Although sex differences in coronary heart disease (CHD) have long been recognized, many of the recommendations for the management of female patients continue to be identical to male patients. Given the paucity of sex-specific data in basic science and clinical studies, however, defining unique diagnostic and therapeutic strategies for women remains problematic for scientists and clinicians. For instance, women represent only 38% of subjects in previously NIH-funded cardiovascular studies (1). Previous studies and clinical trials have also included inadequate numbers of women. Finally, only 25% of previous cardiovascular clinical trials have reported sex-specific results (2).
Recently, researchers have been encouraged to report sex differences in basic and clinical studies. Much of the impetus originates from data indicating that more women die of cardiovascular disease (CVD) than men (3). This disparity in mortality may signal the need for sex-specific guidelines for the diagnosis of CHD. In this review, we will discuss sex differences in the clinical manifestations and outcome of CHD, the limitations of current approaches for the management of female patients, and the potential strategies to improve the evaluation of CHD in women.
SEX DIFFERENCES IN THE CLINICAL MANIFESTATIONS AND OUTCOMES OF CORONARY ARTERY DISEASE
CHD may have different clinical manifestations in younger women (<65 years) compared to older women and men. For example, younger women are more likely to report typical angina than older women and men. In a recent meta-analysis of 74 international studies, which included 13,331 women and 11,511 men, the prevalence of typical angina was 11–27% greater for women <65 years than women ≥75 years of age and men (4). Compared to men, younger women were also more likely to present atypically (e.g., rest pain, prolonged chest pain not relieved with rest, diaphoresis, jaw pain, and fatigue in absence of chest pain) (5).
Although younger women are more likely to have angina, they are less likely to have obstructive disease on coronary angiography. In a detailed analysis of women with suspected ischemic CHD enrolled in the Women’s Ischemic Syndrome Evaluation (WISE), >50% had non-obstructive coronary artery disease (<50% stenosis), while the remaining had minimal to no detectable disease (6). Non-obstructive coronary artery disease (CAD) is also more frequently found in younger women presenting with acute coronary syndrome (ACS). In a recent analysis of national registry data in >450,000 women (average age of 64±13 years), those presenting with ACS had a 50% lower likelihood of having obstructive disease than age-matched men (7). Similarly, women presenting with ST elevation myocardial infarction have higher rates of non-obstructive disease than men, 10–25% compared to 6–10% (8).
Historically, the prognosis for non-obstructive disease was considered benign (9–11). Recent data form the WISE study, however, suggest that women with non-obstructive disease and atypical chest pain have a two-fold greater risk of non-fatal myocardial infarction than asymptomatic women (12). Those who have more typical angina and ischemia have an even higher mortality (13). A recent study reported that the 5-year CVD event rates were 16%, 7.9%, and 2.4% in women with <50% stenosis, women without stenosis, and those without symptoms, respectively (14). In addition, >50% of symptomatic women without obstructive disease continue to have signs and symptoms of ischemia and undergo repeat diagnostic procedures and hospitalizations (15, 16). Comparative prognostic data in men with non-obstructive CHD are currently not available.
LIMITATIONS OF CURRENT APPROACHES FOR THE MANAGEMENT OF WOMEN
It remains unclear why women continue to have higher overall mortality than men despite less obstructive disease (Figure 1) (3). The reduction in mortality from CHD for women has also lagged behind that for men, and has even increased in younger women over the last several years (17). One proposed explanation attributes the higher mortality to advanced age and a higher rate of co-morbidities, because CHD presents 10 years later in women than men (18). However, this does not explain why most of the mortality difference is observed in younger women (17). For example, in a study of >300,000 patients from the National Registry of Myocardial Infarction-2, the adjusted mortality rate was twice as high among women <50 years of age than men (19). In the Thrombolysis In Myocardial Infarction-II trial, women had significantly greater rates of death and re-infarction at 6 weeks and 1 year, even after adjustment for age and co-morbidities (20, 21).
Figure 1.
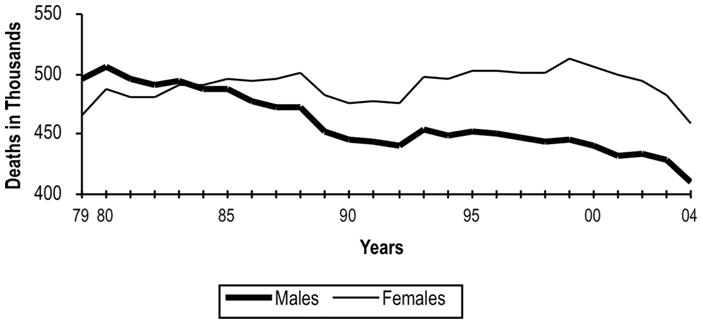
Death from cardiovascular disease in the United States from 1979 to 2005 in women and men (3). Overall mortality from cardiovascular remains higher in women than men. Reduction in mortality has previously lagged behind men but has shown similar declines since 2000.
