Abstract
Background
During hypoxia, upregulation of hypoxia inducible factor-1 alpha (HIF-1α) transcriptional factor can activate several downstream angiogenic genes. However, HIF-1α is naturally degraded by prolyl hydroxylase-2 (PHD2) protein. Here we hypothesize that short hairpin RNA (shRNA) interference therapy targeting PHD2 can be used for treatment of myocardial ischemia and this process can be followed noninvasively by molecular imaging.
Methods and Results
PHD2 was cloned from mouse embryonic stem (ES) cells by comparing the homolog gene in human and rat. The best candidate shRNA sequence for inhibiting PHD2 was inserted into the pSuper vector driven by the H1 promoter, followed by a separate hypoxia response element (HRE)-incorporated promoter driving a firefly luciferase (Fluc) reporter gene. This construct was used to transfect mouse C2C12 myoblast cell line for in vitro confirmation. Compared to the control short hairpin scramble (shScramble) as control, inhibition of PHD2 increased levels of HIF-1α protein and several downstream angiogenic genes by >30% (P<0.01). Afterwards, shRNA targeting PHD2 (shPHD2) plasmid was injected intramyocardially following ligation of left anterior descending (LAD) artery in mice. Animals were randomized into shPHD2 group (n=20) versus shScramble sequence as control (n=20). Bioluminescence imaging detected transgene expression for 4–5 weeks. Echocardiographic study showed the shPHD2 group had improved fractional shortening compared with the shScramble group at week 4 (33.7%±1.9% vs. 28.4%±2.8%; P<0.05). Postmortem analysis showed increased presence of small capillaries and venules in the infarcted zones by CD31 staining. Finally, Western blot anlaysis of explanted hearts also confirm that animals treated with shPHD2 had significantly higher levels of HIF-1α protein.
Conclusions
This is the first study to image the biological role of shRNA therapy for improving cardiac function. Inhibition of PHD2 by shRNA led to significant improvement in angiogenesis and contractility by in vitro and in vivo experiments. With further validation, the combination of shRNA therapy and molecular imaging can be used to track novel cardiovascular gene therapy applications in the future.
Keywords: RNA interference, molecular imaging, hypoxia inducible factor, prolyl hydroxylases, ischemic heart disease
INTRODUCTION
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in the Western world 1. Conventional treatment for CAD consists of medical therapy as the first-line strategy, followed by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG). However, a significant number of patients will still have refractory angina despite these treatments 2. For such patients, the alternative approach of delivering potent angiogenic factors to stimulate new vessel growth has undergone intense investigation over the past decade. With the use of various gene transfer techniques, it is now possible to modify cardiac cells to overexpress beneficial proteins or inhibit pathologic proteins and achieve desired therapeutic effects 3. The field has expanded tremendously from preclinical studies in the early 1990s to large randomized clinical trials in the early 2000s. Although initial phase 1 trials in patients with myocardial ischemia provided encouraging results 4,5, recent phase 2 randomized trials (AGENT, VIVA, KAT) yielded only modest benefits 4–6. These inconsistencies have been attributed to the unclear role of single therapeutic genes such as vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF), as well as the inability to monitor gene transfer in vivo 7.
Newer approaches based on the upstream transcriptional factor HIF-1α may be a more natural choice. HIF-1α is known to control the expression of over 60 genes that affect cell survival and metabolism in adverse conditions, including VEGF 8, FGF 4, insulin-like growth factor (IGF) 9, erythropoietin 10, nitric oxide synthase 11, among others. Unfortunately, HIF-1α has a biological half-life of only~5 minutes under normoxic condition 12. This is because during normoxic condition, HIF-1α is hydroxylated by oxygen-dependent PHD2, ubiquitylated, and subsequently degraded. In this paper, we demonstrated that the inhibition of HIF-1α degradation via shRNA knockdown of PHD2 in the ischemic heart represents a novel angiogenic therapy approach. At the same time, we tracked the shRNA vector in vivo through novel molecular imaging technology.
MATERIALS AND METHODS
RNA interference of mouse PHD2 gene in culture cell
Mouse PHD2 gene was cloned from mouse ES cell after comparing human and rat homolog gene. We designed 4 sequences of RNA interference sites. The targeting sequences are shown in Figure 1a. Sequence for the short hairpin scramble (shScramble) antisense is TGTGAGGAACTTGAGATCT (control). Construction of the H1 promoter driving sense and antisense, respectively, was performed as described 13. The fragment No. 2 knocking down site was inserted after H1 promoter in the vector pSuper as described in the Oligoengine™ manual. SpeI site on the pSuper was cut in order to insert the hypoxia response element HRE-SV40-firefly luciferase cassette.
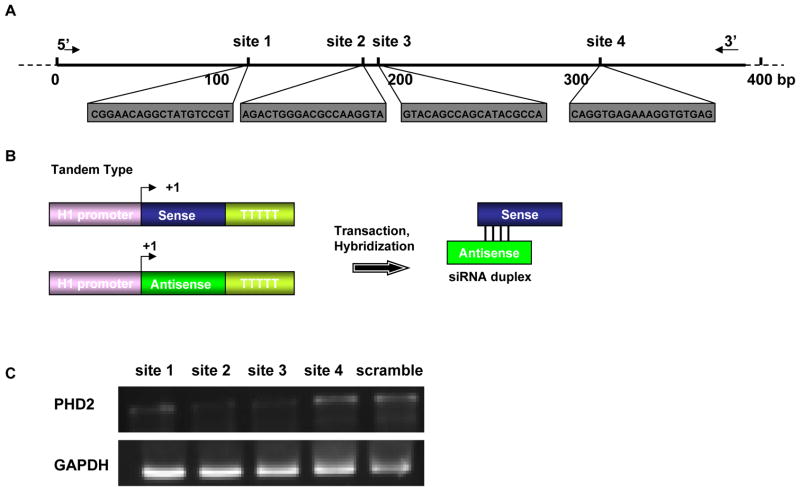
Figure 1. Optimization of knocking-down target of mouse PHD2 gene.
