In recent years, tissue engineering has emerged as a new and ambitious approach that aims to restore damaged organs or tissues by delivering functional cells, supporting scaffolds, and biologically active molecules 1. One of the challenges yet to be overcome in this field is to find a suitable biomaterial scaffold capable of creating enriched microenvironments to improve cell survival in vivo. In order to address this issue, we compared 3 different matrices—Matrigel (an extract of basement membrane proteins derived from the Engelbreth-Holm-Swarm (EHS) tumor), Collagen I (a fibrillar extracellular matrix protein which is also the most abundant collagen type), and Puramatrix (a self assembling peptide with sequence AcN-(RADA)4-CNH2) 2. These matrices were used in conjunction with bone marrow-derived mesenchymal stem cells isolated from L2G85 transgenic mice that constitutively express firefly luciferase (Fluc) and enhanced green fluorescent protein (eGFP) (BM-MSCsFluc+/eGFP+) under the constitutive β-actin promoter. Using longitudinal bioluminescence imaging (BLI), we demonstrate here that the combination of Matrigel with BM-MSCsFluc+/eGFP+ has the best long-term survival.
At present, two critical problems limit the field of cell-based tissue engineering: (1) poor survival of transplanted cells and (2) lack of a non-invasive modality to monitor cell fate. In this study, we hypothesize that biomaterial scaffolds can furnish the biomechanical and structural characteristics of the seeded cells until they are able to fully incorporate into their new environment and that BLI can be adapted to image the efficacy of different biomatrices in living subjects. We evaluated three common biomatrices, including Matrigel (BD Biosciences, San Jose, CA), Collagen I (BD Biosciences, San Jose, CA), and Puramatrix (BD Biosciences, San Jose, CA). We focused on BM-MSCsFluc+/eGFP+ because these cells have the potential to differentiate into many clinically relevant cell types such as muscle, liver, brain and epithelial lineages3,4.
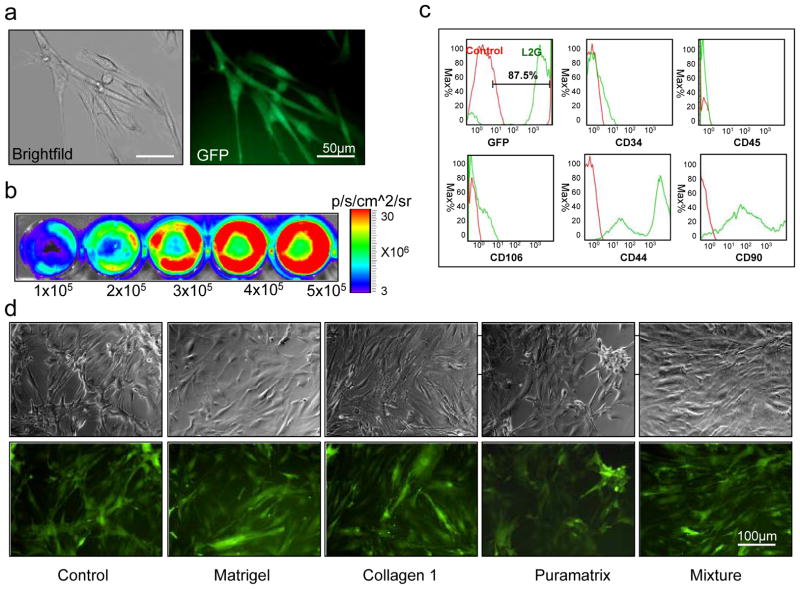
BM-MSCsFluc+/eGFP+ were isolated from adult male L2G85 transgenic FVB mice (n=6) using a modified protocol 5, 6. Fluorescence microscopy showed typical mesenchymal spindle-like morphology of BM-MSCsFluc+/eGFP+ (Figure 1a). Ex vivo BLI of cultured cells demonstrated a robust correlation between cell numbers and Fluc signal activity (r2=0.98), suggesting that BLI could be used to track cell fate in vivo (Figure 1b). Cell characteristics were further analyzed by LSR Flow Cytometry (Becton Dickinson Immunocytometry Systems) and FlowJo analysis software (TreeStar, San Carlos, CA). These cells expressed high levels of MSC-specific markers such as CD44 and CD90, but were negative for CD34 and CD45 markers present on hematopoietic stem cells and white blood cells (Figure 1c). Overall, the patterns of cell surface markers seen in BM-MSCsFluc+/eGFP+ were consistent with those reported in other studies 4, 7. Furthermore, there is no significant changes of BM-MSCGFP+/Fluc+ morphology after culturing in the presence of different biomatrices for 48 hours in same culture condition (Figure 1d).
