b Nineteen patients underwent sternotomy.
c Twelve patients had abdominal abscess and 8 had gastrointestinal rupture.
d Six patients underwent laminectomy.
e Oral cavity, lymph nodes, lungs, mediastinum, uterus, and liver.
Candidemia and Osteomyelitis
Candida osteomyelitis was the first proven Candida site involvement in nearly one-half of patients (Table 1). The remaining half of patients initially had candidemia or other form of candidiasis. Thirty-one patients (15%) had concomitant candidemia at the time of diagnosis of Candida osteomyelitis (Table 2). Most cases of Candida osteomyelitis (71%) were diagnosed before initiation of antifungal therapy, and the remainder occurred as breakthrough infection during antifungal therapy.
Table 2.
Diagnostic Approaches and Microbiology of Candida Species Causing Candida Osteomyelitis
| No. (%) | |
|---|---|
| Biopsya | |
| Percutaneous/closed/guided biopsy | 76 (37) |
| Open biopsy/surgery | 70 (34) |
| Both percutaneous/closed/guided and open biopsy/surgery | 13 (6) |
| Microbiology/histopathologyb | |
| Direct culture | 154 (74) |
| Histology | 8 (4) |
| Direct culture and histology | 44 (21) |
| Candida spp | |
| C. albicans | 134 (65) |
| C. tropicalis | 33 (16) |
| C. glabrata | 17 (8) |
| C. parapsilosis | 14 (7) |
| C. krusei | 2 (1) |
| C. guilliermondii | 2 (1) |
| Not specified | 13 (6) |
| Otherc | 5 (2) |
| Candida spp recovered by culture per patient | |
| 1 | 197 (95) |
| ≥1 | 10 (5) |
| Bacteria as recovered in cultures mixed with Candida spp | |
| Staphylococcus aureus | 7 (3) |
| Staphylococcus epidermidis | 3 (1) |
| Enterococcus faecalis | 3 (1) |
| Proteus mirabilis | 2 (1) |
| Diphtheroids | 2 (1) |
| Pseudomonas aeruginosa | 1 (0.5) |
| Escherichia coli | 1 (0.5) |
| Eikenella corrodens | 1 (0.5) |
| Lactobacillus spp | 1 (0.5) |
| Streptococcus agalactiae | 1 (0.5) |
| Klebsiella oxytoca | 1 (0.5) |
| Streptococcus salivarius | 1 (0.5) |
| Staphylococcus capitis | 1 (0.5) |
| Other fungi as recovered in cultures mixed with Candida spp | |
| Aspergillus spp | 4 (2) |
a Diagnostic approaches included fine needle aspiration and swab cultures, at ≤10% each. For 9 cases (4%), none of the foregoing methods was provided, and the diagnosis of Candida osteomyelitis was based on a positive blood culture for Candida species in association with radiologically compatible signs of osteomyelitis.
b In addition to direct cultures of bone and surrounding tissue, positive blood cultures for Candida species were present in 31 patients (15%) at the time of diagnosis of Candida osteomyelitis. For 1 case, neither direct culture nor histology was performed, and the diagnosis was based on a positive histopathological result from a previous episode of Candida osteomyelitis.
c Candida dubliniensis, Candida lusitaniae, Candida ciferri, Candida inconspicua, Candida holmii.
Classification of Candida Osteomyelitis and Mechanisms of Osteoarticular Infection
One hundred thirty-six cases (66%) were proven and 71 (34%) probable Candida osteomyelitis (Table 3). The apparent mechanisms of Candida osteomyelitis consisted of hematogenous dissemination (67%), direct inoculation (24%), and contiguous infection (9%).
Table 3.
Classification, Apparent Mechanisms, and Anatomical Distribution of Candida Osteomyelitis
| No. (%) | |
|---|---|
| Classification of Candida osteomyelitis | |
| Proven | 136 (66) |
| Probable | 71 (34) |
| Apparent mechanisms of infection | |
| Hematogenous | 138 (67) |
| Direct inoculation | 51 (25) |
| Contiguous infection | 18 (9) |
| No. of bones infected per patient | |
| 1 | 34 (16) |
| 2 | 98 (47) |
| ≥3 | 75 (36) |
| Type of bone infected | |
| Vertebraa | 105 (51) |
| Femur | 30 (14) |
| Rib | 27 (13) |
| Sternum | 23 (11) |
| Humerus | 17 (8) |
| Tibia | 16 (8) |
| Fibula | 8 (4) |
| Phalanx | 10 (5) |
| Pelvis | 8 (4) |
| Cranium | 8 (4) |
| Otherb | 29 (14) |
| Concomitant joint involvement | |
| Intervertebral joint | 82 (40) |
| Costochondral/costosternal joint | 22 (11) |
| Synovial joint | 43 (21) |
| Knee | 22 (11) |
| Hip | 10 (5) |
| Ankle | 7 (3) |
| Shoulder | 3 (1) |
| Elbow | 4 (2) |
| Otherc | 17 (8) |
a Cervical (n = 10), thoracic (n = 44), lumbar (n = 62), and sacral (n = 5). In some cases, >1 vertebral anatomic site was concurrently infected.
b Includes metatarsus, ulna, radius, tarsus, talus, metacarpus, calcaneus, malleolus, patella, olecranon, and scapula bones at <5% each.
c Includes sacroiliac, tarsal, metatarsophalangeal, sternoclavicular, carpal, and skull base (synarthrosis) joints at <5% each.
Osteoarticular Distribution
Consistent with a predominantly hematogenous process, the majority of patients had 2 or more sites of infection. In decreasing order of frequency, Candida osteomyelitis involved the vertebrae, femora, ribs, sternum, and humeri (Table 3). Intervertebral discs were infected in 82 (40%) patients, costochondral, costosternal, and costoclavicular joints in 22 (11%), and synovial joints in 43 (21%). The most common synovial joints infected were knee (11%) and hip (5%).
Diagnostic Procedures
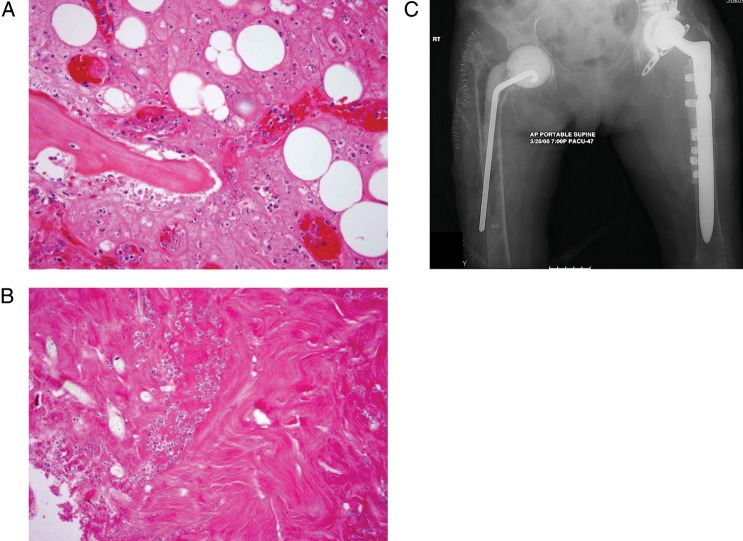
Candida osteomyelitis was diagnosed predominantly via direct culture and less frequently by histopathology with or without culture (Table 2). Among diagnostic approaches used for Candida osteomyelitis, 76 (37%) patients underwent percutaneous/closed/guided biopsy, whereas open biopsy/surgery was performed in 70 (34%) patients. The histopathology of Candida osteomyelitis from one of our original cases is depicted in Figure 2.
Figure 2.
A and B, Representative pathological specimens from a patient with proven Candida species osteomyelitis. All photomicrographs shown are at ×20 magnification under hematoxylin-eosin staining. A, Depicts lamellar bone with scalloped edges and inflammatory infiltration of the marrow space, as well as surrounding bone. B, Depicts fungal forms consistent with Candida species within the necrotic bone. C, Anteroposterior radiograph of the hips from a patient with proven Candida glabrata osteomyelitis and prosthetic joint infection demonstrates markedly demineralized, sclerotic bone with destruction of the femoral head and neck. Attempted placement of a cement spacer resulted in a femoral fracture with protrusion of the rod component through the midshaft.
Clinical Microbiology
Most patients had only 1 Candida species recovered (Table 2). Candida albicans was identified in 65% of cases, while C. tropicalis was recovered in 16%, C. glabrata in 8%, and C. parapsilosis in 7%. Non-albicans Candida species have increased progressively as emerging causes of Candida osteomyelitis throughout the study period (Figure 1). Staphylococcus aureus and other bacteria were identified in mixed cultures among 25 (12%) patients (Table 2).
Clinical Manifestations
Most patients (90%) complained about local pain with confirmatory tenderness, erythema, and edema, but fever was present in only a minority (31%) of patients. The onset of these symptoms was insidious with duration lasting several weeks to months. Limitation of function and movement also was documented in 64 (31%) patients. Sinus tracts and draining pus were observed in 34 (16%) patients (Table 4).
Table 4.