(a) Individual sequences of four siRNA target sites against the PHD2 gene. (b) Schema of the tandem type shRNA structure. Two H1 promoters are used to drive the separate transcription of the sense and antisense strands. The two strands would then anneal to form a double-stranded siRNA complex within the cell. (c) Forty-eight hours after siRNA fragment transfection, comparison of knocking-down efficiency was tested by RT-PCR of mouse PHD2 gene. Data shown here indicate that the site-2 siRNA fragment had the best interference efficiency.
Cell culture, shRNA transfection, and hypoxia exposure
Mouse C2C12 myoblasts were cultured in DMEM medium (high glucose) supplemented with 10% fetal bovine serum as described in the ATCC protocol. The sense and antisense fragments of mouse PHD2 driven by the H1 promoter were co-transfected into C2C12 with the plasmid pCMV-Fluc as control for equal transfection efficiency. Lipofectamine 2000 (Invitrogen) was used for the transfection according to manufacturer’s protocol. Cells were cultured for 1 day after shRNA fragment transfection before being subjected to hypoxia. Hypoxia was achieved by placing cells in a hypoxia chamber filled with 5% CO2, 1% O2, and 94% N2 at 37°C. Cells were then kept under hypoxic conditions for 48 hours. At the end of the hypoxic treatment, cells were harvested immediately to extract RNA and protein.
Reverse-transcription polymerase chain reaction (RT-PCR) analysis of angiogenic genes
RT-PCR was used to compare the expression of antigenic genes (bFGF, tranferin, FLT, KDR, TGF, PAI-1) in transfected cells under nomoxia versus hypoxia. Total RNA was prepared from C2C12 cells with Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The primer sets used in the amplification reaction are shown in Supplementary Table 1. PCR products were separated on 1% agarose gel electrophoresis and quantified with Labworks 4.6 Image Acquisition and analysis software (UVP Bio-Imaging Systems).
Western blot analysis of HIF-1α in vitro and in vivo
After 48 hrs of hypoxia culture, the C2C12 cells were washed by phosphate buffered saline (PBS) and homogenized with 200 μl of homogenization buffer (RIPA), after which the supernatants were fractionated on SDS-PAGE and blotted to a Hybond-P membrane. The membranes were blocked with 5% nonfat dry milk in 1 TBS containing 0.05% Tween 20 and incubated with the antibodies against HIF-1α (Novus, USA). Detection was performed with secondary HRP-conjugated antibodies and the ECL detection system. For the in vivo Western blot, heart samples were taken at day 1, 4, 7, 14, and 28 days after plasmid injection.
Animal surgery to induce myocardial infarction
Ligation of the mid left anterior descending (LAD) artery was performed in adult female FVB mice (Charles River Laboratories, Wilmington, MA) by a single experienced surgeon (GH). Myocardial infarction was confirmed by myocardial blanching and EKG changes. After waiting for 10 minutes, animals were then injected intramyocardially with 25 μg of shRNA plasmid at the peri-infarct zone (n=20) or 25 μg of shScramble plasmid (n=20) as control. In both groups, the volume of injection was 50 μl using a 31-gauge Hamilton syringe. Study protocols were approved by the Stanford Animal Research Committee.
Optical bioluminescence imaging of plasmid gene expression
Cardiac bioluminescence imaging was performed with the Xenogen In Vivo Imaging System (Alameda, CA). After intraperitoneal injection of the reporter probe D-luciferin (150 mg/kg body weight), animals were imaged for 1–10 minutes. The same mice were scanned repetitively for a 4-week period according to the specific study design. Bioluminescence signals were quantified in units of maximum photons per second per centimeter squared per steradian (p/s/cm2/sr) as described previously 14.
Analysis of left ventricular function with echocardiogram
Echocardiography was performed before (day -7) and after (week 2, week 4, week 8) the LAD ligation. The Siemens-Acuson Sequioa C512 system equipped with a multi-frequency (8–14 MHZ) 15L8 transducer was used by an investigator (ZL) blinded to group designation. Analysis of M-mode images was performed using Siemens built-in software. Left ventricular end diastolic diameter (EDD) and end-systolic diameter (ESD) were measured and used to calculate left ventricular fractional shortening by the formula: LVFS = [EDD-ESD]/EDD.
Histological examination
Explanted hearts from study and control groups were embedded into OCT compound (Miles Scientific, Elkhart, IN). Frozen sections (5 um thick) were processed for immunostaining. To detect microvascular density (MVD) in the peri-infarct area, a rat anti-CD31 (BD Pharmingen) was used. The number of capillary vessels was counted by a blinded investigator (LZ) in ten randomly selected areas using a light microscope (x200 magnification). Additional samples were used to examine the infarction size by Masson’s trichrome staining
Statistical Analysis
ANOVA and repeated measures ANOVA with post-hoc testing as well as the two-tailed Student’s t–test were used. Differences were considered significant at P-values of <0.05. Unless specified, data are expressed as mean ± standard deviation.
Statement of Responsibility
The authors had full access to and take full responsibilityfor the integrity of the data. All authors have read and accept the manuscript as written.