Another possible explanation is that women may receive fewer diagnostic tests, experience more treatment delays, and are given less aggressive therapy than men. Previous studies have shown that women with suspected obstructive CHD underwent fewer stress tests and diagnostic angiograms than men (22–25). Women often experience treatment delays and receive less aggressive therapy (26, 27). A previous registry study showed that women were less likely to have an electrocardiogram performed within 10 minutes of presentation (25.2% for women vs. 29.3% for men) and were less commonly cared for by a cardiologist during their inpatient hospitalization (53.4% for women vs. 63.4% for men) (26). Women also received less acute medical treatment after myocardial infarction than men, including less heparin (80% vs. 84%) and glycoprotein IIb/IIIa inhibitors (28.7% vs. 38.6%) (26). At discharge, women did not receive aspirin (87.5% vs. 90.4%), beta blockers (80.5% vs. 82.7%), and statins (55.9% vs. 69.4%) as frequently as men (26). Most of these findings are based on data in early 2000. As more and more women and physicians become aware that CVD remains the leading killer in women, the underutilization of diagnostic and treatment strategies should dissipate, which may account for similar decreases in mortality in women and men since 2000 (Figure 1) (3).
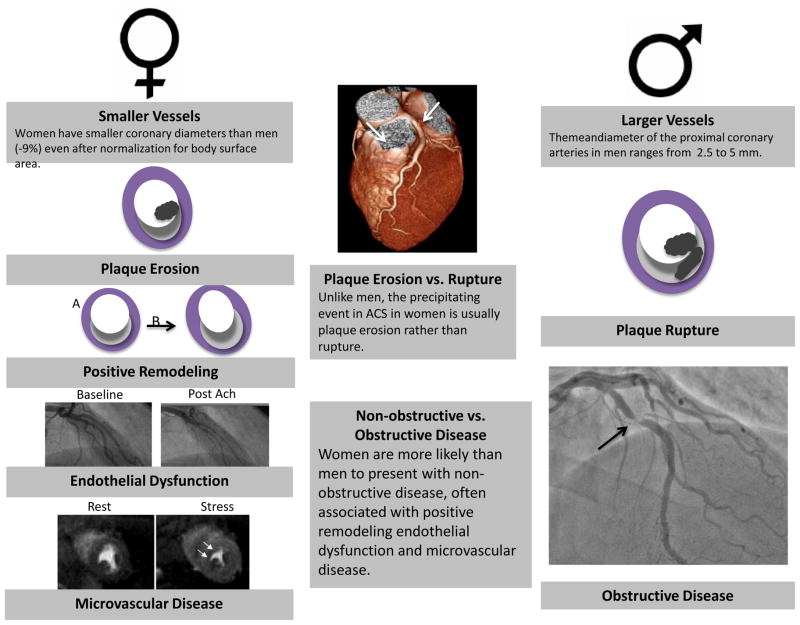
A final explanation for the higher overall mortality in women is that the current diagnostic paradigm may be suitable for men, but may not be appropriate for all women (18, 28). For example, non-traditional risk factors which are more common in women, such as decreased heart rate variability and lower levels of physical activity, are not accounted for by current risk stratification algorithms (29). There may also be unique factors to women, such as cyclic hormones and pregnancy associated vasculature changes, which may alter the pathophysiology of CHD (Figure 2). This may explain why plaque erosion rather than plaque rupture more likely precipitates ACS in women, why women have more positive remodeling and less anatomical obstruction, and why women have more coronary dysfunction (i.e., endothelial dysfunction and microvascular disease) than men (30–32). Finally, anatomically, women have smaller vessels than men, even after correction for body surface area, so even mild disease may be more harmful in women than men (19, 33). Thus, it is possible that setting an intervention threshold of 70% for significant disease, which is based on earlier studies in men, may be too high for women.
Figure 2.
Coronary heart disease may have different clinical manifestations. Women may have different clinical manifestations of coronary heart disease than men. Because women have smaller arteries, they may be more susceptible to even the slightest mismatch in demand and supply. Women may also be more prone to plaque erosion and the development of coronary dysfunction than men.
POTENTIAL STRATEGIES TO IMPROVE THE EVALUATION OF CHD IN WOMEN
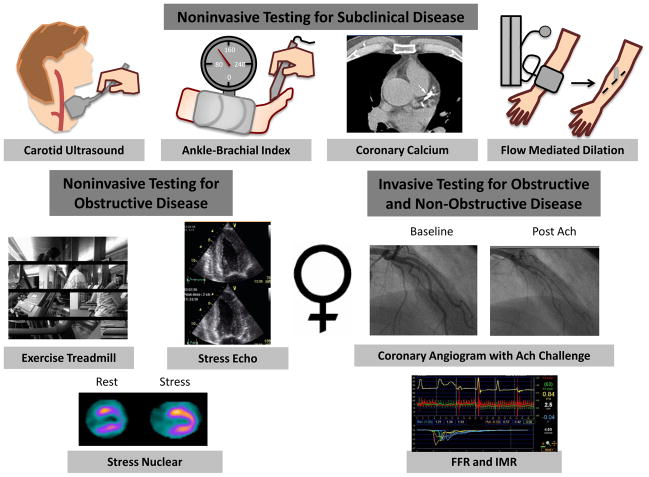
Although further studies are needed, cardiovascular medicine experts have recently proposed changing the paradigm for the diagnostic evaluation of CHD in women (18, 34). These modifications include amending risk stratification models in women, changing current recommendations for diagnostic testing to improve sensitivity and specificity in women, and adding coronary function testing to evaluate non-obstructive disease (<50% stenosis) in women (Figure 3).
Figure 3.
A new paradigm for the diagnosis and risk stratification of women with suspected CHD. Asympatomatic women may benefit from further risk stratification with noninvasive tests designed to detect subclinical disease. Symptomatic women may benefit from the addition of coronary function testing including measuring fractional flow reserve, response to acetycholine, and the index of microvascular resistance to guide therapy. Ach: acetycholine, FFR: fractional flow reserve, IMR: index of microvascular resistance.
