Abstract
Background
Embryonic stem (ES) cells are distinguished by their capacity for self-renewal and pluripotency. Here we characterize the differentiation of ES cell-derived endothelial cells (ESC-ECs), use novel molecular imaging techniques to examine their survival following transplantation into the heart, and determine the therapeutic efficacy of ESC-ECs for restoration of cardiac function following ischemic injury.
Methods and Results
Murine ES cells were transfected with vascular endothelial cadherin promoter driving enhanced green fluorescence protein (pVE-cadherin-eGFP). Differentiation of ES cells to ECs was performed by FACS analysis of both Flk-1 (early EC marker at day 4) and VE-cadherin (late EC marker at day 8). After isolation, these ESC-ECs expressed endothelial cell markers similar to adult mouse lung endothelial cells, formed vascular-like channels, and had high metabolism of Dil-Ac-LDL. For in vivo imaging, ES cells were transduced with an ubiquitin promoter driving firefly luciferase and monomeric red fluorescence protein (pUb-Fluc-mRFP). A robust correlation exists between Fluc signals and cell numbers by ex vivo imaging analysis (R2=0.98) and by in vitro enzyme assay (R2=0.94). Afterwards, 5x105 ESC-ECs or PBS (as control) was injected into the hearts of mice undergoing LAD ligation. (n=15 per group). Bioluminescence imaging showed longitudinal survival of transplanted ESC-ECs for ~8 weeks. Echocardiography demonstrated significant functional improvement in the ESC-EC group compared to control (P<0.05). Finally, postmortem analysis confirmed increased presence of small capillaries and venules in the infracted zones by CD31 staining.
Conclusion
This is the first study to track the fate and function of transplanted ESC-ECs in the heart. With further validation, these ESC-ECs could become a valuable source of cell therapy for induction of angiogenesis in the treatment of myocardial ischemia.
Keywords: embryonic stem cells, differentiation, endothelial cells, ischemic heart disease, molecular imaging
INTRODUCTION
Coronary artery disease (CAD) is the leading cause of morbidity and mortality in the US. Current treatments fail to address the underlying cause of heart failure, which are cell loss and tissue scarring 1. Stem cells may overcome these problems by promoting neovascularization, inhibiting cardiomyocyte apoptosis, and recruiting resident stem cells 2. Several elegant studies demonstrate that endothelial progenitor cells from bone marrow 3,4 or peripheral blood 5,6 can enhance angiogenesis and contribute to functional regeneration of infarcted myocardium. These exciting results have led to a pilot clinical trial showing that injection of endothelial progenitor cells can improve global left ventricular function in patients with myocardial infarction 7. However, other studies have suggested that the endothelial progenitor cells present in patients with CAD are both diminished in quantity 8 and angiogenic capacity 9. These factors may limit the therapeutic usefulness of these cells as shown in a recent clinical trial 10.
With their unlimited self-renewal and pluripotency capacity, ES cells represent a new and exciting avenue of stem cell therapy. In cell culture, mouse and human ES cells can differentiate to endothelial cells through successive maturation steps as manifest by endothelial-specific markers such as fetal liver kinase 1 (Flk-1), platelet endothelial cell adhesion molecule (PECAM), VE-cadherin, von Willebrand Factor (vWF), and endothelial nitric oxide synthase (eNOS) 11,12. In addition, these cells can participate in vasculogenesis by matrigel assay 13 and incorporate Dil-ac-LDL 14, two hallmarks of mature endothelial cell function. However, no study has addressed the behavior of these cells after transplantation into the heart.
In this study, we hypothesize that ESC-ECs can be a viable source of stem cell therapy for improving cardiac function after myocardial infarction. Importantly, fate of transplanted cells can also be tracked longitudinally and quantitatively using novel molecular imaging techniques.
METHODS
Culture and maintenance of mouse ES cells
The murine ES-D3 cell line (CRL-1394) derived from SV129 mice was obtained from the American Type Culture Collection (ATCC; Manassas, VA). The ES cells were electroporated with linearized pVE-eGFP-IE plasmid carrying pVEcadherin-eGFP-5′Int1 enhancer (gift from S. Nishikawa, Kyoto, University) with neomycin resistant gene for selection of stable clones 15.
Differentiation and isolation of ESC-ECs
ES cell differentiation was performed with minor modifications from previous protocols 11,12. Briefly, 3×104 cells/well were transferred to collagen IV-coated 6-well plates (Becton-Dickinson, Mountain View, CA), cultured for 4 days, and sorted for Flk-1 (early EC marker). Flk-1+ cells were re-plated in the same medium supplemented with 50 ng/mL of VEGF (R&D Systems). After another 4 days of culturing, Flk-1+ cells were stained with VE-cadherin antibody (BD Pharmingen, San Jose, CA). Next, VE-cadherin (late EC marker) and eGFP double positive cells were isolated using FACS Vantage (Becton Dickinson). The cells were maintained on EGM-2 medium (Clonetics, San Diego, CA) for 1–2 passages on fibronectin-coated plates (Invitrogen, Carlsbad CA) for subsequent experiments. Control, untransfected ES cells were differentiated under same conditions.