RESULTS
Mouse PHD2 gene isolation and knocking down in culture cells
Since the mouse PHD2 cDNA cannot be found in gene bank, we compared the exact PhD2 sequence that has been isolated from human and rat. Base on the reported nucleotide sequence of PHD2 gene in rat and human (http://www.genebank), we isolated the PHD2 DNA clone from mouse ES cell (Sv129 line). According to the PHD2 sequence, we designed four siRNA sites (Figure 1a) using a commercially available web-based software (http://www.ambion.com/techlib/misc/siRNA_finder.html). To determine the site that possesses the optimal knocking-down efficiency, we cloned the sense and antisense downstream of the H1 promoter, respectively, by PCR method (Figure 1b). These 4 shRNA constructs were used to transfect C2C12 myoblasts in 6-well plates along with pCMV-luciferase plasmid used to confirm for equal transfection efficiency (data not shown). After 48 hours of cell culture, mRNA levels of PHD2 within C2C12 cells were measured by RT-PCR. Using the densitometric analysis software, site 2 and site 3 inhibition could degrade 50–60% of the mouse PHD2 mRNA, which were significantly better than site 1 (15–25%) and site 4 (20–30%) (Figure 1c).
In vitro characterization of shPHD2 under nomoxia and hypoxia conditions
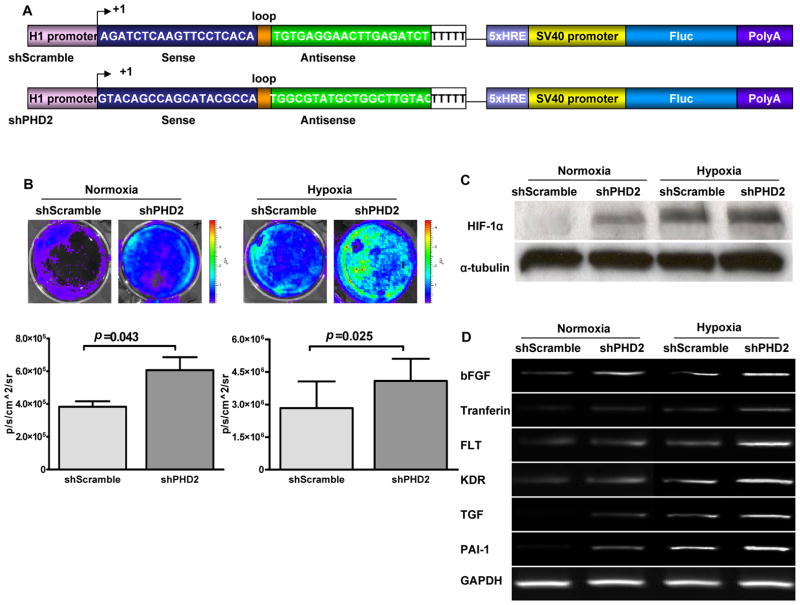
Previously, Warnecke and colleagues demonstrated that pharmacological activation of HIF-1α by hydroxlyase inhibitors can effectively increase vascularization in a sponge model for angiogenesis 15. In order to achieve a more permanent inhibition in vivo via non-viral transfection, we constructed plasmid targeting PHD2 (shPHD2) by inserting the short hairpin structure downstream of H1 promoter in a pSuper vector. A hypoxia sensing 5xHRE-SV40 promoter driving Fluc cassette was also inserted into the backbone of pSuper vector. The 5 copies of hypoxia response element (5xHRE) derived from the erythropoietin gene are activated through binding of the HIF-1 complex 16, and thus allow us to monitor the efficacy of the upstream shPHD2 knockdown compared to the upstream shScramble control (Figure 2a). C2C12 cells were transfected with shPHD2 and shScramble plasmid, respectively, and cultured in the incubator with 1% oxygen for 48 hrs or under normoxic (23% oxygen) condition as control. In the normoxic condition, cells transfected with shPHD2 had significantly higher Fluc bioluminescence signals compared to cells transfected with shScramble control, indicating increased binding of 5xHRE-SV40 promoter by HIF-1α following shPHD2 knockdown (Figure 2b). As expected, a similar but more robust trend was observed when the cells were exposed to hypoxic condition. To confirm the imaging signals, nuclear extracts were isolated and Western blot analysis performed for detection of HIF-1α protein. As shown in Figure 2c, robust HIF-1α stabilization was observed following exposure to hypoxia in shPHD2 transfected cells. The protein level was increased up to 50% after shPHD2 transfection. Upregulation of the HIF-1α pathway has been shown to activate several downstream genes responsible for stimulation of angiogenesis 17. To examine if upregulation of HIF-1α via shRNA knockdown of PHD2 can exert similar effects, total RNAs were extracted from C2C12 cells transfected with shPHD2. As shown in Figure 2d, six genes related to angiogenesis (bFGF, tranferin, FLT, KDR, TGF, PAI-1) were increased by ~30% after shPHD2 treatment. Thus, both physiologic hypoxia and PHD2 knockdown can effectively stabilize HIF-1α and induce HIF-1α dependent gene activation in cell cultures.
Figure 2. In vitro characterization of mouse shPHD2.
(a) Schema of classic hairpin carrying the site-2 sequence (shPHD2) and control hairpin carrying the scramble sequence (shScramble). The H1 promoter drives the expression of a hairpin structure in which the sense and antisense strands of the siRNA are connected by a 9-bp long loop sequence. In addition, a separate 5xHRE-SV40 promoter driving firefly luciferase (Fluc) is used to track shRNA activity in vitro and in vivo. 5x HRE, 5 repeat of hypoxia response elements; Sv40, simian virus 40. (b) In vitro imaging results indicate that Fluc signals increased significantly in respone to shPHD2 therapy during both normoxia and hypoxia conditions via binding of HIF-1α protein on the 5xHRE binding site. In addition, there were significant Fluc signal differences between shPHD2 and shScramble under normoxia (P=0.043) and hypoxia (P=0.025) states. (c) Similarly, Western blot data show that levels of HIF-1α protein were more robust after shPHD2 plasmid transfection during normoxia and and 6 hr hypoxia incubation. (d) RT-PCR analysis confirmed significant upregulation among 6 genes involved in angiogenesis due to activation of the HIF-1α protein from knocking down PHD2. GAPDH was used as the loading control.