Risk Stratification for Asymptomatic Women
One proposed modification is the addition of nontraditional risk factors, biomarkers, and noninvasive imaging to improve risk stratification in asymptomatic women. Traditional risk factor counting and the Framingham risk score may underestimate risk in women. In a previous survey of >13,000 participants, the Framingham Risk Score classified >90% of women <69 years of age as low risk (17). Specifically, the study showed that 2%, 8.5%, and 44.1% of women compared to 59.4%, 90.8%, and 97.5% of men aged 50–59, 60–69, and 70–79 years, respectively, were classified as intermediate risk.
Current risk stratification models may underestimate risk in women because there are significant sex differences in the prevalence of traditional and nontraditional risk factors and the effect of these risk factors on outcome. For example, cardiovascular mortality in diabetic women is almost three times higher than in diabetic men (34, 35). In addition, high triglyceride and low levels of high-density lipoprotein cholesterol are more prominent and more potent independent risk factors for CHD in women than men (36). Women with metabolic disturbances, including abdominal obesity, features of the metabolic syndrome, low estrogen, and low testosterone, may also be at a higher risk (28). In addition, women have greater mean C reactive protein (CRP), an inflammatory marker, which increases proportionally with the risk of future cardiac events and has been associated with accelerated CHD risk in women when combined with traditional risk factors (18, 37). Based on these findings, a sex specific risk score (i.e., the Reynolds Risk Score) was derived (n=24, 588) and later validated (n=8,158) in large cohorts of women (38). The score incorporates high sensitivity CRP, systolic blood pressure, high density lipoprotein cholesterol, total cholesterol, hemoglobin A1C, and smoking. When compared with the Framingham Risk Score, the Reynolds score resulted in the correct reclassification of >40% of women at intermediate risk (38).
Another approach to improve risk stratification is to use noninvasive imaging to detect subclinical disease, which includes the application of the ankle-brachial index, carotid intimal thickness, coronary artery calcium score (CAC), and brachial flow mediated dilation (Figure 3) (18). For women, an abnormal ankle brachial index of ≤0.90 increases with age and has a prevalence ranging between <5% for women <60 years to 10%–35% for those 60–80 years old (18). For an ankle brachial index ≤0.90, the hazard ratio for death is 2.7 (95% CI: 2.0 to 3.6) for women and 3.3 (95% CI: 2.7 to 4.1) for men (39). For carotid intimal thickness (cIMT), another validated measure of subclinical disease, a negative cIMT is associated with a ~1% and ~3% risk in women and ~11% and ~14% risk in men, respectively (40). Coronary artery calcium is another imaging measure that detects subclinical disease. Similar to obstructive CAD, its incidence in women lags behind men. Based on a NHLBI Multi-Ethnic Study of Atherosclerosis, women with a CAC score ≥300 had an annual CHD event rate of 2.2%, placing them at a high risk for CHD, thus warranting more aggressive treatment (41). Of note, women with a high CAC score and multiple risk factors have a 10% greater CHD event risk than men with a similar risk profile (42). Finally, flow-mediated dilation, a noninvasive test for endothelial dysfunction (which is the earliest manifestation of CHD), may emerge as another promising measure to improve risk stratification in asymptomatic women, although currently it has mainly research applications.
Diagnosis and Risk Stratification in Symptomatic Women
In addition to improving the risk stratification of asymptomatic patients, amending the correct paradigm for the diagnosis and risk stratification of symptomatic women may be warranted (Figure 3). Similar to men, only symptomatic women with intermediate to high pre-test probability of CHD should undergo noninvasive testing. Unlike symptomatic men, symptomatic women may have more non-obstructive disease in addition to single vessel disease than age-matched men, which can decrease the diagnostic accuracy and result in a higher false positive rate (34).
Treadmill testing is the most common noninvasive evaluation for suspected ischemia, but its continued application in women remains contentious. In a meta-analysis evaluating ECG testing for women, sensitivity and specificity were 61% and 70%, respectively (43). In comparison, a meta-analysis in men showed a slightly higher sensitivity and specificity of 72% and 77%, respectively (44). One previous study directly compared the sensitivity and specificity of treadmill testing in 3,213 women vs. 5,458 men using myocardial perfusion as the reference standard. Although more women (14%) than men (10%) had a false positive ECG (p<0.001), the false-negative rate was considerably lower in women (17% vs. 32%, p <0.001) (45). Compared with men, women had lower test sensitivity (30% vs. 42%, p <0.001) and positive predictive value (34% vs. 70%, p <0.001) but higher specificity (82% vs. 78%, p = 0.002), negative predictive value (78% vs. 52%, p <0.001), and accuracy (69% vs. 58%, p <0.001). In the smaller subset of patients referred for coronary angiography (205 women, 838 men), the false-positive electrocardiographic rate was again higher in women (13% vs. 7%, p = 0.003), but neither specificity (69% vs. 74%, p = NS) nor accuracy (60% vs. 66%, p = NS) was different between the sexes.