Clinical Manifestations, Radiological Features, and Inflammatory Markers of Candida Osteomyelitis
| Vertebral Osteomyelitis,a n = 105 (51%) | Nonvertebral Osteomyelitis, n = 102 (49%) | Neutropenia, n = 21 (10%) | All Osteomyelitis, n = 207 (100%) | |
|---|---|---|---|---|
| Clinical manifestation | ||||
| Local symptoms (pain, tenderness, erythema, edema) | 98 (93) | 88 (86) | 20 (95) | 186 (90) |
| Fever | 29 (28) | 36 (35) | 11 (52) | 65 (31) |
| Limitation of function/ movement | 40 (38) | 24 (24) | 3 (14) | 64 (31) |
| Draining pus/sinus tract | 1 (1) | 33 (32) | 0 (0) | 34 (16) |
| Fracture proceeding (as a sequela of Candida osteomyelitis) | 3 (3) | 2 (2) | 1 (5) | 5 (2) |
| None | 2 (2) | 3 (3) | 1 (5) | 5 (2) |
| Radiological featuresb | ||||
| Osteolysis/bone destruction/ bone erosion | 69 (66) | 42 (41) | 13 (62) | 111 (54) |
| Extension into soft tissues | 24 (23) | 31 (30) | 0 (0) | 55 (27) |
| Increase of nuclear scan uptake (Tc99m/Ga67) | 29 (28) | 19 (19) | 6 (29) | 48 (23) |
| Decrease of intervertebral space | 44 (42) | … | 4 (19) | 44 (21) |
| Epidural abscess | 24 (23) | … | 2 (10) | 24 (12) |
| Spinal cord compression | 9 (9) | … | 1 (5) | 9 (4) |
| Paraspinal and psoas abscess | 12 (11) | 0 (0) | 2 (10) | 12 (6) |
| Fracture | 11 (10) | 8 (8) | 1 (5) | 19 (9) |
| Periosteal reaction | 3 (3) | 11 (11) | 2 (10) | 14 (7) |
| Decrease of signal intensity on T1-weighted MRI | 12 (11) | 0 (0) | 2 (10) | 12 (6) |
| Increase of signal intensity on T2-weighted MRI | 8 (8) | 4 (4) | 1 (5) | 12 (6) |
| Increase of contrast-enhanced T1-weighted MRI | 9 (9) | 2 (2) | 3 (14) | 11 (5) |
| Bone abscess | 4 (4) | 3 (3) | 0 (0) | 7 (3) |
| Decrease of articular space | 0 (0) | 4 (4) | 0 (0) | 4 (2) |
| Increase of articular space | 1 (1) | 4 (4) | 0 (0) | 5 (2) |
| Sequestrum | 1(1) | 3 (3) | 0 (0) | 4 (2) |
| Involucrum | 0 (0) | 2 (2) | 0 (0) | 2 (1) |
| Inflammatory biomarkersc, median (range) | ||||
| WBC count, /mm3 | 10 100 (2650–36 000) | 14 700 (900–32 700) | Not applicable | 10 900 (900–36 000) |
| PMNs, % | 73 (12–93) | 73 (16–91) | Not applicable | 73 (12–93) |
| ESR, mm/h | 92 (12–150) | 61 (3–120) | Not available | 65 (3–150) |
| CRP, mg/dL | 12 (1.2–46) | 6.5 (2–11.8) | Not applicable | 8.8 (1.2–46) |
| Hgb, g/dL | 10.15 (6.6–13.4) | 9.3 (6.5–17.2) | Not available | 9.5 (6.5–17.2) |
Data are No. (%) unless otherwise specified.
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hgb, hemoglobin; MRI, magnetic resonance imaging; PMN, polymorphonuclear lymphocyte; WBC, white blood cell.
a Vertebral involvement with diskitis vs no diskitis: Of 82 cases of vertebral osteomyelitis with diskitis, 23 (28%) had fever, 76 (93%) had local symptoms, 31 (38%) had limitation of function, 2 (2%) had fracture proceeding, and 2 (2%) had no symptoms. Hgb median value was 10.6 (range, 6.6–13.4), WBC 12 850 (range, 2650–36 000), PMNs (%) 84 (range, 12–93), ESR 119 (range, 23–150), and CRP 10.43 (range, 1.2–17). Of 21 cases of vertebral osteomyelitis without diskitis, fever was present in 6 (29%), local symptoms in 21 (100%), draining pus in 1 (5%), limitation of function in 8 (38%), and fracture proceeding in 1 (5%). Hgb median value was 9.25 (range, 7.3–9.5), WBC 6700 (range, 4900–11 400), ESR 65 (range, 12–129), CRP 46 (range, 29.8–46). For 2 cases of vertebral involvement, data for presence of diskitis were not available.
b Radiological methods included conventional radiography (135), radionuclide scanning (62), computed tomography (57), MRI (48), ultrasound (4), and positron emission tomography (2).
c Eight patients (4%) had normal Hgb, 15 patients (7%) had normal WBC, 9 patients (4%) had normal PMNs, 3 patients (1%) had normal ESR, and 1 patient (0.5%) had normal CRP.
Markers of Inflammation
Markers of inflammation in most patients with Candida osteomyelitis were only moderately to minimally elevated. White blood cell (WBC) counts were mildly elevated (10 900; range, 900–36 000 cells/mm3), with 73% neutrophils (range, 12%–93%). Median values of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and hemoglobin were 65 (range, 3–150 mm/hour), 8.8 (range, 1.2–46 mg/dL), and 9.5 (range, 6.5–17.2 g/dL), respectively (Table 4).
Diagnostic Imaging
The most common radiological abnormalities were bone destruction, extension into soft tissues, increase of radionuclide scan uptake, decrease of intervertebral space, and epidural abscess (Table 4). Decreased signal intensity on T1-weighted images, as well as increased signal intensity on T2-weighted images, was observed on magnetic resonance imaging (MRI). Figure 2 demonstrates radiological changes in one of the original cases.
Effect of Age
Vertebrae were the most commonly infected bone sites in adults (OR, 0.09; 95% CI, .04–.25), whereas femora were most common in pediatrics (OR, 20.6; 95% CI, 8.4–48.1) (Table 5). The most common distribution of infected sites for adults was vertebrae, ribs, and sternum. For pediatric patients (≤18 years), the pattern was femur, humerus, and vertebra/ribs. Irrespective of age, local symptoms were usually present, and overall outcome was similar (OR, 0.98; 95% CI, .47–2.1).
Table 5.
Effect of Age on Effect by Site of Infection, Clinical Manifestations, and Outcome in Candida Osteomyelitis
| Outcome (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Populationa (No.) | Mechanism (No.) | Bone Site, Localization (No.) | No. of Sites Infected per Patient (No.) | Clinical Manifestation (No.) | Therapeutic Intervention (No.) | CRb | PR | Rel | D |
| All pediatric patientsc (37) | Hematogenous (29) | Vertebra (3)d | Only AFT (25) | 52 | 24 | 36 | 12 | ||
| Contiguous (5) | Femur (21)e | 1 bone involved (15) | Local symptoms (31)f | Only surgery (1) | 0 | 100 | 0 | 0 | |
| Direct inoculation (3) | Rib (3) | 2 bones involved (8) | Limitation of function/movement (13)g | AFT + surgery (10) | 40 | 10 | 50 | 20 | |
| Sternum (2) | ≥3 bones involved (14) | Fever (12) | Polyenes (22) | 41 | 23 | 41 | 18 | ||
| Humerus (17)h | Draining pus (3) | Azoles (6) | 50 | 17 | 50 | 0 | |||
| Others (19) | Combination AFTi (3) | 100 | 0 | 0 | 0 | ||||
| Adults (164)j,k | Hematogenous (103) | Vertebra (95)d | 1 bone involved (54) | Local symptoms (156)f | Only AFT (61) | 26 | 74 | 15 | 8 |
| Direct inoculation (49) | Femur (7)e | 2 bones involved (88) | Limitation of function/movement (52)g | Only surgery (9) | 56 | 22 | 22 | 22 | |
| Contiguous (12) | Rib (28) | ≥3 bones involved (22) | Fever (53) | AFT + surgery (90) | 29 | 61 | 46 | 7 | |
| Sternum (21) | Draining pus (31) | Polyenes (53) | 25 | 70 | 32 | 15 | |||
| Humerus (0)h | Azoles (44) | 23 | 68 | 32 | 2 | ||||
| Combination AFTi (48) | 33 | 58 | 35 | 4 | |||||
Abbreviations: AFT, antifungal therapy; CI, confidence interval; CR, complete response; D, death; OR, odds ratio; PR, partial response; Rel, relapsed.
a Six cases are not included in this analysis for lack of the age data point.
b Complete response (OR, 0.98; 95% CI, .47–2.0).
c Pediatric patients are defined as age ≤18 years, which also corresponds to the onset of closure of the epiphysis.
d Vertebra (OR, 0.09; 95% CI, .04–.25).
e Femur (OR, 20.6; 95% CI, 8.4–48.1).
f Local symptoms (OR, 0.26; 95% CI, .12–.79).
g Limitations of function or movement (OR, 0.98; 95% CI, .48–2.0).
h Humerus (OR, 46.3; 95% CI, 12.9–169).
i Combination AFT = polyenes + azoles.
j Four adult cases lacked sufficient outcome data.
k Four adult patients received no therapy, and detailed medical treatment data were not available in 2 adult cases.