Figure 1.
Isolation and characterization of bone marrow derived mesenchymal stem cells from L2G85 transgenic mice (MSCsFluc+/eGFP+). (a) Morphology of MSCsFluc+/eGFP+ in brightfield and fluorescence microscopy. (b) MSCsFluc+/eGFP+ show robust correlation of firefly luciferase activities and cell numbers. Bioluminescence imaging was performed on varying numbers of MSCsFluc+/eGFP+ plated on 24-well plates. (c) MSCsFluc+/eGFP+ showed 87±8% GFP positive, 94±11% CD44, 90±5% CD90, 3±0.4% CD34, and 0.1±0.05% CD45. All samples were performed in triplicates. (d) MSCsFluc+/eGFP+ morphology after culturing in the presence of different biomatrices for 48 hours. The same culture condition and same cell number was tested for all group. Scale bars: 50 μm in panel a and 100 μm in panel d.
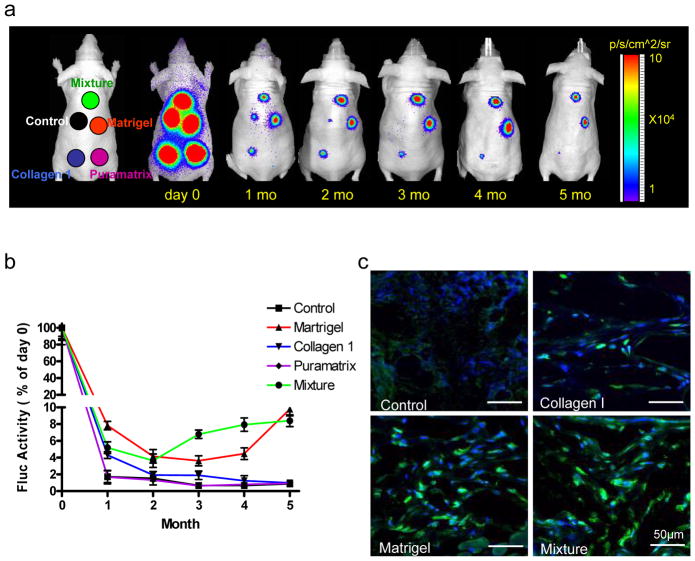
To assess stem cell viability in different biomatrices in vivo, the same numbers (5×105) of MSCsFluc+/eGFP+ were suspended with (i) PBS as a control, (ii) Matrigel alone, (iii) Collagen I alone, (iv) Puramatrix alone, or (v) with an equal mixture of Matrigel, Collagen I, and Puramatrix (20 μl total volume for all groups). Cells and biomatrices were implanted subcutaneously into the backs of adult athymic nude mice (n=10) at 5 different sites. After transplantation, mice were imaged repeatedly using the Xenogen In Vivo Imaging System (IVIS; Xenogen, Alameda, CA). The reporter probe was D-Luciferin (375 mg/kg body weight). BLI was acquired at 1-min intervals and activities expressed as photons per second per centimeter square per steridian (p/s/cm2/sr) as described before 8. BLI signals were normalized to day 0 and expressed as % activity of day 0. Within 2 hours (day 0) after cell transplantation, BLI signals (mean±SD) in all five groups were similar: 5.57±0.58×105 p/sec/cm2/sr in PBS control, 6.90±0.43×105 in Matrigel, 5.35±0.62×105 in Collagen I, 2.76±0.23×105 in Puramatrix, and 4.44±0.41×105 in the mixture group (P=NS) (Figure 2a). However, the BLI signals decreased progressively in all groups subsequently. By one month, the PBS control and Puramatrix groups had significantly lower activities (1.6 to 1.8%) compared to the others (5.1 to 8.2%) (P<0.05) as shown in Figure 2b. Interestingly, after 5 months of follow-up, cell signals in the Matrigel and mixture groups (5 to 9%) maintained significantly higher signal activities compared to the other groups (all <1%) (P<0.01). BLI data were also confirmed by postmortem histology. Immunostaining showed engraftment of MSCsFluc+/eGFP+ in the Matrigel and mixture groups. Fewer MSCsFluc+/eGFP+ were seen in the Collagen I, Puramatrix, and PBS control groups (Figure 2c).