The accuracy of treadmill testing in women can be improved by adding multiple parameters, such as chronotropic and hemodynamic response and maximal exercise capacity, to ST segment evaluation (34). For example, integrative tests scores, such as the Duke Treadmill score, have been shown to improve accuracy and provide sex-specific data (34). In a study of 976 symptomatic women who underwent treadmill testing and were then referred to angiography, significant stenosis was present in 19%, 35%, and 89% of low-, moderate-, and high-risk women, respectively, based on the Duke treadmill risk categories (46). The 5-year CHD death rates ranged from 5% to ≥10% for women vs. 9% to ≥25% for men with low to high risk Duke treadmill scores. Based on these data, women with a high Duke treadmill score have a high probability of obstructive disease and should be referred for invasive testing (34). Those with an intermediate Duke treadmill score should be referred for stress testing with imaging. Other important parameters include the maximal exercise capacity and heart rate recovery measurement (1–2 minutes after exercise), which provide near- and long-term outcome in large cohorts of women (47, 48). Women who exercise <5 metabolic equivalents (METs) are at an increased risk for death and should be referred for pharmacologic stress testing (49). Those who have ischemia at low workloads (<5 METs) also have a high likelihood of obstructive disease and should be referred for invasive angiography (34). Thus, current guidelines encourage the use of comprehensive data from treadmill testing to risk stratify women with suspected ischemia (34).
For women with suspected CAD and an abnormal resting, both stress echocardiography and myocardial perfusion provide valuable diagnostic and prognostic data. Based on aggregate data, stress echocardiography provides improved sensitivity and specificity and diagnostic accuracy with no differences between the sexes; however, most studies have not been corrected for post-test referral bias and post-test verification bias (34). Similarly, prognostic information by stress echocardiography is comparable in women and men. The presence of an abnormal stress echocardiography is associated with a high risk of future adverse cardiac events; conversely, a normal study confers a low risk (50–53). Stress echocardiography has been shown to provide incremental prognostic data beyond that provided by clinical and exercise variables (27, 34, 54). Based on a multi-center registry data, exercise stress echocardiography may also be more cost effective than treadmill exercise testing, given that the higher rate of false positives using treadmill exercise testing likely leads to more unnecessary angiography tests and expense (55). Nevertheless, there is insufficient data to recommend exercise stress echocardiography as the initial test in all women, and it should still be reserved for women with suspected CAD and an abnormal resting ECG (34).
The assessment of myocardial perfusion by gated single positron emission tomography (SPECT) is another noninvasive technique for the diagnosis and risk stratification of women with suspected obstructive CAD. Historically, myocardial perfusion imaging has been reported to have a high number of false positives in women, possibly due to breast attenuation and a smaller average heart size (56). Specificity has improved with advancement in nuclear imaging technology and is now only slightly lower than stress echocardiography (Table 1). Similar to stress echocardiography, myocardial perfusion provides incremental prognostic information to clinical and exercise variables for both women and men (34). In a recent multi-center registry of 5009 men and 3402 women, the number of territories with perfusion defects was associated with cardiac mortality in women and men (57). In women, the number of abnormal territories remained the strongest correlate of mortality after adjustment for exercise variables. In the setting of a normal perfusion study, the annual cardiac event rate is <1% compared to a significantly increased risk of cardiac death in the setting of an abnormal perfusion study (58).
TABLE 1.
Studies comparing sensitivity and specificity in women and men
| Noninvasive Test | Reference | Women | Men | ||
|---|---|---|---|---|---|
|
| |||||
| Sensitivity | Specificity | Sensitivity | Specificity | ||
|
| |||||
| Exercise Treadmill | Miller, et al 2002 (45) | 0.30 | 0.82 | 0.42 | 0.78 |
| Stress Echo | |||||
| Exercise Echo | Luotalahti, et al 1996(63) | 0.77 | 0.80 | 0.96 | 0.60 |
| Exercise Echo | Roger, et al 1997 (64) | 0.79 | 0.37 | 0.78 | 0.44 |
| Dobutamine Echo | Salustri et al 1992 (65) | 0.83 | 0.75 | 0.48 | 0.86 |
| Dobutamine Echo | Mazeika, et al, 1992 (66) | 0.75 | 1.00 | 0.63 | 0.92 |
| Dobutamine Echo | Marwick, et al, 1993(67) | 0.55 | 0.75 | 0.75 | 0.88 |
| Dobutamine Echo | Dionisopoulous, et al, 1997 (68) | 0.90 | 0.79 | 0.85 | 0.96 |
| Dobutamine Echo | Elhendy, et al, 1997 (69) | 0.76 | 0.94 | 0.73 | 0.81 |
| Dobutamine Echo | Seknus, et al 1997 (70) | 0.78 | 0.55 | 0.88 | 0.46 |
| Dobutamine Echo | Rollan, et al, 1999(71) | 0.69 | 0.89 | 0.77 | 0.77 |
| Myocardial Perfusion Imaging (MPI) | |||||