Tracking shPHD2 vector using bioluminescence imaging in living animals
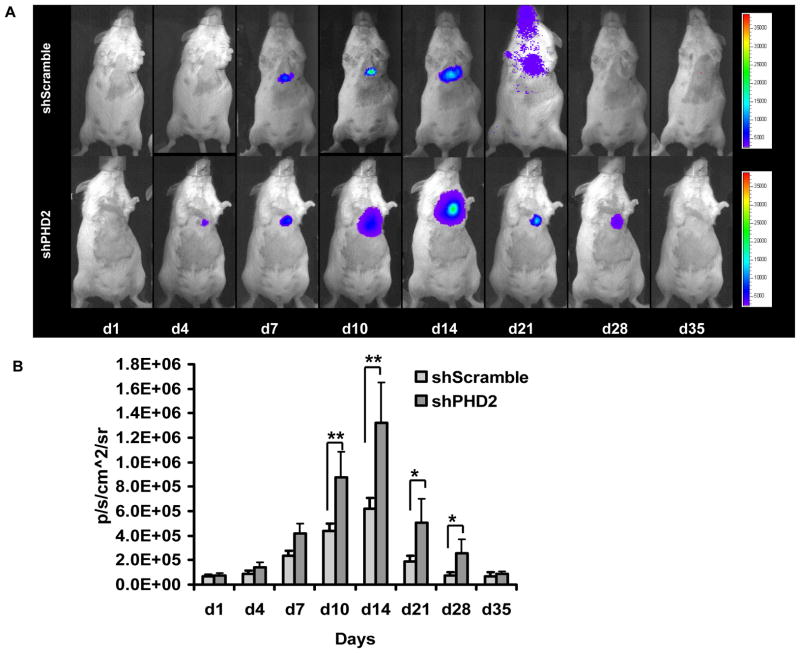
Previously, Natarajan and colleagues have demonstrated the feasibility of small interfering RNA (siRNA) therapy for attenuating myocardial ischemia reperfusion injury. However, subsequent analysis showed that the actual knockdown target was to murine pro-collagen prolyl 4-hydroxylase-2 rather than HIF prolyl 4-hydroxylase-2 18. Here we confirmed our selection target with the GenBank database. Instead of using siRNA fragments which are only stable in vivo for 72 hours, we selected shRNA plasmid. However, at present, the duration of shRNA mediated expression is unknown. Thus, we incorporated the 5xHRE-SV40 driving Fluc gene to track the shRNA expression activity. To evaluate the pharmacokinetics of shRNA in vivo, we injected the two shRNA plasmids into mice with myocardial infarction and followed their gene expression via Fluc bioluminescence imaging (Figure 3a). As expected, mice injected with shPHD2 plasmid (bottom row) had significantly higher Fluc activity compared to mice injected with shScramble plasmid (top row). This can be attributed to the efficient knockdown of PHD2, resulting in more HIF-1α protein binding to the 5xHRE-Sv40 promoter site. For control animals injected with shScramble, endogenous activation of HIF-1α following myocardial infarction led to visible but lower Fluc signals. Quantitative analyses of Fluc activities for both groups are shown in Figure 3b. Overall, infarcted animals had significantly higher activation of Fluc compared to non-infarcted animals during the first 2–4 week period.
Figure 3. Molecular imaging of shRNA plasmid fate after intramyocardial delivery.
(a) Following myocardial infarction, activation of HIF-1α protein binds to 5xHRE site to activate Fluc expression. Fluc signals are more robust at weeks 1 and 2. Furthermore, infarcted mice injected with shPHD2 (bottom row) had more robust Fluc signals compared to infarcted mice injected with shScramble (top row) due to knocking down of PHD2, which result in more HIF-1α protein binding to 5xHRE site. (b) Detailed quantitative analysis of Fluc bioluminescence signals from all animals injected with shScramble or shPHD2 plasmid with LAD ligation. Signal activity is expressed as p/sec/cm2/sr.