In vitro characterization of ESC-ECs
The Dill-labeled acetylated low-density lipoprotein (Dil-Ac-LDL) uptake assay, matrigel assay, and RT-PCR were used to confirm endothelial cell phenotype of these Flk-1+/VE-cadherin+ purified cells. Briefly, ESC-ECs were incubated with 10 μg/ml of Dil-Ac-LDL (Molecular Probes, Eugene, OR) at 37 °C for 6 hours prior to detection with fluorescence microscopy as described 14. The formation of vascular-like structures was assessed by seeding cells in 12-well plates coated with Matrigel (BD Pharmingen) and incubating them at 37° for 24 hours as described 12. For RT-PCR analysis, total RNA was purified using Trizol (Invitrogen) from undifferentiated ES cells at day 0, differentiated ES cells at day 4 (after Flk+ sort), differentiated ES cells at day 8 (after VE-cadherin+/eGFP+ double sort), and adult mouse microvascular endothelial cells (obtained from lung lavage) as positive control. Primer sequences for these specific genes (CD31, Flk-1, eNOS, vWF, Oct-4, β-actin) are listed in Supplementary Table 1.
Transduction of ES cells with novel double fusion reporter gene
In order to track transplanted cells in vivo, ES cells were also transduced with self-inactivating lentiviral vector carrying an ubiquitin promoter driving Fluc and eGFP at multiplicity of infection (MOI) of 10. Stable clones were isolated by FACS for eGFP expression. Fluc reporter gene activity was confirmed ex vivo using the Xenogen IVIS 200 system (Xenogen, Alameda, CA) and in vitro using Fluc enzyme assay as described 16. Fluc enzyme activity was expressed as relative light unit per milligram protein (RLU/mg).
Animal surgery and ESC-EC transplantation
Ligation of the mid left anterior descending (LAD) artery was performed in adult female SV129 mice (Charles River Laboratories, Wilmington, MA) by a single experienced surgeon (AYS). Infarction was confirmed by myocardial blanching and EKG changes. Animals were then injected with 5.0x105 ESC-ECs at the peri-infarct zone (n=15) or 25 μL of PBS as control (n=15). Study protocols were approved by the StanfordAnimal Research Committee.
Optical bioluminescence imaging of transplanted cell survival
Cardiac bioluminescence imaging was performed using the Xenogen IVIS 200 system. After intraperitoneal injection of reporter probe D-Luciferin (150 mg luciferin/kg), animals were imaged for 1–10 minutes. The same mice were imaged for up to 8 weeks. Imaging signals were quantified in units of maximum photons per second per centimeter square per steridian (photons/sec/cm2/sr) as described 16.
Left ventricular functional analysis with echocardiogram
Echocardiography was performed by a blinded investigator (AYS) using the Siemens-Acuson Sequioa C512 system equipped with a multi-frequency (8–14 MHz) 15L8 transducer. Analysis of M-mode images was performed using Siemens built-in software. Left ventricular end diastolic diameter (EDD) and end-systolic diameter (ESD) were measured and used to calculate fractional shortening (FS) by the following formula: FS= [EDD-ESD]/EDD 17.
Histological examination
Explanted hearts from study and control groups were embedded into OCT compound (Miles Scientific, Elkhart, IN). Frozen sections (5 μm thick) were processed for immunostaining. To detect microvascular density (MVD) in the peri-infart area, a rat anti-CD31 (BD Pharmingen) was used. The number of capillary vessels was counted by a blinded investigator (FC) in ten randomly selected areas using a light microscope (x200 magnification).
Statistical analysis
Unless specified, data are expressed as mean ± standard deviation. For statistical analysis, ANOVA and repeated measures ANOVA with post-hoc testing as well as the two-tailed Student’s t–test was used. Differences were considered significant at P-values of <0.05.