| Exercise MPI | Kiat et al, 1990 (72) | 1.00 | 0.67 | 0.92 | 0.50 |
| Exercise MPI | Van Train et al, 1990 (73) | 0.95 | 0.62 | 0.95 | 0.43 |
| Exercise and Vasodilator Stress | Gupta et al, 1992 (74) | 0.85 | 0.67 | 0.81 | 0.86 |
| Exercise and Vasodilator Stress | Sciammarella et al, 1992 (75) | 1.00 | 0.40 | 0.95 | 0.33 |
| Exercise and Vasodilator Stress | Hambye et al, 1996 (76) | 0.61 | 0.67 | 0.94 | 0.82 |
| Exercise and Vasodilator Stress | Astarita et al, 1998 (77) | 1.00 | 0.47 | 1.0 | 0.50 |
Overall, noninvasive testing appears to be a more valuable for risk stratification than diagnosis of obstructive CAD. The sensitivity and specificity can be as low as ~55% and even lower if diagnostic accuracy is corrected for referral bias. The “false” positives in noninvasive testing, however, may in part reflect the presence of coronary dysfunction, which is associated with increased morbidity and mortality, rather than the limitation of noninvasive testing
Measuring coronary function has emerged as an important step in the evaluation of patients with suspected ischemia. In a recent study, the incorporation of fractional flow reserve, a measure of the functional significance of an anatomical lesion, to guide percutaneous coronary intervention has been shown to reduce the number of stents used as well as to improve morbidity and mortality (59). Detecting endothelial dysfunction and microvascular disease as a cause of chest pain and ischemia even in the absence of significant stenosis may also provide important diagnostic and prognostic information as well as reassurance to patients. In the cardiac catheterization lab, the measurement of endothelial function and microvascular function is achieved by measuring the change in coronary diameter in response to acetylcholine challenge and by measuring the index of microvascular resistance using a coronary pressure wire. In patients undergoing diagnostic angiography, single vessel percutaneous coronary intervention, or post myocardial event, the presence of coronary dysfunction was associated with major adverse cardiac events (60, 61). Although treatment regimens have not clearly been identified, improvement of coronary function has been shown to decrease the rate of major adverse cardiac events when compared with no improvement (62). Although invasive coronary angiography remains the gold standard for the evaluation of coronary function for the time being, the development of a noninvasive measure is needed to risk stratify asymptomatic patients, diagnose coronary dysfunction in those presenting with chest pain, and monitor therapy (27, 54).
CONCLUSION
CHD remains the leading cause of death in both men and women. Sex differences in the clinical presentation and manifestation of CAD may warrant the development of guidelines specific for women as opposed to men. Future investigation should evaluate diagnostic and treatment strategies to optimize outcome in women and men.
References
- 1.Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000 Aug 17;343(7):475–480. doi: 10.1056/NEJM200008173430706. [DOI] [PubMed] [Google Scholar]
- 2.Blauwet LA, Hayes SN, McManus D, Redberg RF, Walsh MN. Low rate of sex-specific result reporting in cardiovascular trials. Mayo Clin Proc. 2007 Feb;82(2):166–170. doi: 10.4065/82.2.166. [DOI] [PubMed] [Google Scholar]
- 3.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Jan 29;117(4):e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 4.Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation. 2008 Mar 25;117(12):1526–1536. doi: 10.1161/CIRCULATIONAHA.107.720953. [DOI] [PubMed] [Google Scholar]
- 5.Canto JG, Goldberg RJ, Hand MM, et al. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med. 2007 Dec 10;167(22):2405–2413. doi: 10.1001/archinte.167.22.2405. [DOI] [PubMed] [Google Scholar]
- 6.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory) Am J Cardiol. 2001 Apr 15;87(8):937–941. A933. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 7.Shaw LJ, Shaw RE, Merz CN, et al. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation. 2008 Apr 8;117(14):1787–1801. doi: 10.1161/CIRCULATIONAHA.107.726562. [DOI] [PubMed] [Google Scholar]
- 8.Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. Jama. 2005 Jan 26;293(4):477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- 9.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986 Mar;7(3):479–483. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 10.Lichtlen PR, Bargheer K, Wenzlaff P. Long-term prognosis of patients with anginalike chest pain and normal coronary angiographic findings. J Am Coll Cardiol. 1995 Apr;25(5):1013–1018. doi: 10.1016/0735-1097(94)00519-v. [DOI] [PubMed] [Google Scholar]
- 11.Kaski JC, Rosano GM, Collins P, Nihoyannopoulos P, Maseri A, Poole-Wilson PA. Cardiac syndrome X: clinical characteristics and left ventricular function. Long-term follow-up study. J Am Coll Cardiol. 1995 Mar 15;25(4):807–814. doi: 10.1016/0735-1097(94)00507-M. [DOI] [PubMed] [Google Scholar]