Injection of shPHD2 plasmid improved left ventricular ejection function
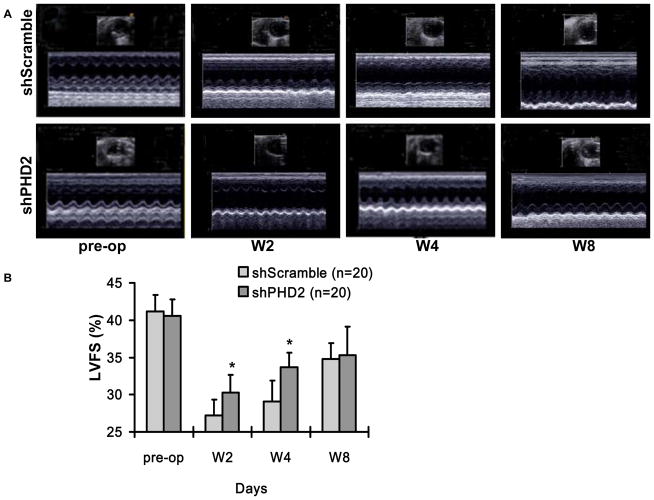
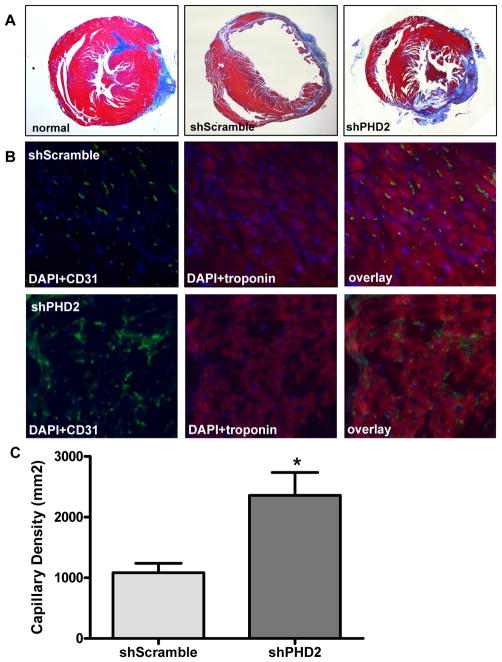
Following degradation of PHD2 by hypoxia or pharmacologic inhibition, the HIF-1α becomes stabilized and binds to the β-subunit of HIF-1 to activate transcription of several genes favorable for anti-apoptosis, neovascularization, and other physiological processes 19, 20. To examine whether shPHD2 therapy can also improve cardiac function following myocardial infarction, echocardiography was performed before (day -7) and after (week 2, week 4, week 8) the LAD ligation. At day -7, there was comparable LVFS between the control shScramble group and the shPHD2 group. Following LAD ligation, the shPHD2 group had significantly higher LVFS (P=0.03) compared to the scramble group at week 2 and week 4 (Figure 4). However, this beneficial effect was no longer maintained by week 8 (shScramble: 36.8±2.1% vs. shPHD2: 38.3±3.8%, P=0.23). This is likely due to limited expression of plasmid mediated shRNA expression within the first 4 weeks only, as shown by our imaging results on Figure 3b. To confirm the functional imaging data, trichrome staining showed less infarction size for shPHD2 group compared to shScramble group at week 4. Immunohistochemistry of the peri-infarct region by CD31 staining also showed more neovascularization for the shPHD2 group compared to shScramble group (Figure 5).
Figure 4. Evaluation of cardiac function in infarcted mice following shRNA therapy.
(a) Representative echocardiogram (M-mode) of mice with LAD ligation following injection of shScramble plasmid (upper panel) versus shPHD2 plasmid (lower panel) at pre-surgery, week 2, week 4 and week 8. (b) Quantitative analysis of left ventricular fractional shortening (LVFS) between the two groups. Animals injected with shPHD2 had significant improvement in LVFS at week 4 but not at week 8.
Figure 5. Injection of shPHD2 plasmid improves myocardial neovascularization.
(a) Representative histology of infarcted heart injected with shScramble, infarcted heart injected with shPHD2, and control non-infarcted heart injected with PBS at week 4. Trichrome stains of the peri-infarct area indicate the infarction size based on collagen staining. (b) Immunofluorescence staining of CD31 endothelial marker (green) indicate small vessels in the myocardium. Cardiomyocyte staining is identified by troponin (red; 400x magnification). Nuclear staining is identified by DAPI (blue; 400x magnification). (c) Quantitative analysis of capillary density was significantly higher in the shPHD2 group compared with the control shScramble group at week 4 (P<0.01) (20x magnification).
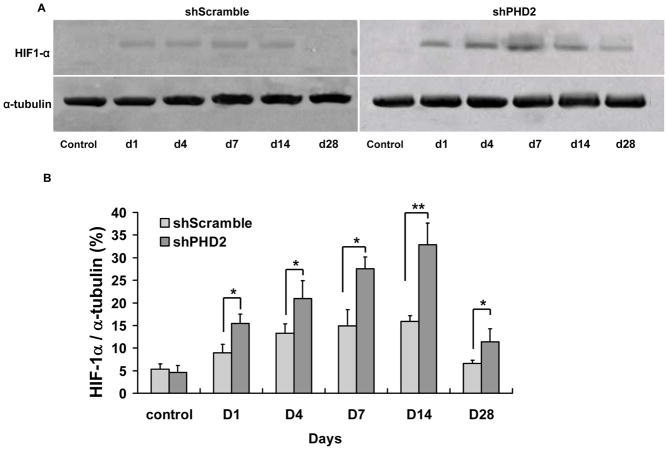
shPHD2 knockdown mediates HIF-1a upregulation in myocardial tisues
Our novel shPHD2 construct has two main components: H1 promoter driving knockdown of PHD2 and 5xHRE-SV40 promoter driving Fluc for noninvasive imaging. Based on our Fluc imaging data from Figure 3b, the peak expression of shRNA plasmid following myocardial infarction occurs around week 2. To further confirm the in vivo imaging data, we assayed for HIF-1α protein expression of explanted hearts at day 1, day 4, day 7, day 14, and day 28 following shPHD2 plasmid therapy (Figure 6a). Quantitative analysis of the Western blot indicates that HIF-1α proteins were significantly higher in the shPHD2 treated hearts compared to shScramble treated hearts starting at day 1. Protein levels peaked at day 14 and returned back to baseline levels by week 4 (Figure 6b). Overall, these results are consistent with the changes in molecular imaging signals. Taken together, these data suggest that shPHD2 plasmid mediated knockdown of PHD2 resulted in early activation of HIF-1α expression, which in turn led to downstream activation of several angiogenic genes, generating neovascularization and subsequent improvement of cardiac function at week 4 following myocardial infarction.