Treatment and Outcome
Ninety-two (44%) patients were treated with antifungal agents only, 10 (5%) underwent surgical treatment only, and 100 (48%) were treated with both antifungal therapy and surgery (Table 6). Debridement was the most common surgical procedure (44%) followed by drainage, bone grafting, stabilization, decompression, and intervertebral fusion. Median duration of therapy was 90 days (range, 7–720 days). There was no apparent benefit on outcome of any particular antifungal agent.
Table 6.
Treatment and Outcome of Candida Osteomyelitis
| Therapeutic Interventiona | No. (%) | Favorable Response (Complete Response + Partial Response), No. (%) | Failure, No. (%) | Relapse, No. (%)b |
|---|---|---|---|---|
| Only antifungal agents | 92 (44) | 89 (97) | 3 (3) | 16 (17) |
| Only surgeryc | 10 (5) | 8 (80) | 1 (10) | 2 (20) |
| Antifungal agents and surgery | 100 (48) | 90 (90) | 10 (10) | 43 (43) |
| Class of antifungal agent(s) used; median duration of treatment (range) | ||||
| Polyenesd; 42 days (range, 9–360 days) | 46 (22) | 40 (87) | 6 (13) | 12 (26) |
| Azolesd; 330 days (range, 42–480 days) | 42 (20) | 39 (93) | 3 (7) | 12 (29) |
| Flucytosined; 42 days (range, 33–97 days) | 8 (4) | 8 (100) | 0 (0) | 2 (25) |
| Echinocandin; 7 days (range, 7 days) | 1 (0.5) | 1 (100) | 0 (0) | 1 (100) |
| Combinatione; 200 days (range, 19–540 days) | 94 (45) | 90 (96) | 4 (4) | 32 (34) |
| Surgical intervention | ||||
| Debridementf | 92 (44) | 81 (88) | 10 (11) | 40 (44) |
| Femoral debridementg | 9 (10) | 5 (60) | 3 (30) | 4 (50) |
| Vertebral debridement | 42 (46) | 42 (100) | 0 (0) | 17 (40) |
| Femoral+vertebral debridement | 1 (1) | 0 (0) | 1 (100) | 0 (0) |
| Drainage | 28 (14) | 27 (96) | 1 (4) | 12 (43) |
| Bone grafting | 26 (13) | 25 (96) | 1 (4) | 13 (50) |
| Stabilization | 19 (9) | 18 (95) | 1 (5) | 10 (53) |
| Decompression | 18 (9) | 17 (94) | 1 (6) | 11 (61) |
| Intervertebral body fusion | 12 (6) | 12 (100) | 0 (0) | 4 (33) |
| Fixation | 9 (4) | 9 (100) | 0 (0) | 6 (67) |
| Insertion of metal hardware/prosthesis | 6 (3) | 6 (100) | 0 (0) | 4 (67) |
| Removal of metal hardware/prosthesis | 6 (3) | 4 (67) | 2 (33) | 2 (33) |
| Amputation | 6 (3) | 2 (33) | 4 (67) | 3 (50) |
| Irrigation | 6 (3) | 6 (100) | 0 (0) | 2 (33) |
| Total outcome | ||||
| Median duration of therapy, 90 days (range, 7–720 days) | ||||
| Complete response, 90 days (range, 19–540 days) | 66 (32; 32% relapsed) | |||
| Partial response, 90 days (range, 7–720 days) | 123 (59; 27% relapsed) | |||
| Failure, 42 days (range, 9–480 days) | 15 (7) | |||
| Lost to follow-up | 3 (1) |
a Three patients received no therapy, and detailed medical treatment data were not available in 2 cases.
b Defined as recurrence of infection after complete or partial response.
c Outcome data were not available for 1 patient who underwent surgical intervention. Among 9 patients who had only debridement of bone, 5 (56%) had complete response, 2 (22%) had partial response, 2 (22%) relapsed, and 2 (22%) died (1 of unrelated cause [gastrointestinal hemorrhage] and 1 of Candida-related cause). Of the 83 patients who had debridement plus antifungal therapy, 26 (31%) had complete response, 48 (58%) had partial response, 38 (46%) relapsed, and 6 (7%) died (3 of Candida-related causes and 3 of unrelated causes [heroin use, methicillin-resistant Staphylococcus aureus sepsis, and renal failure]). Patients may be placed in more than 1 category.
d Among 46 patients receiving polyenes, 16 (35%) discontinued therapy because of adverse effects (10 [62.5%] due to renal failure, and 6 [6.25%] due to hepatic failure, hepatic and renal failure, allergy, anemia, jaundice, and hypokalemia [n = 1 each]). Among 42 patients receiving azoles, 2 (5%) discontinued treatment: 1 (50%) due to cholestasis, and 1 (50%) due to vomiting. Among 8 patients receiving flucytosine, 3 (37.5%) ceased therapy (2 [67%] due to myelosuppression, and 1 [33%] due to severe diarrhea).
e Among the 94 patients undergoing combination antifungal therapy, the following classes were used: polyenes-azoles (39), polyenes-flucytosine (35), polyenes-azoles-flucytosine (10), azoles-flucytosine (6), azoles-echinocandins (2), polyenes-azoles-echinocandins (1), and polyenes-azoles-flucytosine-echinocandins (1).
f Non-femoral and non-vertebral debridement included sternum, tibia, fibula, talus, rib, tarsus, calcaneus, phalanges, pelvis, metatarsus, malleolus, hallux, clavicle, and cranium.
g Outcome data were not available for 1 patient who underwent femoral debridement.
Complete response of Candida osteomyelitis was documented in 66 patients (32%), partial response in 123 (59%), and failure in 15 (7%) (Table 6). During their courses of antifungal therapy, relapses occurred among 32% and 27% of patients who ultimately achieved complete response and partial response, respectively. Premature discontinuation of therapy was the most common cause for these relapses.
Among the 10 patients who received only surgical therapy, 2 of these patients suffered postoperative relapse and 2 died (gastrointestinal bleeding and Candida-related infection).
Effect of Hardware
Among the 11 patients (5%) who had hardware in place, 7 were hardware-infected, and 2 had cobacterial infection (1 patient with S. aureus as soft tissue infection, and 1 with Escherichia coli bacteremia). Of these 11 patients, 3 (27%) achieved complete response and 7 achieved (64%) partial response; 5 (45%) relapsed, and 1 (9%) died (Candida-related).
Effect of Bacterial Infections
Thirty patients (14%) had concurrent bacterial infection. Among these 30 cases, 10 (33%) had complete response, 16 (53%) had partial response, 11 (37%) relapsed, and 3 (10%) died (1 of bacterial sepsis, and 2 with candidiasis plus aspergillosis).
DISCUSSION
This analysis of 207 cases identifies important features of Candida osteomyelitis that have not been previously observed or well established in smaller studies. There is a strong male predominance with >2:1 male:female ratio. Most patients present with localizing symptoms of insidious onset and subacute to chronic course with only moderate or minimal response of biomarkers of inflammation. The mechanism of infection of bones follows a pattern of hematogenous dissemination, direct inoculation, and contiguous infection. Coinciding with hematogenous dissemination as being the most common mechanism of infection, most patients have ≥2 bones infected, thus warranting a search for other sites once a single focus is identified. When analyzed by age, the most common distribution of infected sites for adults is vertebrae, rib, and sternum; for pediatric patients the pattern is femur, humerus, and vertebra/ribs. Underscoring the need for biopsy and culture, non-albicans Candida species were found to be an increasingly frequent cause of Candida osteomyelitis with bacteria, including S. aureus, as additional copathogens. The overall complete response rate of Candida osteomyelitis of 32% is relatively low and consistent with the difficulty in treating this infection. The study also documents the importance of relapse despite treatment, possibly as the result of inadequate duration of therapy.
This study found that establishing a diagnosis of Candida osteomyelitis may be difficult. Among those patients with a preceding episode of candidemia, there was usually a delay in diagnosis of 1 week to several months. While intact polymorphonuclear neutrophils occupy a critical role in innate host defenses against invasive forms of Candida [134, 135], our review demonstrated that Candida osteomyelitis develops predominantly in patients who were not neutropenic or otherwise immunocompromised. A high index of suspicion for this infection should be maintained for all candidemic patients with subsequent localizing osteoarticular symptoms. Similarly, patients with subsequent localizing osteoarticular symptoms following surgery should be further evaluated for Candida osteomyelitis.
Neonates and infants with risk factors for candidemia, including umbilical vein catheterization, very low birthweight, and necrotizing enterocolitis, who also display localizing osteoarticular symptoms should be evaluated for concomitant Candida osteomyelitis. Users of illicit intravenous drugs also develop a distinctive syndrome consisting of a febrile illness of disseminated cutaneous, follicular, nodular, ocular and osteoarticular lesions, with C. albicans being the predominant isolate. Costal cartilage, costochondral joints, knees, and sacroiliac joint are usually involved [103].