- 12.Robinson JG, Wallace R, Limacher M, et al. Cardiovascular risk in women with non-specific chest pain (from the Women’s Health Initiative Hormone Trials) Am J Cardiol. 2008 Sep 15;102(6):693–699. doi: 10.1016/j.amjcard.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 13.Johnson BD, Shaw LJ, Buchthal SD, et al. Prognosis in women with myocardial ischemia in the absence of obstructive coronary disease: results from the National Institutes of Health-National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004 Jun 22;109(24):2993–2999. doi: 10.1161/01.CIR.0000130642.79868.B2. [DOI] [PubMed] [Google Scholar]
- 14.Gulati M, Cooper-DeHoff RM, McClure C, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the Women’s Ischemia Syndrome Evaluation Study and the St James Women Take Heart Project. Arch Intern Med. 2009 May 11;169(9):843–850. doi: 10.1001/archinternmed.2009.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson BD, Shaw LJ, Pepine CJ, et al. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006 Jun;27(12):1408–1415. doi: 10.1093/eurheartj/ehl040. [DOI] [PubMed] [Google Scholar]
- 16.Shaw LJ, Merz CN, Pepine CJ, et al. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006 Aug 29;114(9):894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 17.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002: concealed leveling of mortality rates. J Am Coll Cardiol. 2007 Nov 27;50(22):2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009 Oct 20;54(17):1561–1575. doi: 10.1016/j.jacc.2009.04.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 Participants. N Engl J Med. 1999 Jul 22;341(4):217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 20.Woodfield SL, Lundergan CF, Reiner JS, et al. Gender and acute myocardial infarction: is there a different response to thrombolysis? J Am Coll Cardiol. 1997 Jan;29(1):35–42. doi: 10.1016/s0735-1097(96)00449-4. [DOI] [PubMed] [Google Scholar]
- 21.Becker RC, Burns M, Every N, et al. Early clinical outcomes androutine management of patients with non-ST-segment elevation myocardial infarction: a nationwide perspective. Arch Intern Med. 2001 Feb 26;161(4):601–607. doi: 10.1001/archinte.161.4.601. [DOI] [PubMed] [Google Scholar]
- 22.Daly C, Clemens F, Lopez Sendon JL, et al. Gender differences in the management and clinical outcome of stable angina. Circulation. 2006 Jan 31;113(4):490–498. doi: 10.1161/CIRCULATIONAHA.105.561647. [DOI] [PubMed] [Google Scholar]
- 23.Roger VL, Farkouh ME, Weston SA, et al. Sex differences in evaluation and outcome of unstable angina. Jama. 2000 Feb 2;283(5):646–652. doi: 10.1001/jama.283.5.646. [DOI] [PubMed] [Google Scholar]
- 24.Maynard C, Beshansky JR, Griffith JL, Selker HP. Influence of sex on the use of cardiac procedures in patients presenting to the emergency department. A prospective multicenter study. Circulation. 1996 Nov 1;94(9 Suppl):II93–98. [PubMed] [Google Scholar]
- 25.Hvelplund A, Galatius S, Madsen M, et al. Women with acute coronary syndrome are less invasively examined and subsequently less treated than men. Eur Heart J. 2009 Mar;31(6):684–690. doi: 10.1093/eurheartj/ehp493. [DOI] [PubMed] [Google Scholar]
- 26.Blomkalns AL, Chen AY, Hochman JS, et al. Gender disparities in the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: large-scale observations from the CRUSADE (Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes With Early Implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol. 2005 Mar 15;45(6):832–837. doi: 10.1016/j.jacc.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen PK, Meyer C, Engvall J, Yang P, McConnell MV. Noninvasive assessment of coronary vasodilation using cardiovascular magnetic resonance in patients at high risk for coronary artery disease. J Cardiovasc Magn Reson. 2008;10:28. doi: 10.1186/1532-429X-10-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellasi A, Raggi P, Merz CN, Shaw LJ. New insights into ischemic heart disease in women. Cleve Clin J Med. 2007 Aug;74(8):585–594. doi: 10.3949/ccjm.74.8.585. [DOI] [PubMed] [Google Scholar]
- 29.Ramaekers D, Ector H, Aubert AE, Rubens A, Van de Werf F. Heartrate variability and heart rate in healthy volunteers. Is the female autonomic nervous system cardioprotective? Eur Heart J. 1998 Sep;19(9):1334–1341. doi: 10.1053/euhj.1998.1084. [DOI] [PubMed] [Google Scholar]
- 30.Arbustini E, Dal Bello B, Morbini P, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999 Sep;82(3):269–272. doi: 10.1136/hrt.82.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke AP, Farb A, Malcom GT, Liang Y, Smialek J, Virmani R. Effect of risk factors on the mechanism of acute thrombosis and sudden coronary death in women. Circulation. 1998 Jun 2;97(21):2110–2116. doi: 10.1161/01.cir.97.21.2110. [DOI] [PubMed] [Google Scholar]
- 32.Han SH, Bae JH, Holmes DR, Jr, et al. Sex differences in atheroma burden and endothelial function in patients with early coronary atherosclerosis. Eur Heart J. 2008 Jun;29(11):1359–1369. doi: 10.1093/eurheartj/ehn142. [DOI] [PubMed] [Google Scholar]
- 33.Dodge JT, Jr, Brown BG, Bolson EL, Dodge HT. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992 Jul;86(1):232–246. doi: 10.1161/01.cir.86.1.232. [DOI] [PubMed] [Google Scholar]
- 34.Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005 Feb 8;111(5):682–696. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 35.Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. ArchIntern Med. 2002 Aug 12–26;162(15):1737–1745. doi: 10.1001/archinte.162.15.1737. [DOI] [PubMed] [Google Scholar]