Figure 6. Confirmation of HIF-1α activation in postmortem explanted hearts.
(a) In vivo Western blots for day 1, day 4, day 7, day 14, and day 28 heart samples injected with shScramble (top) versus shPHD2 (bottom). Significant upregulation of HIF-1α can be seen in the shPHD2 therapy group at day 14, coinciding with most robust Fluc bioluminescence imaging signal during the same time period. (b) Quantitative densitometric analysis of HIF-1α protein levels following shScramble and shPHD2 injections show a similar trend compared to the in vivo imaging results.
DISCUSSION
In this paper, we describe a novel shRNA therapy method which can also be tracked by noninvasive molecular imaging in a murine model of myocardial infarction. The major findings can be concluded as follows: (1) shRNA can be expressed consistently with two H1 promoters driving sense and anti-sense fragment, respectively. The sense and anti-sense fragment anneal automatically in cytoplasm to exert their knocking down effects; (2) down-regulation of the mouse PHD2 gene by plasmid mediated short hairpin RNA interference (shPHD2) leads to activation of downstream angiogenic genes and proteins involved in the hypoxia response pathway as assessed by both in vitro and in vivo assays; (3) direct injection of shRNA targeting PHD2 can improve ventricular function and enhance neoangiogenesis in a mouse model of myocardial infarction during 4 week follow-up; (4) importantly, the pharmacokinetics of shRNA plasmid delivery can be monitored noninvasively in living subjects by a novel 5xHRE-SV40 binding site driving Fluc reporter gene; (5) finally, a time-dependent decrease of Fluc signal activity was observed within a 4-week period due to plasmid degradation, which likely explains for the loss of cardiac functional recovery at 8 week follow-up.
RNA interference (RNAi) is an innate biological phenomenon that has evolved during mammalian evolution 21. Biologically, RNAi has an important role for the transient and long-term blocking protein expression. It is achieved by loading the RNA interference silencing complex (RISC) with a short single stranded antisense RNA that is complementary to a target mRNA 22, 23. One common therapeutic approach is to inject small inhibitory RNA (siRNA) fragments into mice by intraperitoneal or tail vein routes 18, 24. However, this approach is often limited by the instability of siRNA fragments in target organs. For instance, siRNA is stable in the heart for only 72 hours 18 whereby naked plasmids can remain stable in the heart up to 8 weeks 25. Building upon the experience from these previous studies, we combined the siRNA and naked plasmid approach to induce more effective long-term RNA interference. We were able to activate an upstream HIF-1α pathway gene by knocking down PHD2 and induce neovasculogenesis. Overall, our data concur with other studies describing short-term targeting of the murine pro-collagen prolyl 4-hydroxylase-2 by siRNA 18, 24 and further expand by providing longitudinal pharmacokinetics data of shRNA therapy using molecular imaging analysis.
In this study, we selected PHD2 as the knockdown target. PHD2 is an upstream negative regulatory gene in HIF-1 pathway. During hypoxia, when HIF-1α is stabilized, HIF-1 mediates transcriptional responses by binding to hypoxia-responsive elements (HRE) present on a series of target genes involved in metabolic adaptation, hematopoiesis, angiogenesis, and apoptosis 12, 26. Under normal oxygenated conditions, HIF-1α is hydroxylated on two conserved proline residues, proline 402 or proline 564, by a family of prolyl-4-hydroxylases (PHDs) 27–29. Several studies have demonstrated that PHD inhibition recapitulate various cellular and physiology responses to hypoxia or preconditioning stimuli. These include HIF-1α stabilization, the induction of hypoxia inducible genes (e.g. HO-1 and GLUT-1), stimulation of angiogenesis, and protection against metabolic stress 30–32. Importantly, recent evidence suggests that the expression of a single angiogenic factor such as VEGF alone may not be sufficient for the functional revascularization of ischemic tissues 33. Thus, newer approaches based on upregulation of the upstream transcriptional factor HIF-1 may be a more natural choice. HIF-1 is known to control the expression of over 60 genes that affect cell survival and metabolism in adverse conditions, including VEGF 8, insulin-like growth factor (IGF) 9, fibroblast growth factor (FGF) 4, erythropoietin 10, nitric oxide synthase (NOS) 11, among others. Based on those previous studies, HIF-1 plays a critical role in a variety of physiological processes, and upregulation HIF-1α through PHD2 knock-down represents a potentially new target in the field of cardiovascular gene therapy.
In this study, we were able to track the HIF-1α upregulation through a novel noninvasive molecular imaging approach, avoiding the sampling biases and errors that may occur when groups of animals are sacrificed at different time points 34. 5xHRE-SV40 promoter was inserted in front of the Fluc reporter gene. This hypoxia sensing construct can reflect the effects of shRNA plasmid expression through HIF-1α binding to the HRE element. For in vivo imaging signals, the plasmid expression reached peak activities between week 1 and week 2 (Figure 3b). These results concur with the Western blot data of explanted hearts shown in Figure 6, which indicate that the HIF-1α activity (upregulated by shPHD2 knock-down) also increased during week 1 to week 2 and became degraded by week 4. Furthermore, the echocardiography data showed heart function improvement within the first month, confirming the Western blot and molecular imaging results.