Our findings that markers of systemic inflammatory response (WBC count, ESR, and CRP) may be minimally elevated or even normal suggest that reliance on history and physical findings remain key factors for early diagnosis. As our study demonstrates that most patients with hematogenous Candida osteomyelitis have 2 or more infected bones, a search for multiple osseous sites is important. The insidious onset and multifocal nature of Candida osteomyelitis mimicked metastatic cancer in adults and chronic multifocal bacterial osteomyelitis in children.
Age-related differences in children and adults with Candida osteomyelitis have not been previously described with sufficient statistical power. Vertebrae are >7 times likely to be infected in adults than in children, while femur and humerus are 14 and 46 times more likely, respectively, to be infected in children. The lumbar vertebrae are more frequently infected than other sites in adults. These age-related patterns of localization parallel those of bacterial osteomyelitis [136]. As previously proposed for Brucella osteomyelitis, lumbar degenerative joint disease in older males may also predispose to development of Candida osteomyelitis [137].
We also found that the femoral metaphysis is typically infected in the pediatric population with associated septic arthritis as opposed to epiphysis in adults. Infection begins in the cartilaginous portion of the distal part of long bones resulting in destruction and subsequently growth inhibition [138, 139]. In neonates and infants, the hemodynamics of metaphysis of longs bones is characterized by a dilated vascular plexus that penetrates the cartilaginous epiphyseal plate, facilitating invasion of Candida into the synovium of the adjacent joint space [140]. The joint capsule in infants surrounds the epiphysis and metaphysis, making the joint more susceptible to spread from adjacent osteomyelitis. Thus, Candida osteomyelitis in neonates was usually multifocal and associated with arthritis.
Differences in vertebral hemodynamics also may explain why this particular bone site was frequently infected by Candida species in the adults of our study. The vascular network that penetrates the endplates of vertebrae in pediatric patients is absent in adults. Bacterial infection begins in the subchondral plate of the vertebral body and classically invades into the disk space [141]. Initially, organisms infect the subchondral areas of vertebral bodies and subsequently invade the intervertebral disk, revealing the classic radiological finding of bone destruction of both endplates with decrease of disk space.
Our study also demonstrates that unlike bacterial osteomyelitis in adults where the intervertebral disk and adjacent 2 vertebral bodies are infected, 20% of our cases of Candida vertebral osteomyelitis did not have disk involvement. Thus, as compared to bacterial osteomyelitis, Candida vertebral osteomyelitis is often characterized by more limited bone destruction.
This study permits clinically practical comparisons between Candida osteomyelitis and bacterial osteomyelitis, especially that caused by S. aureus. Candida tends to display multifocality, sparing of intervertebral disks, and muted markers of inflammation. The multimodality of Candida osteomyelitis suggests that a radionuclide bone scan in addition to MRI should be performed for optimal detection of infected bones.
Our study found that management of Candida osteomyelitis in some cases can be accomplished with prolonged antifungal therapy alone. Surgical intervention, specifically in more complicated cases, was considered to be warranted for successful eradication and structural stability. This was particularly apparent in patients with severe neurological deficits, spinal instability, persistent symptoms, or clinical deterioration despite administration of antifungal agents.
Given the retrospective and literature review nature of this study, definitive conclusions about antifungal outcome are not feasible. Although there may be a bias to report successfully reported cases, there is also a tendency to report cases that are difficult to treat. The strength of this study is the large number of well-defined reviewed cases of a relatively infrequent disease, providing the opportunity to analyze several of its epidemiological, microbiological, and therapeutic features, and thus improving our knowledge on Candida osteomyelitis.
This study underscores the need for more extended courses of antifungal therapy for Candida osteomyelitis than is commonly provided. Antifungal agents were administered for a median duration of 3 months. This practice was associated with relapses that occurred among 32% and 27% of patients who ultimately achieved complete response and partial response, respectively. Premature discontinuation of therapy was the most common cause for these relapses. Based upon expert experience (B-III, level of evidence), the Infectious Diseases Society of America (IDSA) 2009 guidelines for Candida osteomyelitis recommend 1 of 2 primary regimens of antifungal therapy: fluconazole 400 mg (6 mg/kg) daily for 6–12 months or a lipid formulation of amphotericin B, 3–5 mg/kg daily for several weeks followed by fluconazole for 6–12 months [142]. Given the relatively low complete response rate of 32% in this large series, a median duration of antifungal therapy of 3 months may be inadequate. A longer duration of 6–12 months per the IDSA guidelines may be more effective for achieving complete response. Echinocandins may offer a new therapeutic option in treatment of Candida osteomyelitis [143].
Penetration of antifungal agents may help guide choices of antifungal therapy; however, there is a paucity of data from comparative experimental or clinical studies. Fluconazole showed superior penetration into the nucleus pulposus in an uninfected rabbit model when compared with amphotericin B and amphotericin B lipid complex [144]. Lipid formulations of amphotericin B and amphotericin B achieved high concentrations relative to minimum inhibitory concentrations of most Candida species into the bone marrow of noninfected rabbits [145].
Finally, no single center has had sufficient numbers of patients with Candida osteomyelitis from which to draw meaningful conclusions. This study, however, lays the foundation for a prospective multicenter observational study that would monitor all cases of Candida osteomyelitis and a therapeutic clinical trial with uniform response criteria.
In summary, Candida osteomyelitis is a chronic form of invasive candidiasis with localizing symptoms in most cases. The most common symptom is local pain, whereas systemic inflammatory response is usually absent. Reported cases are steadily increasing. Candida osteomyelitis frequently affects nonimmunosuppressed pediatric and adult patients. Candida albicans and Candida tropicalis are the predominant recovered species. Vertebral osteomyelitis is the most common type in adults, whereas the femoral and humeral bones are typically infected in pediatrics. Emergence of Candida osteomyelitis may occur during antifungal treatment. Timely diagnosis of Candida osteomyelitis with extended courses of 6–12 months of antifungal therapy, and surgical intervention, when indicated, may improve outcome.
Notes
Financial support. This work was supported by the Special Account for Research Funds (to M. N. G., N. V. S.) of the National and Kapodistrian University of Athens; National Institutes of Health through an MD Anderson Cancer Center Support Grant (CA016672); Save Our Sick Children Foundation (T. J. W.); Weill Cornell Clinical and Translational Science Center; and Postdoctoral Scientist Award (KL2RR024997 to E. A.).