- 36.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986 Feb;111(2):383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 37.Wong ND, Pio J, Valencia R, Thakal G. Distribution of C-reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001 Summer;4(3):109–114. doi: 10.1111/j.1520-037x.2001.00570.x. [DOI] [PubMed] [Google Scholar]
- 38.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. Jama. 2007 Feb 14;297(6):611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 39.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. Jama. 2008 Jul 9;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon A, Chironi G, Levenson J. Comparative performance of subclinical atherosclerosis tests in predicting coronary heart disease in asymptomatic individuals. Eur Heart J. 2007 Dec;28(24):2967–2971. doi: 10.1093/eurheartj/ehm487. [DOI] [PubMed] [Google Scholar]
- 41.Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framinghamrisk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007 Dec 10;167(22):2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 42.Bellasi A, Lacey C, Taylor AJ, et al. Comparison of prognostic usefulness of coronary artery calcium in men versus women (results from a meta-and pooled analysis estimating all-cause mortality and coronary heart disease death or myocardial infarction) Am J Cardiol. 2007 Aug 1;100(3):409–414. doi: 10.1016/j.amjcard.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 43.Kwok Y, Kim C, Grady D, Segal M, Redberg R. Meta-analysis of exercise testing to detect coronaryartery disease in women. Am J Cardiol. 1999 Mar 1;83(5):660–666. doi: 10.1016/s0002-9149(98)00963-1. [DOI] [PubMed] [Google Scholar]
- 44.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002 Oct 1;106(14):1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 45.Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002 Mar;112(4):290–297. doi: 10.1016/s0002-9343(01)01111-1. [DOI] [PubMed] [Google Scholar]
- 46.Alexander KP, Shaw LJ, Shaw LK, Delong ER, Mark DB, Peterson ED. Value of exercise treadmilltesting in women. J Am Coll Cardiol. 1998 Nov 15;32(6):1657–1664. doi: 10.1016/s0735-1097(98)00451-3. [DOI] [PubMed] [Google Scholar]
- 47.Panzer C, Lauer MS, Brieke A, Blackstone E, Hoogwerf B. Association of fasting plasma glucose with heart rate recovery in healthy adults: a population-based study. Diabetes. 2002 Mar;51(3):803–807. doi: 10.2337/diabetes.51.3.803. [DOI] [PubMed] [Google Scholar]
- 48.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003 Sep 30;108(13):1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 49.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index) Am J Cardiol. 1989 Sep 15;64(10):651–654. doi: 10.1016/0002-9149(89)90496-7. [DOI] [PubMed] [Google Scholar]
- 50.Heupler S, Mehta R, Lobo A, Leung D, Marwick TH. Prognostic implications of exercise echocardiography in women with known or suspected coronary artery disease. J Am Coll Cardiol. 1997 Aug;30(2):414–420. doi: 10.1016/s0735-1097(97)00167-8. [DOI] [PubMed] [Google Scholar]
- 51.Poldermans D, Fioretti PM, Boersma E, et al. Long-term prognostic value of dobutamine-atropine stress echocardiography in 1737 patients with known or suspected coronary artery disease: A single-center experience. Circulation. 1999 Feb 16;99(6):757–762. doi: 10.1161/01.cir.99.6.757. [DOI] [PubMed] [Google Scholar]
- 52.Arruda-Olson AM, Juracan EM, Mahoney DW, McCully RB, Roger VL, Pellikka PA. Prognostic value of exercise echocardiography in 5,798 patients: is there a gender difference? J Am Coll Cardiol. 2002 Feb 20;39(4):625–631. doi: 10.1016/s0735-1097(01)01801-0. [DOI] [PubMed] [Google Scholar]
- 53.Shaw LJ, Vasey C, Sawada S, Rimmerman C, Marwick TH. Impact of gender on risk stratification by exercise and dobutamine stress echocardiography: long-term mortality in 4234 women and 6898 men. Eur Heart J. 2005 Mar;26(5):447–456. doi: 10.1093/eurheartj/ehi102. [DOI] [PubMed] [Google Scholar]
- 54.Terashima M, Nguyen PK, Rubin GD, et al. Impaired coronary vasodilation by magnetic resonance angiography is associated with advanced coronary artery calcification. JACC Cardiovasc Imaging. 2008 Mar;1(2):167–173. doi: 10.1016/j.jcmg.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Marwick TH, Shaw L, Case C, Vasey C, Thomas JD. Clinical and economic impact of exercise electrocardiography and exercise echocardiography in clinical practice. Eur Heart J. 2003 Jun;24(12):1153–1163. doi: 10.1016/s0195-668x(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 56.Taillefer R, DePuey EG, Udelson JE, Beller GA, Latour Y, Reeves F. Comparative diagnostic accuracy of Tl-201 and Tc-99m sestamibi SPECT imaging (perfusion and ECG-gated SPECT) in detecting coronary artery disease in women. J Am Coll Cardiol. 1997 Jan;29(1):69–77. doi: 10.1016/s0735-1097(96)00435-4. [DOI] [PubMed] [Google Scholar]
- 57.Marwick TH, Shaw LJ, Lauer MS, et al. The noninvasive prediction of cardiac mortality in men and women with known or suspected coronary artery disease. Economics of Noninvasive Diagnosis (END) Study Group. Am J Med. 1999 Feb;106(2):172–178. doi: 10.1016/s0002-9343(98)00388-x. [DOI] [PubMed] [Google Scholar]