Although the shPHD2 plasmid produced a therapeutic effect in this study, our experiment has two limitations. First, we observed robust activation of downstream angiogenic genes such as bFGF, transferin, FLT, KDR, TGF, and PAI-1 after shRNA plasmid transfection in vitro. However, surprisingly, we did not observe significant changes in VEGF level in vitro after shPHD2 delivery. The lack of VEGF activation following inhibition of PHD2 has been described previously in the cancer literature27. Other studies have also demonstrated that another pathway known as asparaginyl hydroxylase might also inhibit HIF-1α transactivation 35, 36. Therefore, it is possible that knocking down two hydroxylase genes (prolyl hydroxylase-2 and asparaginyl hydroxylase) simultaneously may yield even more powerful therapeutic results. Second, a time-dependent decrease of bioluminescence signal activity was observed within this time period, indicating a loss of the shRNA plasmid. Adoption of newer vectors that are less immunogenic such as minicircles 37, 38, lentivirus 39, or AAV40, may prolong gene expression and provide a more persistent functional recovery. Ongoing studies are evaluating dual knockdown targets carried by these less immunogenic vectors.
In summary, non-viral gene therapy through shRNA is a rapidly evolving area of investigation. With further validation, knocking down one or more regulatory factors involved in angiogenesis pathways as described here could provide a new avenue for treating myocardial ischemia. Furthermore, we believe molecular imaging can be a valuable tool in monitoring the localization and activityof the shRNA vectors used for cardiovascular therapy. The in vivo information gathered is already generating useful insights and will enable better understanding of shRNA activity and mechanism in living subjects.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NIH HL089027 (JCW), NIH HL074883 (JCW), and AHA Postdoctoral Fellowship (MH).
References
- 1.Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell CJ, Roger V, Rumsfeld J, Sorlie P, Steinberger J, Thom T, Wasserthiel-Smoller S, Hong Y. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115(5):e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Kim MC, Kini A, Sharma SK. Refractory angina pectoris: mechanism and therapeutic options. J Am Coll Cardiol. 2002 Mar 20;39(6):923–934. doi: 10.1016/s0735-1097(02)01716-3. [DOI] [PubMed] [Google Scholar]
- 3.Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003 Jun;9(6):694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- 4.Grines CL, Watkins MW, Helmer G, Penny W, Brinker J, Marmur JD, West A, Rade JJ, Marrott P, Hammond HK, Engler RL. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation. 2002 Mar 19;105(11):1291–1297. doi: 10.1161/hc1102.105595. [DOI] [PubMed] [Google Scholar]
- 5.Hedman A, Kivela A, Vanninen E, Mussalo H, Kauppila E, Simula S, Narvanen O, Rantala A, Peuhkurinen K, Nieminen MS, Laakso M, Yla-Herttuala S. Safety and feasibility of catheter-based local intracoronary vascular endothelial growth factor gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia: phase II results of the Kuopio Angiogenesis Trial (KAT) Circulation. 2003 Jun 3;107(21):2677–2683. doi: 10.1161/01.CIR.0000070540.80780.92. [DOI] [PubMed] [Google Scholar]
- 6.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: Vascular endothelial growth factor in Ischemia for Vascular Angiogenesis. Circulation. 2003 Mar 18;107(10):1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 7.Pislaru S, Janssens SP, Gersh BJ, Simari RD. Defining gene transfer before expecting gene therapy: putting the horse before the cart. Circulation. 2002 Jul 30;106(5):631–636. doi: 10.1161/01.cir.0000019621.18368.b7. [DOI] [PubMed] [Google Scholar]
- 8.Vincent KA, Feron O, Kelly RA. Harnessing the response to tissue hypoxia: HIF-1 alpha and therapeutic angiogenesis. Trends Cardiovasc Med. 2002 Nov;12(8):362–367. doi: 10.1016/s1050-1738(02)00186-x. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda R, Hirota K, Fan F, Jung YD, Ellis LM, Semenza GL. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J Biol Chem. 2002 Oct 11;277(41):38205–38211. doi: 10.1074/jbc.M203781200. [DOI] [PubMed] [Google Scholar]
- 10.Stolze I, Berchner-Pfannschmidt U, Freitag P, Wotzlaw C, Rossler J, Frede S, Acker H, Fandrey J. Hypoxia-inducible erythropoietin gene expression in human neuroblastoma cells. Blood. 2002 Oct 1;100(7):2623–2628. doi: 10.1182/blood-2001-12-0169. [DOI] [PubMed] [Google Scholar]
- 11.Sandau KB, Fandrey J, Brune B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001 Feb 15;97(4):1009–1015. doi: 10.1182/blood.v97.4.1009. [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Experimental & molecular medicine. 2004 Feb 29;36(1):1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 13.Miyagishi M, Taira K. U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nature biotechnology. 2002;20(5):497–500. doi: 10.1038/nbt0502-497. [DOI] [PubMed] [Google Scholar]
- 14.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113(7):1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warnecke C, Griethe W, Weidemann A, Jürgensen JS, Willam C, Bachmann S, Ivashchenko Y, Wagner I, Frei U, Wiesener M, Eckardt K-U. Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. The FASEB journal. 2003;17(9):1186–1188. doi: 10.1096/fj.02-1062fje. [DOI] [PubMed] [Google Scholar]