Potential conflicts of interest. D. P. K. is a consultant and board member and received payment for lectures from Schering-Plough, Pfizer, and Astellas Pharma US; and has received grant support from Astellas Pharma US and Merck; and has received honorarium from Enzon Pharmaceuticals. T. J. W. has received research grant support from Astellas Pharma US and Novartis and has served as consultant to iCo, Draius, Trius, Astellas Pharma US, and Sigma Tau Pharmaceuticals. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Gathe JC, Jr, Harris RL, Garland B, Bradshaw MW, Williams TW., Jr Candida osteomyelitis. Report of five cases and review of the literature. Am J Med. 1987;82:927–37. doi: 10.1016/0002-9343(87)90154-9. [DOI] [PubMed] [Google Scholar]
- 2.Collignon P. Candida osteomyelitis. Am J Med. 1987;83:1173. doi: 10.1016/0002-9343(87)90970-3. [DOI] [PubMed] [Google Scholar]
- 3.Hendrickx L, Van Wijngaerden E, Samson I, Peetermans WE. Candidal vertebral osteomyelitis: report of 6 patients, and a review. Clin Infect Dis. 2001;32:527–33. doi: 10.1086/318714. [DOI] [PubMed] [Google Scholar]
- 4.Bellini C, Antonini P, Ermanni S, Dolina M, Passega E, Bernasconi E. Malignant otitis externa due to Aspergillus niger. Scand J Infect Dis. 2003;35:284–8. doi: 10.1080/00365540310000247. [DOI] [PubMed] [Google Scholar]
- 5.Dailey NJ, Young EJ. Candida glabrata spinal osteomyelitis. Am J Med Sci. 2011;341:78–82. doi: 10.1097/MAJ.0b013e3181f6c6ea. [DOI] [PubMed] [Google Scholar]
- 6.Chia SL, Tan BH, Tan CT, Tan SB. Candida spondylodiscitis and epidural abscess: management with shorter courses of anti-fungal therapy in combination with surgical debridement. J Infect. 2005;51:17–23. doi: 10.1016/j.jinf.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Burton MJ, Shah P, Swiatlo E. Misidentification of Candida parapsilosis as C. famata in a clinical case of vertebral osteomyelitis. Am J Med Sci. 2011;341:71–3. doi: 10.1097/MAJ.0b013e3181f54dab. [DOI] [PubMed] [Google Scholar]
- 8.Matta RF, El Hajje MJ, Safadieh L, et al. Primary sternal osteomyelitis: a report of two cases with literature review. Pediatr Infect Dis J. 2010;29:976–8. doi: 10.1097/inf.0b013e3181e0c928. [DOI] [PubMed] [Google Scholar]
- 9.Wellinghausen N, Moericke A, Bundschuh S, Friedrich W, Schulz AS, Gatz SA. Multifocal osteomyelitis caused by Candida dubliniensis. J Med Microbiol. 2009;58:386–90. doi: 10.1099/jmm.0.003970-0. [DOI] [PubMed] [Google Scholar]
- 10.Metcalfe S, Morgan-Hough C. Cervical epidural abscess and vertebral osteomyelitis following non-traumatic oesophageal rupture: a case report and discussion. Eur Spine J. 2009;18(suppl 2):224–7. doi: 10.1007/s00586-009-0889-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhogal RH, Nayeemuddin M, Akhtar I, Grainger M, Downing R. Continued lumbar spinal erosion after repair of chronic contained rupture of a mycotic abdominal aortic aneurysm. Surg Infect (Larchmt) 2008;9:475–80. doi: 10.1089/sur.2007.054. [DOI] [PubMed] [Google Scholar]
- 12.Ozdemir N, Celik L, Oğuzoğlu S, Yildirim L, Bezircioğlu H. Cervical vertebral osteomyelitis and epidural abscess caused by Candida albicans in a patient with chronic renal failure. Turk Neurosurg. 2008;18:207–10. [PubMed] [Google Scholar]
- 13.Yener S, Comlekci A, Yesil S, Tocpu A, Manisali M. Candida albicans osteomyelitis in a diabetic foot ulcer. J Diabetes Complications. 2009;23:137–9. doi: 10.1016/j.jdiacomp.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Cha JG, Hong HS, Koh YW, Kim HK, Park JM. Candida albicans osteomyelitis of the cervical spine. Skeletal Radiol. 2008;37:347–50. doi: 10.1007/s00256-007-0429-9. [DOI] [PubMed] [Google Scholar]
- 15.Sica G, Meissner S, Dawas K, Maynard N. Candida osteo-chondromyelitis complicating thoraco-abdominal esophageal surgery. Surg Infect (Larchmt) 2007;8:479–82. doi: 10.1089/sur.2006.022. [DOI] [PubMed] [Google Scholar]
- 16.Schilling A, Seibold M, Mansmann V, Gleissner B. Successfully treated Candida krusei infection of the lumbar spine with combined caspofungin/posaconazole therapy. Med Mycol. 2008;46:79–83. doi: 10.1080/13693780701552996. [DOI] [PubMed] [Google Scholar]
- 17.Ghersin E, Lessick J, Agmon Y, Engel A, Kophit A, Adler Z. Candida prosthetic valve endocarditis: the complementary role of multidetector computed tomography and transoesophageal echocardiography in preoperative evaluation. Australas Radiol. 2007;51(suppl):B231–4. doi: 10.1111/j.1440-1673.2007.01780.x. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Ramos FM, Botwin K, Shah CP. Candida spondylodiscitis: an unusual case of thoracolumbar pain with review of imaging findings and description of the clinical condition. Pain Physician. 2004;7:257–60. [PubMed] [Google Scholar]
- 19.Gursel T, Kaya Z, Kocak U, Erbaş G, Akyurek N, Tali ET. Candida vertebra osteomyelitis in a girl with factor X deficiency. Haemophilia. 2005;11:629–32. doi: 10.1111/j.1365-2516.2005.01148.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang SC, Shao PL, Hsueh PR, Lin KH, Huang LM. Successful treatment of Candida tropicalis arthritis, osteomyelitis and costochondritis with caspofungin and fluconazole in a recipient of bone marrow transplantation. Acta Paediatr. 2006;95:629–30. doi: 10.1080/08035250500491629. [DOI] [PubMed] [Google Scholar]
- 21.Tietz HJ, Czaika V, Sterry W. Case report. Osteomyelitis caused by high resistant Candida guilliermondii. Mycoses. 1999;42:577–80. doi: 10.1046/j.1439-0507.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- 22.Khazim RM, Debnath UK, Fares Y. Candida albicans osteomyelitis of the spine: progressive clinical and radiological features and surgical management in three cases. Eur Spine J. 2006;15:1404–10. doi: 10.1007/s00586-005-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaikh Z, Shaikh S, Pujol F, Trauber D, Sam M. Candida tropicalis osteomyelitis: case report and review of literature. Am J Med. 2005;118:795–8. doi: 10.1016/j.amjmed.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 24.Cone LA, Dreisbach L, Dreisbach P, Wuesthoff M. Another patient with Candida vertebral osteomyelitis treated with liposomal amphotericin B. Surg Neurol. 2005;63:592. doi: 10.1016/j.surneu.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Cone LA, Byrd RG, Potts BE, Wuesthoff M. Diagnosis and treatment of Candida vertebral osteomyelitis: clinical experience with a short course therapy of amphotericin B lipid complex. Surg Neurol. 2004;62:234–7. doi: 10.1016/j.surneu.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Arias F, Mata-Essayag S, Landaeta ME, et al. Candida albicans osteomyelitis: case report and literature review. Int J Infect Dis. 2004;8:307–14. doi: 10.1016/j.ijid.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Lerch K, Kalteis T, Schubert T, Lehn N, Grifka J. Prosthetic joint infections with osteomyelitis due to Candida albicans. Mycoses. 2003;46:462–6. doi: 10.1046/j.0933-7407.2003.00928.x. [DOI] [PubMed] [Google Scholar]
- 28.Seravalli L, Van Linthoudt D, Bernet C, et al. Candida glabrata spinal osteomyelitis involving two contiguous lumbar vertebrae: a case report and review of the literature. Diagn Microbiol Infect Dis. 2003;45:137–41. doi: 10.1016/s0732-8893(02)00497-2. [DOI] [PubMed] [Google Scholar]
- 29.Petrikkos G, Skiada A, Sabatakou H, Antoniadou A, Dosios T, Giamarellou H. Case report. Successful treatment of two cases of post-surgical sternal osteomyelitis, due to Candida krusei and Candida albicans, respectively, with high doses of triazoles (fluconazole, itraconazole) Mycoses. 2001;44:422–5. doi: 10.1046/j.1439-0507.2001.00673.x. [DOI] [PubMed] [Google Scholar]
- 30.Boix V, Tovar J, Martín-Hidalgo A. Candida spondylodiscitis. Chronic illness due to heroin analgesia in an HIV positive person. J Rheumatol. 1990;17:563–5. [PubMed] [Google Scholar]
- 31.Parry MF, Grant B, Yukna M, et al. Candida osteomyelitis and diskitis after spinal surgery: an outbreak that implicates artificial nail use. Clin Infect Dis. 2001;32:352–7. doi: 10.1086/318487. [DOI] [PubMed] [Google Scholar]
- 32.Miller DJ, Mejicano GC. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis. 2001;33:523–30. doi: 10.1086/322634. [DOI] [PubMed] [Google Scholar]