Figure 2.
Bioluminescence imaging of transplanted MSCsFluc+/eGFP+ in living animals. (a) To assess longitudinal cell survival, animals were imaged for 5 months after subcutaneous injection of 5×105 MSCsFluc+/eGFP+ mixed with PBS control, Matrigel, Collagen 1, Puramatrix, and mixture. (b) Quantification of BLI signals showed a drastic decrease of Fluc activities from day 2 to month 1. After that, the BLI signals in the Matrigel and mixture groups remained stable compared with the other three groups. BLI signals were all normalized to day 0 in each group. (c) Postmortem immunohistochemistry staining of eGFP by confocal fluorescence microscopy revealed more robust engraftment of MSCsFluc+/eGFP+ within the Matrigel and mixture groups compared to the PBS control and Collagen 1 groups, consistent with the noninvasive BLI data. Scale bars: 50 μm.
In summary, we have demonstrated that Matrigel-supported stem cell engraftment is superior to cells alone (PBS control) or cells supported with other matrices (Collagen I and Puramatrix). This can be attributed to the special properties of the Matrigel basement membrane matrix, which is known to release various growth factors and to provide structural support for cell seeding and differentiation 9. These data support the notion that loss of transplanted cells occurring within the heterotopic sites is likely due to the lack of appropriate extracellular matrix components needed for the cells to attach and develop in their new environment. We succeeded in assessing the longitudinal survival course and proliferation of engrafted cells by performing high throughput in vivo BLI. This technology is superior to the traditional histology-based assessment of cell grafts, which cannot provide longitudinal information within the same animal 10. To fully realize the clinical potential of tissue engineering, careful in vivo evaluation of the viability of engrafted cells and/or tissues will be needed. Further optimization and validation of both the supporting biomatrices and imaging technology will advance the development of this burgeoning and exciting field.
References
- 1.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006 Mar;7(3):211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 2.Kim MS, Yeon JH, Park JK. A microfluidic platform for 3-dimensional cell culture and cell-based assays. Biomed Microdevices. 2007 Feb;9(1):25–34. doi: 10.1007/s10544-006-9016-4. [DOI] [PubMed] [Google Scholar]
- 3.Tropel P, Noël D, Platet N, et al. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Experimental cell research. 2004;295(2):395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 4.Tropel P, Noel D, Platet N, et al. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004 May 1;295(2):395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Cao YA, Wagers AJ, Beilhack A, et al. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):221–226. doi: 10.1073/pnas.2637010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagers AJ, Sherwood RI, Christensen JL, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002 Sep 27;297(5590):2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002 Jul 4;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 8.Cao F, Lin S, Xie X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006 Feb 21;113(7):1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Philp D, Chen SS, Fitzgerald W, et al. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005 Feb;23(2):288–296. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]
- 10.Contag PR, Olomu IN, Stevenson DK, et al. Bioluminescent indicators in living mammals. Nature Medicine. 1998;4(2):245–247. doi: 10.1038/nm0298-245. [DOI] [PubMed] [Google Scholar]