- 58.Shaw LJ, Iskandrian AE. Prognostic value ofgated myocardial perfusion SPECT. J Nucl Cardiol. 2004 Mar-Apr;11(2):171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009 Jan 15;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 60.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000 Apr 25;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 61.Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. 2008 Jun 17;117(24):3152–3156. doi: 10.1161/CIRCULATIONAHA.107.742312. [DOI] [PubMed] [Google Scholar]
- 62.Modena MG, Bonetti L, Coppi F, Bursi F, Rossi R. Prognostic role of reversible endothelial dysfunction in hypertensive postmenopausal women. J Am Coll Cardiol. 2002 Aug 7;40(3):505–510. doi: 10.1016/s0735-1097(02)01976-9. [DOI] [PubMed] [Google Scholar]
- 63.Luotolahti M, Saraste M, Hartiala J. Exercise echocardiography in the diagnosis of coronary artery disease. Ann Med. 1996 Feb;28(1):73–77. doi: 10.3109/07853899608999078. [DOI] [PubMed] [Google Scholar]
- 64.Roger VL, Pellikka PA, Bell MR, Chow CW, Bailey KR, Seward JB. Sex and test verification bias. Impact on the diagnostic value of exercise echocardiography. Circulation. 1997 Jan 21;95(2):405–410. doi: 10.1161/01.cir.95.2.405. [DOI] [PubMed] [Google Scholar]
- 65.Salustri A, Fioretti PM, McNeill AJ, Pozzoli MM, Roelandt JR. Pharmacological stress echocardiography in the diagnosis of coronary artery disease and myocardial ischaemia: a comparison between dobutamine and dipyridamole. Eur Heart J. 1992 Oct;13(10):1356–1362. doi: 10.1093/oxfordjournals.eurheartj.a060066. [DOI] [PubMed] [Google Scholar]
- 66.Mazeika PK, Nadazdin A, Oakley CM. Stress Doppler echocardiography using dobutamine in coronary patients with and without ischaemia induction. Eur Heart J. 1992 Aug;13(8):1020–1027. doi: 10.1093/oxfordjournals.eurheartj.a060308. [DOI] [PubMed] [Google Scholar]
- 67.Marwick T, D’Hondt AM, Baudhuin T, et al. Optimal use of dobutamine stress for the detection and evaluation of coronary artery disease: combinationwith echocardiography or scintigraphy, or both? J Am Coll Cardiol. 1993 Jul;22(1):159–167. doi: 10.1016/0735-1097(93)90830-t. [DOI] [PubMed] [Google Scholar]
- 68.Dionisopoulos PN, Collins JD, Smart SC, Knickelbine TA, Sagar KB. The value of dobutamine stress echocardiography for the detection of coronary artery disease in women. J Am Soc Echocardiogr. 1997 Oct;10(8):811–817. doi: 10.1016/s0894-7317(97)70040-3. [DOI] [PubMed] [Google Scholar]
- 69.Elhendy A, Geleijnse ML, van Domburg RT, et al. Gender differences in the accuracy of dobutamine stress echocardiography for the diagnosis of coronary artery disease. Am J Cardiol. 1997 Dec 1;80(11):1414–1418. doi: 10.1016/s0002-9149(97)00707-8. [DOI] [PubMed] [Google Scholar]
- 70.Secknus MA, Marwick TH. Influence of gender on physiologic response and accuracy of dobutamine echocardiography. Am J Cardiol. 1997 Sep 15;80(6):721–724. doi: 10.1016/s0002-9149(97)00502-x. [DOI] [PubMed] [Google Scholar]
- 71.Rollan MJ, San Roman JA, Vilacosta I, et al. The influence of sex on the performance of dobutamine echocardiography for the diagnosis of ischemic cardiopathy. Rev Esp Cardiol. 1999 Dec;52(12):1060–1065. [PubMed] [Google Scholar]
- 72.Kiat H, Van Train KF, Maddahi J, et al. Development and prospective application of quantitative 2-day stress-rest Tc-99m methoxy isobutyl isonitrile SPECT for the diagnosis of coronary artery disease. Am Heart J. 1990 Dec;120(6 Pt 1):1255–1266. doi: 10.1016/0002-8703(90)90234-o. [DOI] [PubMed] [Google Scholar]
- 73.Van Train KF, Maddahi J, Berman DS, et al. Quantitative analysis of tomographic stress thallium-201 myocardial scintigrams: a multicenter trial. J Nucl Med. 1990 Jul;31(7):1168–1179. [PubMed] [Google Scholar]
- 74.Gupta NC, Esterbrooks DJ, Hilleman DE, Mohiuddin SM. Comparison of adenosine and exercise thallium-201 single-photon emission computed tomography (SPECT) myocardial perfusion imaging. The GE SPECTMulticenter Adenosine Study Group. J Am Coll Cardiol. 1992 Feb;19(2):248–257. doi: 10.1016/0735-1097(92)90474-2. [DOI] [PubMed] [Google Scholar]
- 75.Sciammarella MG, Fragasso G, Gerundini P, et al. 99Tcm-MIBI single photon emission tomography (SPET) for detecting myocardial ischaemia and necrosis in patients with significant coronary artery disease. Nucl Med Commun. 1992 Dec;13(12):871–878. doi: 10.1097/00006231-199212000-00004. [DOI] [PubMed] [Google Scholar]
- 76.Hambye AS, Vervaet A, Lieber S, Ranquin R. Diagnostic value and incremental contribution of bicycle exercise, first-pass radionuclide angiography, and 99mTc-labeled sestamibi single-photon emission computed tomography in the identification of coronary artery disease in patients without infarction. J Nucl Cardiol. 1996 Nov-Dec;3(6 Pt 1):464–474. doi: 10.1016/s1071-3581(96)90056-2. [DOI] [PubMed] [Google Scholar]
- 77.Astarita C, Nicolai E, Liguori E, Gambardella S, Rumolo S, Maresca FS. Dipyridamole-echocardiography and thallium exercise myocardial scintigraphy in the diagnosis of obstructive coronary or microvascular disease in hypertensive patients with left ventricular hypertrophy and angina. G Ital Cardiol. 1998 Sep;28(9):996–1004. [PubMed] [Google Scholar]