- 33.Arranz-Caso JA, Lopez-Pizarro VM, Gomez-Herruz P, García-Altozano J, Martinez-Martinez J. Candida albicans osteomyelitis of the zygomatic bone. A distinctive case with a possible peculiar mechanism of infection and therapeutic failure with fluconazole. Diagn Microbiol Infect Dis. 1996;24:161–4. doi: 10.1016/0732-8893(96)00012-0. [DOI] [PubMed] [Google Scholar]
- 34.Harris MC, Pereira GR, Myers MD, et al. Candidal arthritis in infants previously treated for systemic candidiasis during the newborn period: report of three cases. Pediatr Emerg Care. 2000;16:249–51. doi: 10.1097/00006565-200008000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Hayes WS, Berg RA, Dorfman HD, Freedman MT. Case report 291. Diagnosis: Candida discitis and vertebral osteomyelitis at L1-L2 from hematogenous spread. Skeletal Radiol. 1984;12:284–7. doi: 10.1007/BF00349511. [DOI] [PubMed] [Google Scholar]
- 36.Eisen DP, MacGinley R, Christensson B, Larsson L, Woods ML. Candida tropicalis vertebral osteomyelitis complicating epidural catheterisation with disease paralleled by elevated D-arabinitol/L-arabinitol ratios. Eur J Clin Microbiol Infect Dis. 2000;19:61–3. doi: 10.1007/s100960050013. [DOI] [PubMed] [Google Scholar]
- 37.Jonnalagadda S, Veerabagu MP, Rakela J, Kusne S, Randhawa P, Rabinovitz M. Candida albicans osteomyelitis in a liver transplant recipient: a case report and review of the literature. Transplantation. 1996;62:1182–4. doi: 10.1097/00007890-199610270-00028. [DOI] [PubMed] [Google Scholar]
- 38.Curran MP, Lenke LG. Torulopsis glabrata spinal osteomyelitis involving two contiguous vertebrae. A case report. Spine (Phila Pa 1976) 1996;21:866–70. doi: 10.1097/00007632-199604010-00019. [DOI] [PubMed] [Google Scholar]
- 39.Kaji M, Shoji H, Oizumi K. Intractable meningitis and intracranial abscess following sinusitis due to Candida species. Kurume Med J. 1998;45:279–81. doi: 10.2739/kurumemedj.45.279. [DOI] [PubMed] [Google Scholar]
- 40.Sanz-Rodriguez C, Hernandez-Surmann F, Bueno AG, Goizueta C, Noguerado A. Candida and bacterial mandibular osteomyelitis in an AIDS patient. Eur J Clin Microbiol Infect Dis. 1998;17:531–2. doi: 10.1007/BF01691142. [DOI] [PubMed] [Google Scholar]
- 41.McCullers JA, Flynn PM. Candida tropicalis osteomyelitis: case report and review. Clin Infect Dis. 1998;26:1000–1. doi: 10.1086/517629. [DOI] [PubMed] [Google Scholar]
- 42.Clancy CJ, Nguyen MH, Morris AJ. Candidal mediastinitis: an emerging clinical entity. Clin Infect Dis. 1997;25:608–13. doi: 10.1086/513770. [DOI] [PubMed] [Google Scholar]
- 43.Dorigo B, Cameli AM, Trapani M, Raspanti D, Torri M, Mosconi G. Efficacy of femoral intra-arterial administration of teicoplanin in gram-positive diabetic foot infections. Angiology. 1995;46:1115–22. doi: 10.1177/000331979504601207. [DOI] [PubMed] [Google Scholar]
- 44.Sorrell TC, Dunlop C, Collignon PJ, Harding JA. Exogenous ocular candidiasis associated with intravenous heroin abuse. Br J Ophthalmol. 1984;68:841–5. doi: 10.1136/bjo.68.11.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Connell CJ, Cherry AV, Zoll JG. Letter: osteomyelitis of cervical spine: Candida guilliermondii. Ann Intern Med. 1973;79:748. doi: 10.7326/0003-4819-79-5-748_1. [DOI] [PubMed] [Google Scholar]
- 46.Tang C. Successful treatment of Candida albicans osteomyelitis with fluconazole. J Infect. 1993;26:89–92. doi: 10.1016/0163-4453(93)97064-5. [DOI] [PubMed] [Google Scholar]
- 47.Turner DL, Johnson SA, Rule SA. Successful treatment of candidal osteomyelitis with fluconazole following failure with liposomal amphotericin. B J Infect. 1999;38:51–3. doi: 10.1016/s0163-4453(99)90032-4. [DOI] [PubMed] [Google Scholar]
- 48.Munk PL, Lee MJ, Poon PY, et al. Candida osteomyelitis and disc space infection of the lumbar spine. Skeletal Radiol. 1997;26:42–6. doi: 10.1007/s002560050189. [DOI] [PubMed] [Google Scholar]
- 49.Mullins RF, Still JM, Jr, Savage J, Davis JB, Law EJ. Osteomyelitis of the spine in a burn patient due to Candida albicans. Burns. 1993;19:174–6. doi: 10.1016/0305-4179(93)90045-a. [DOI] [PubMed] [Google Scholar]
- 50.Diament MJ, Weller M, Bernstein R. Candida infection in a premature infant presenting as discitis. Pediatr Radiol. 1982;12:96–8. doi: 10.1007/BF00972443. [DOI] [PubMed] [Google Scholar]
- 51.Neale TJ, Muir JC, Mills H, Horne JG, Jones MR. Candida albicans vertebral osteomyelitis in chronic renal failure. Postgrad Med J. 1987;63:695–8. doi: 10.1136/pgmj.63.742.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirschmann JV, Everett ED. Candida vertebral osteomyelitis. J Bone Joint Surg Am. 1976;58:573–5. [PubMed] [Google Scholar]
- 53.Hennequin C, Bouree P, Hiesse C, Dupont B, Charpentier B. Spondylodiskitis due to Candida albicans: report of two patients who were successfully treated with fluconazole and review of the literature. Clin Infect Dis. 1996;23:176–8. doi: 10.1093/clinids/23.1.176. [DOI] [PubMed] [Google Scholar]
- 54.Kashimoto T, Kitagawa H, Kachi H. Candida tropicalis vertebral osteomyelitis and discitis. A case report and discussion on the diagnosis and treatment. Spine (Phila Pa 1976) 1986;11:57–61. doi: 10.1097/00007632-198601000-00016. [DOI] [PubMed] [Google Scholar]
- 55.Friedman BC, Simon GL. Candida vertebral osteomyelitis: report of three cases and a review of the literature. Diagn Microbiol Infect Dis. 1987;8:31–6. doi: 10.1016/0732-8893(87)90044-7. [DOI] [PubMed] [Google Scholar]
- 56.Armstrong N, Schurr M, Helgerson R, Harms B. Fungal sacral osteomyelitis as the initial presentation of Crohn's disease of the small bowel: report of a case. Dis Colon Rectum. 1998;41:1581–4. doi: 10.1007/BF02237311. [DOI] [PubMed] [Google Scholar]
- 57.Rowe IF, Wright ED, Higgens CS, Burnie JP. Intervertebral infection due to Candida albicans in an intravenous heroin abuser. Ann Rheum Dis. 1988;47:522–5. doi: 10.1136/ard.47.6.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smilack JD, Gentry LO. Candida costochondral osteomyelitis. Report of a case and review of the literature. J Bone Joint Surg Am. 1976;58:888–90. [PubMed] [Google Scholar]
- 59.Ward RM, Sattler FR, Dalton AS., Jr Assessment of antifungal therapy in an 800-gram infant with candidal arthritis and osteomyelitis. Pediatrics. 1983;72:234–8. [PubMed] [Google Scholar]
- 60.Simpson MB, Jr, Merz WG, Kurlinski JP, Solomon MH. Opportunistic mycotic osteomyelitis: bone infections due to Aspergillus and Candida species. Medicine (Baltimore) 1977;56:475–82. [PubMed] [Google Scholar]
- 61.Gustke KA, Wu KK. Torulopsis glabrata osteomyelitis: report of a case. Clin Orthop Relat Res. 1981;154:197–200. [PubMed] [Google Scholar]
- 62.Shaikh BS, Appelbaum PC, Aber RC. Vertebral disc space infection and osteomyelitis due to Candida albicans in a patient with acute myelomonocytic leukemia. Cancer. 1980;45:1025–8. doi: 10.1002/1097-0142(19800301)45:5<1025::aid-cncr2820450532>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 63.Dan M, Priel I. Failure of fluconazole therapy for sternal osteomyelitis due to Candida albicans. Clin Infect Dis. 1994;18:126–7. doi: 10.1093/clinids/18.1.126. [DOI] [PubMed] [Google Scholar]
- 64.Belzunegui J, Gonzalez C, Lopez L, Plazaola I, Maiz O, Figueroa M. Osteoarticular and muscle infectious lesions in patients with the human immunodeficiency virus. Clin Rheumatol. 1997;16:450–3. doi: 10.1007/BF02238936. [DOI] [PubMed] [Google Scholar]
- 65.Imahori SC, Papademetriou T, Ogliela DM. Torulopsis glabrata osteomyelitis. A case report. Clin Orthop Relat Res. 1987;219:214–20. [PubMed] [Google Scholar]
- 66.Sugar AM, Saunders C, Diamond RD. Successful treatment of Candida osteomyelitis with fluconazole. A noncomparative study of two patients. Diagn Microbiol Infect Dis. 1990;13:517–20. doi: 10.1016/0732-8893(90)90084-9. [DOI] [PubMed] [Google Scholar]
- 67.Corso FA, Shaul DB, Wolfe BM. Spinal osteomyelitis after TPN catheter-induced septicemia. J Parenter Enteral Nutr. 1995;19:291–5. doi: 10.1177/0148607195019004291. [DOI] [PubMed] [Google Scholar]