- 16.Ruan H, Su H, Hu L, Lamborn KR, Kan YW, Deen DF. A hypoxia-regulated adeno-associated virus vector for cancer-specific gene therapy. Neoplasia. 2001;3(3):255–263. doi: 10.1038/sj.neo.7900157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brahimi-Horn C, Berra E, Pouysségur J. Hypoxia: the tumor’s gateway to progression along the angiogenic pathway. Trends in cell biology. 2001;11(11):S32–S36. doi: 10.1016/s0962-8924(01)02126-2. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA., 3rd Hypoxia inducible factor-1 activation by prolyl 4-hydroxylase-2 gene silencing attenuates myocardial ischemia reperfusion injury. Circulation research. 2006 Jan 6;98(1):133–140. doi: 10.1161/01.RES.0000197816.63513.27. [DOI] [PubMed] [Google Scholar]
- 19.Zagórska A, Dulak Jz. HIF-1: the knowns and unknowns of hypoxia sensing. Acta biochimica polonica. 2004;51(3):563–585. [PubMed] [Google Scholar]
- 20.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nature medicine. 2003;9(6):677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 21.Grimm D, Streetz KL, Jopling CL, Storm TA, Pandey K, Davis CR, Marion P, Salazar F, Kay MA. Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature. 2006;441(7092):537–541. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 22.Gou D, Narasaraju T, Chintagari NR, Jin N, Wang P, Liu L. Gene silencing in alveolar type II cells using cell-specific promoter in vitro and in vivo. Nucleic acids research. 2004;32(17):e134. doi: 10.1093/nar/gnh129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannon GJ. RNA interference. Nature. 2002 Jul 11;418(6894):244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 24.Natarajan R, Salloum FN, Fisher BJ, Ownby ED, Kukreja RC, Fowler AA., 3rd Activation of hypoxia-inducible factor-1 via prolyl-4 hydoxylase-2 gene silencing attenuates acute inflammatory responses in postischemic myocardium. American journal of physiology. 2007 Sep;293(3):H1571–1580. doi: 10.1152/ajpheart.00291.2007. [DOI] [PubMed] [Google Scholar]
- 25.Li K, Welikson RE, Vikstrom KL, Leinwand LA. Direct gene transfer into the mouse heart. Journal of Molecular and Cellular Cardiology. 1997;29(5):1499–1504. doi: 10.1006/jmcc.1997.0389. [DOI] [PubMed] [Google Scholar]
- 26.Indovina P, Collini M, Chirico G, Santini MT. Three-dimensional cell organization leads to almost immediate HRE activity as demonstrated by molecular imaging of MG-63 spheroids using two-photon excitation microscopy. FEBS letters. 2007 Feb 20;581(4):719–726. doi: 10.1016/j.febslet.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007 Apr 26; doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 28.Chan DA, Sutphin PD, Denko NC, Giaccia AJ. Role of prolyl hydroxylation in oncogenically stabilized hypoxia-inducible factor-1alpha. The Journal of biological chemistry. 2002 Oct 18;277(42):40112–40117. doi: 10.1074/jbc.M206922200. [DOI] [PubMed] [Google Scholar]
- 29.Chan DA, Sutphin PD, Yen SE, Giaccia AJ. Coordinate regulation of the oxygen-dependent degradation domains of hypoxia-inducible factor 1 alpha. Molecular and cellular biology. 2005 Aug;25(15):6415–6426. doi: 10.1128/MCB.25.15.6415-6426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asikainen TM, Ahmad A, Schneider BK, Ho WB, Arend M, Brenner M, Gunzler V, White CW. Stimulation of HIF-1alpha, HIF-2alpha, and VEGF by prolyl 4-hydroxylase inhibition in human lung endothelial and epithelial cells. Free radical biology & medicine. 2005 Apr 15;38(8):1002–1013. doi: 10.1016/j.freeradbiomed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Asikainen TM, Schneider BK, Waleh NS, Clyman RI, Ho WB, Flippin LA, Gunzler V, White CW. Activation of hypoxia-inducible factors in hyperoxia through prolyl 4-hydroxylase blockade in cells and explants of primate lung. Proceedings of the National Academy of Sciences of the United States of America. 2005 Jul 19;102(29):10212–10217. doi: 10.1073/pnas.0504520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasiganesan H, Sridharan V, Wright G. Prolyl hydroxylase inhibitor treatment confers whole-animal hypoxia tolerance. Acta physiologica (Oxford, England) 2007 Jun;190(2):163–169. doi: 10.1111/j.1748-1716.2007.01676.x. [DOI] [PubMed] [Google Scholar]
- 33.Luttun A, Tjwa M, Moons L, Wu Y, Angelillo-Scherrer A, Liao F, Nagy JA, Hooper A, Priller J, De Klerck B, Compernolle V, Daci E, Bohlen P, Dewerchin M, Herbert J-M, Fava R, Matthys P, Carmeliet G, Collen Ds, Dvorak HF, Hicklin DJ, Carmeliet P. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nature medicine. 2002;8(8):831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 34.Chang GY, Xie X, Wu JC. Overview of stem cells and imaging modalities for cardiovascular diseases. Journal of nuclear cardiology. 2006;13(4):554–569. doi: 10.1016/j.nuclcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Vincent KA, Feron O, Kelly RA. Harnessing the response to tissue hypoxia: HIF-1 alpha and therapeutic angiogenesis. Trends in cardiovascular medicine. 2002;12(8):362–367. doi: 10.1016/s1050-1738(02)00186-x. [DOI] [PubMed] [Google Scholar]
- 36.Hirota K, Semenza GL. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochemical and biophysical research communications. 2005;338(1):610–616. doi: 10.1016/j.bbrc.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z-Y, He C-Y, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Human gene therapy. 2005;16(1):126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- 38.Chen Z-Y, He C-Y, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Molecular therapy. 2003;8(3):495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 39.Higuchi K, Medin JA. Lentiviral vectors for gene therapy of heart disease. Journal of cardiology. 2007;49(1):1–11. [PubMed] [Google Scholar]
- 40.Su H, Huang Y, Takagawa J, Barcena A, Arakawa-Hoyt J, Ye J, Grossman W, Kan YW. AAV serotype-1 mediates early onset of gene expression in mouse hearts and results in better therapeutic effect. Gene therapy. 2006;13(21):1495–1502. doi: 10.1038/sj.gt.3302787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.