- 68.Ackerman G, Bayley JC. Candida albicans osteomyelitis in a vertebral body previously infected with Serratia marcescens. Spine (Phila Pa 1976) 1990;15:1362–3. doi: 10.1097/00007632-199012000-00024. [DOI] [PubMed] [Google Scholar]
- 69.Chmel H, Grieco MH, Zickel R. Candida osteomyelitis. Report of a case. Am J Med Sci. 1973;266:299–304. doi: 10.1097/00000441-197310000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Freeman JB, Wienke JW, Soper RT. Candida osteomyelitis associated with intravenous alimentation. J Pediatr Surg. 1974;9:783–4. doi: 10.1016/0022-3468(74)90119-5. [DOI] [PubMed] [Google Scholar]
- 71.Svirsky-Fein S, Langer L, Milbauer B, Khermosh O, Rubinstein E. Neonatal osteomyelitis caused by Candida tropicalis. Report of two cases and review of the literature. J Bone Joint Surg Am. 1979;61:455–9. [PubMed] [Google Scholar]
- 72.Weber ML, Abela A, de Repentigny L, Garel L, Lapointe N. Myeloperoxidase deficiency with extensive candidal osteomyelitis of the base of the skull. Pediatrics. 1987;80:876–9. [PubMed] [Google Scholar]
- 73.Owen PG, Willis BK, Benzel EC. Torulopsis glabrata vertebral osteomyelitis. J Spinal Disord. 1992;5:370–3. doi: 10.1097/00002517-199209000-00018. [DOI] [PubMed] [Google Scholar]
- 74.Edwards JE, Turkel SB, Elder HA, Rand RW, Guze LB. Hematogenous Candida osteomyelitis. Report of three cases and review of the literature. Am J Med. 1975;59:89–94. doi: 10.1016/0002-9343(75)90325-3. [DOI] [PubMed] [Google Scholar]
- 75.Ferra C, Doebbeling BN, Hollis RJ, Pfaller MA, Lee CK, Gingrich RD. Candida tropicalis vertebral osteomyelitis: a late sequela of fungemia. Clin Infect Dis. 1994;19:697–703. doi: 10.1093/clinids/19.4.697. [DOI] [PubMed] [Google Scholar]
- 76.Williams RL, Fukui MB, Meltzer CC, Swarnkar A, Johnson DW, Welch W. Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. Am J Neuroradiol. 1999;20:381–5. [PMC free article] [PubMed] [Google Scholar]
- 77.Flanagan PG, Barnes RA. Hazards of inadequate fluconazole dosage to treat deep-seated or systemic Candida albicans infection. J Infect. 1997;35:295–7. doi: 10.1016/s0163-4453(97)93270-9. [DOI] [PubMed] [Google Scholar]
- 78.Eismont FJ, Bohlman HH, Soni PL, Goldberg VM, Freehafer AA. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19–29. [PubMed] [Google Scholar]
- 79.Holzman RS, Bishko F. Osteomyelitis in heroin addicts. Ann Intern Med. 1971;75:693–6. doi: 10.7326/0003-4819-75-5-693. [DOI] [PubMed] [Google Scholar]
- 80.Glower DD, Douglas JM, Jr, Gaynor JW, Jones RN, Oldham HN., Jr Candida mediastinitis after a cardiac operation. Ann Thorac Surg. 1990;49:157–63. doi: 10.1016/0003-4975(90)90382-g. [DOI] [PubMed] [Google Scholar]
- 81.Lertratanakul Y, Glassford GH, Rubinstein HM. Arthritis and osteomyelitis due to Candida albicans: a case report. J Rheumatol. 1977;4:317–20. [PubMed] [Google Scholar]
- 82.Fogarty M. Candidial osteomyelitis: a case report. Aust N Z J Surg. 1983;53:141–3. doi: 10.1111/j.1445-2197.1983.tb02415.x. [DOI] [PubMed] [Google Scholar]
- 83.Lafont A, Olivé A, Gelman M, Roca-Burniols J, Cots R, Carbonell J. Candida albicans spondylodiscitis and vertebral osteomyelitis in patients with intravenous heroin drug addiction. Report of 3 new cases. J Rheumatol. 1994;21:953–6. [PubMed] [Google Scholar]
- 84.Morrow JD, Manian FA. Vertebral osteomyelitis due to Candida glabrata. A case report. J Tenn Med Assoc. 1986;79:409–10. [PubMed] [Google Scholar]
- 85.Wang YC, Lee ST. Candida vertebral osteomyelitis: a case report and review of the literature. Chang Gung Med J. 2001;24:810–5. [PubMed] [Google Scholar]
- 86.Bortel DT. Candida osteomyelitis pubis following a Marshall-Marchetti procedure. Orthopedics. 1993;16:1353–5. doi: 10.3928/0147-7447-19931201-12. [DOI] [PubMed] [Google Scholar]
- 87.Muñoz Fernandez S, Quiralte J, del Arco A, et al. Osteoarticular infection associated with the human immunodeficiency virus. Clin Exp Rheumatol. 1991;9:489–93. [PubMed] [Google Scholar]
- 88.Weisse ME, Person DA, Berkenbaugh JT., Jr Treatment of Candida arthritis with flucytosine and amphotericin B. J Perinatol. 1993;13:402–4. [PubMed] [Google Scholar]
- 89.Thomas FE, Jr, Martin CE, Fisher RD, Alford RH. Candida albicans infection of sternum and costal cartilages: combined operative treatment and drug therapy and 5-fluorocytosine. Ann Thorac Surg. 1977;23:163–6. doi: 10.1016/s0003-4975(10)64093-3. [DOI] [PubMed] [Google Scholar]
- 90.Berant M, Kristal C, Wagner Y. Candida osteomyelitis as a complication of parenteral nutrition in an infant. Successful treatment with flucytosine. Helv Paediatr Acta. 1979;34:155–60. [PubMed] [Google Scholar]
- 91.Gallo WJ, Shapiro DN, Moss M. Suppurative candidiasis: review of the literature and report of case. J Am Dent Assoc. 1976;92:936–9. doi: 10.14219/jada.archive.1976.0104. [DOI] [PubMed] [Google Scholar]
- 92.Nissen TP, Lehman CR, Otsuka NY, Cerruti DM. Fungal osteomyelitis of the distal femoral epiphysis. Orthopedics. 2001;24:1083–4. doi: 10.3928/0147-7447-20011101-23. [DOI] [PubMed] [Google Scholar]
- 93.Bannatyne RM, Clarke HM. Ketoconazole in the treatment of osteomyelitis due to Candida albicans. Can J Surg. 1989;32:201–2. [PubMed] [Google Scholar]
- 94.Adler S, Randall J, Plotkin SA. Candidal osteomyelitis and arthritis in a neonate. Am J Dis Child. 1972;123:595–6. doi: 10.1001/archpedi.1972.02110120119017. [DOI] [PubMed] [Google Scholar]
- 95.Lasday SD, Jay RM. Candida osteomyelitis. J Foot Ankle Surg. 1994;33:173–6. [PubMed] [Google Scholar]
- 96.Kerr J. Fungal osteomyelitis of the temporal bone: a review of reported cases. Ear Nose Throat J. 1994;73:339. [PubMed] [Google Scholar]
- 97.Cooper P, Schofield B, Lennox DW, Ebert-Smith T. Candida albicans osteomyelitis in a patient with avascular necrosis of the hip. Orthopedics. 1991;14:352–5. [PubMed] [Google Scholar]
- 98.Noble HB, Lyne ED. Candida osteomyelitis and arthritis from hyperalimentation therapy. Case report. J Bone Joint Surg Am. 1974;56:825–9. [PubMed] [Google Scholar]
- 99.Hanna E, Hughes G, Eliachar I, Wanamaker J, Tomford W. Fungal osteomyelitis of the temporal bone: a review of reported cases. Ear Nose Throat J. 1993;72:532. 537–41. [PubMed] [Google Scholar]
- 100.Edelstein H, McCabe R. Candida albicans septic arthritis and osteomyelitis of the sternoclavicular joint in a patient with human immunodeficiency virus infection. J Rheumatol. 1991;18:110–1. [PubMed] [Google Scholar]
- 101.Oliverson TJ, Joshi A, Nana A, Lindsey RW. Chronic tibial osteomyelitis caused by Candida parapsilosis. Orthopedics. 2002;25:763–4. doi: 10.3928/0147-7447-20020701-19. [DOI] [PubMed] [Google Scholar]
- 102.Hashimoto Y, Tanioka H. Vertebral osteomyelitis associated with disseminated candidiasis in an oral cancer patient. J Oral Maxillofac Surg. 1991;49:901–3. doi: 10.1016/0278-2391(91)90026-i. [DOI] [PubMed] [Google Scholar]
- 103.Dupont B, Drouhet E. Cutaneous, ocular, and osteoarticular candidiasis in heroin addicts: new clinical and therapeutic aspects in 38 patients. J Infect Dis. 1985;152:577–91. doi: 10.1093/infdis/152.3.577. [DOI] [PubMed] [Google Scholar]
- 104.Bruns J, Hemker T, Dahmen G. Fungal spondylitis. A case of Torulopsis glabrata and Candida tropicalis infection. Acta Orthop Scand. 1986;57:563–5. doi: 10.3109/17453678609014795. [DOI] [PubMed] [Google Scholar]
- 105.Pennisi AK, Davis DO, Wiesel S, Moskovitz P. CT appearance of Candida diskitis. J Comput Assist Tomogr. 1985;9:1050–4. doi: 10.1097/00004728-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 106.Herzog W, Perfect J, Roberts L. Intervertebral diskitis due to Candida tropicalis. South Med J. 1989;82:270–3. doi: 10.1097/00007611-198902000-00029. [DOI] [PubMed] [Google Scholar]
- 107.Liudahl KJ, Limbird TJ. Torulopsis glabrata vertebral osteomyelitis. Case report and review of the literature. Diabet Med Spine (Phila Pa 1976) 1987;12:593–5. [PubMed] [Google Scholar]
- 108.Heald AH, O'Halloran DJ, Richards K, et al. Fungal infection of the diabetic foot: two distinct syndromes. Diabet Med. 2001;18:567–72. doi: 10.1046/j.1464-5491.2001.00523.x. [DOI] [PubMed] [Google Scholar]
- 109.Meberg A, Langslet A, Sovde A, Kolstad A. Candida-septicemia with chorioretinitis, osteomyelitis and arthritis treated with systemic miconazole and intraarticular amphotericin B. Mykosen. 1976;20:257–60. doi: 10.1111/j.1439-0507.1977.tb01549.x. [DOI] [PubMed] [Google Scholar]
- 110.Thurston AJ, Gillespie WJ. Torulopsis glabrata osteomyelitis of the spine: a case report and review of the literature. Aust N Z J Surg. 1981;51:374–6. doi: 10.1111/j.1445-2197.1981.tb04972.x. [DOI] [PubMed] [Google Scholar]
- 111.Kraus WE, Valenstein PN, Corey GR. Purulent pericarditis caused by Candida: report of three cases and identification of high-risk populations as an aid to early diagnosis. Rev Infect Dis. 1988;10:34–41. doi: 10.1093/clinids/10.1.34. [DOI] [PubMed] [Google Scholar]
- 112.Frederickson B, Yuan H, Olans R. Management and outcome of pyogenic vertebral osteomyelitis. Clin Orthop Relat Res. 1978;131:160–7. [PubMed] [Google Scholar]
- 113.Garbino J, Schnyder I, Lew D, Bouchuiguir-Wafa K, Rohner P. An unusual cause of vertebral osteomyelitis: Candida species. Scand J Infect Dis. 2003;35:288–91. doi: 10.1080/00365540310000067. [DOI] [PubMed] [Google Scholar]
- 114.Tchang FK, Gilardi GL. Osteomyelitis due to Torulopsis inconspicua. Report of a case. J Bone Joint Surg Am. 1973;55:1739–43. [PubMed] [Google Scholar]
- 115.Almekinders LC, Greene WB. Vertebral Candida infections. A case report and review of the literature. Clin Orthop Relat Res. 1991;267:174–8. [PubMed] [Google Scholar]
- 116.El-Zaatari MM, Hulten K, Fares Y, et al. Successful treatment of Candida albicans osteomyelitis of the spine with fluconazole and surgical debridement: case report. J Chemother. 2002;14:627–30. doi: 10.1179/joc.2002.14.6.627. [DOI] [PubMed] [Google Scholar]
- 117.Brill PW, Winchester P, Krauss AN, Symchych P. Osteomyelitis in a neonatal intensive care unit. Radiology. 1979;131:83–7. doi: 10.1148/131.1.83. [DOI] [PubMed] [Google Scholar]
- 118.Dijkmans BA, Koolen MI, Mouton RP, Falke TH, van den Broek PJ, van der Meer JW. Hematogenous Candida vertebral osteomyelitis treated with ketoconazole. Infection. 1982;10:290–2. doi: 10.1007/BF01640877. [DOI] [PubMed] [Google Scholar]
- 119.Machi T, Kitagawa S, Hamaoka H, Akasaki T, Miyamoto Y. Postoperative Candida osteomyelitis in femoral fracture: a case report. Kansenshogaku Zasshi. 1994;68:1122–5. doi: 10.11150/kansenshogakuzasshi1970.68.1122. [DOI] [PubMed] [Google Scholar]
- 120.Oleinik EM, Della-Latta P, Rinaldi MG, Saiman L. Candida lusitaniae osteomyelitis in a premature infant. Am J Perinatol. 1993;10:313–5. doi: 10.1055/s-2007-994749. [DOI] [PubMed] [Google Scholar]
- 121.Pohjola-Sintonen S, Ruutu P, Tallroth K. Hematogenous Candida spondylitis. A case report. Acta Med Scand. 1984;215:85–7. [PubMed] [Google Scholar]
- 122.Dwyer K, McDonald M, Fitzpatrick T. Presentation of Candida glabrata spinal osteomyelitis 25 months after documented candidaemia. Aust N Z J Med. 1999;29:571–2. doi: 10.1111/j.1445-5994.1999.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 123.Collignon PJ, Sorrell TC. Disseminated candidiasis: evidence of a distinctive syndrome in heroin abusers. Br Med J (Clin Res Ed) 1983;287:861–2. doi: 10.1136/bmj.287.6396.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aryan HE, Lu DC, Acosta FL, Jr, Ames CP. Corpectomy followed by the placement of instrumentation with titanium cages and recombinant human bone morphogenetic protein-2 for vertebral osteomyelitis. J Neurosurg Spine. 2007;6:23–30. doi: 10.3171/spi.2007.6.1.23. [DOI] [PubMed] [Google Scholar]
- 125.Quindós G, Rowe IF, Higgens CS, Pontón J, Cisterna R, Mackenzie DW. Candidal infection of bone. Assessment of serologic tests in diagnosis and management. Diagn Microbiol Infect Dis. 1990;13:297–302. doi: 10.1016/0732-8893(90)90020-v. [DOI] [PubMed] [Google Scholar]
- 126.Potasman I, Leibovitz Z, Sharf M. Candida sepsis in pregnancy and the postpartum period. Rev Infect Dis. 1991;13:146–9. doi: 10.1093/clinids/13.1.146. [DOI] [PubMed] [Google Scholar]
- 127.Yousefzadeh DK, Jackson JH. Neonatal and infantile candidal arthritis with or without osteomyelitis: a clinical and radiographical review of 21 cases. Skeletal Radiol. 1980;5:77–90. doi: 10.1007/BF00347327. [DOI] [PubMed] [Google Scholar]
- 128.Pittard WB, Thullen JD, Fanaroff AA. Neonatal septic arthritis. J Pediatr. 1976;88:621–4. doi: 10.1016/s0022-3476(76)80022-4. [DOI] [PubMed] [Google Scholar]
- 129.Malani PN, McNeil SA, Bradley SF, Kauffman CA. Candida albicans sternal wound infections: a chronic and recurrent complication of median sternotomy. Clin Infect Dis. 2002;35:1316–20. doi: 10.1086/344192. [DOI] [PubMed] [Google Scholar]
- 130.Prystowsky SD, Vogelstein B, Ettinger DS, et al. Invasive aspergillosis. N Engl J Med. 1976;295:655–8. doi: 10.1056/NEJM197609162951206. [DOI] [PubMed] [Google Scholar]
- 131.Basu S, Kumar A. Osteomyelitis as a manifestation of perinatal human immunodeficiency virus disease. J Infect. 2011;63:163–6. doi: 10.1016/j.jinf.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 132.Estrov Z, Resnitzky P, Shenker Y, Berrebi A, Hurwitz N. Candidemia and sternal Candida albicans osteomyelitis in a patient with chronic lymphatic leukemia. Isr J Med Sci. 1984;20:711–4. [PubMed] [Google Scholar]
- 133.Evdoridou J, Roilides E, Bibashi E, Kremenopoulos G. Multifocal osteoarthritis due to Candida albicans in a neonate: serum level monitoring of liposomal amphotericin B and literature review. Infection. 1997;25:112–6. doi: 10.1007/BF02113589. [DOI] [PubMed] [Google Scholar]
- 134.Edwards JE, Jr, Lehrer RI, Stiehm ER, Fischer TJ, Young LS. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978;89:91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- 135.Roilides E, Holmes A, Blake C, Pizzo PA, Walsh TJ. Effects of granulocyte colony-stimulating factor and interferon-gamma on antifungal activity of human polymorphonuclear neutrophils against pseudohyphae of different medically important Candida species. J Leukoc Biol. 1995;57:651–6. doi: 10.1002/jlb.57.4.651. [DOI] [PubMed] [Google Scholar]
- 136.Kahn DS, Pritzker KP. The pathophysiology of bone infection. Clin Orthop. 1973;96:12–19. [PubMed] [Google Scholar]
- 137.Ioannou S, Karadima D, Pneumaticos S, et al. Efficacy of prolonged antimicrobial chemotherapy for brucellar spondylodiscitis. Clin Microbiol Infect. 2011;17:756–62. doi: 10.1111/j.1469-0691.2010.03272.x. [DOI] [PubMed] [Google Scholar]
- 138.Ho NK, Low YP, See HF. Septic arthritis in the newborn—a 17 years’ clinical experience. Singapore Med J. 1989;30:356–8. [PubMed] [Google Scholar]
- 139.Longjohn DB, Zionts LE, Stott NS. Acute hematogenous osteomyelitis of the epiphysis. Clin Orthop Relat Res. 1995;316:227–34. [PubMed] [Google Scholar]
- 140.Waldvogel FA, Medoff G, Swartz MN. Osteomyelitis: a review of clinical features, therapeutic considerations and unusual aspects. N Engl J Med. 1970;282:198–206. doi: 10.1056/NEJM197001222820406. [DOI] [PubMed] [Google Scholar]
- 141.Sapico FL, Montgomerie JZ. Vertebral osteomyelitis. Infect Dis Clin North Am. 1990;4:539–50. [PubMed] [Google Scholar]
- 142.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503–35. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cornely OA, Lasso M, Betts R, Klimko N, Vazquez J, et al. Caspofungin for the treatment of less common forms of invasive candidiasis. J Antimicrob Chemother. 2007;60:363–9. doi: 10.1093/jac/dkm169. [DOI] [PubMed] [Google Scholar]
- 144.Conaughty JM, Khurana S, Banovac K, Martinez OV, Eismont FJ. Antifungal penetration into normal rabbit nucleus pulposus. Spine. 2004;29:E289–93. doi: 10.1097/01.brs.0000131210.59316.2d. [DOI] [PubMed] [Google Scholar]
- 145.Piscitelli SC, Groll A, Mickiene D, Walsh TJ. Penetration of lipid formulations of amphotericin B into bone marrow and fat tissue in rabbits. Antimicrob Agents Chemother. 2000;44:408–10. doi: 10.1128/aac.44.2.408-410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]