RESULTS
Differentiation of embryonic stem cells to endothelial cells (ESC-ECs)
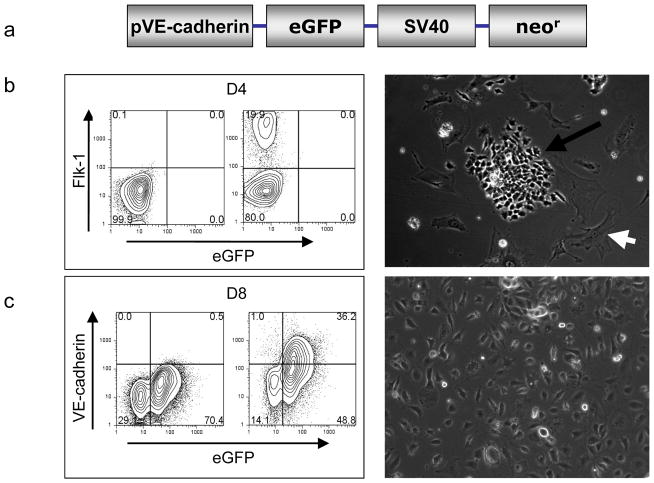
In mouse ES cells, there is a distinct expression pattern of endothelial specific genes: Flk-1 starting at day 2–3, PECAM at day 4, and VE-cadherin at day 5 12,18. To develop a reliable system of purifying mature ECs from ES cells, we used the VE-cadhern promoter driving eGFP construct for selection marker as shown in Figure 1a 15. After 4 days of culturing on collagen IV-coated plates and in differentiation medium, ~20% of ES cells expressed early EC marker (Flk-1) but not late EC marker (VE-cadherin). The outgrowth from Flk-1+ cells exhibited predominantly two morphologies: cobblestone-like endothelial colony and striated smooth muscle-like structures (Figure 1b). The Flk-1+ positive cells were subcultured with VEGF supplementation for another 4 days. Afterwards, ~36% cells were isolated by FACS using both VE-cadherin surface antibody as well as intracellular eGFP expression driven by the VE-cadherin promoter. After expansion in cell culture, these VE-cadherin+/eGFP+ cells have a morphology typical of mature endothelial cells (Figure 1c).
Figure 1.
ES cell differentiation into endothelial-like cells. (a) Schema of the endothelial cell lineage-specific promoter with VE-cadherin promoter driving eGFP and SV40 promoter driving neomycin resistant gene for antibiotic selection. (b) After 4 days on collagen IV plates, Flk-1+ positive cells can be identified by FACS and brightfield microscopy (100x magnification). (c) After another 4 days of culturing on differentiation medium, ~36% of cells express both VE- cadherin surface marker and intracellular eGFP. These double positive cells show typical endothelial cell morphology and can be expanded for additional 5–8 passages (100x magnification).
In vitro characterization of ES cell-derived endothelial cells
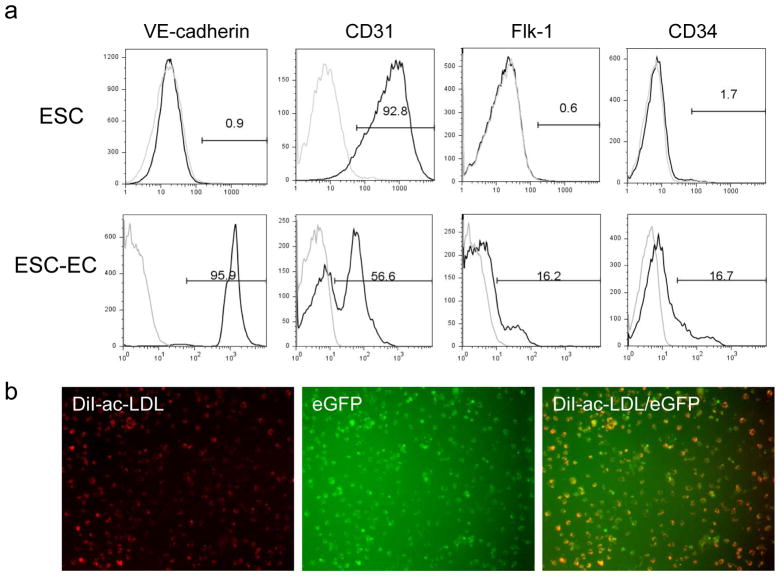
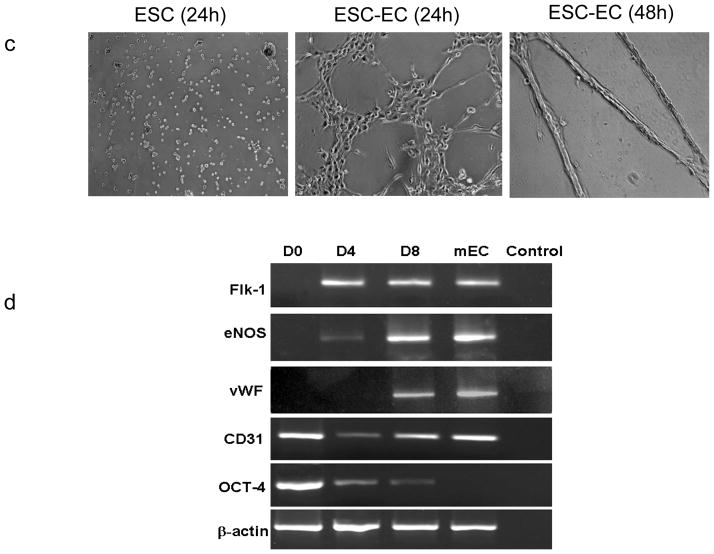
To further characterize these differentiated cells, flow cytometry was performed to analyze endothelial markers including VE-cadherin, Flk-1, CD31, and CD34 (Figure 2a). Isolated cells at day 8 typically express robust levels of VE-cadherin and modest levels of Flk-1, CD31, and CD34. By contrast, undifferentiated ES cells typically express low levels of VE-cadherin, Flk-1, and CD34 but high levels of CD31. Uptake of Lil-ac-LDL has been used to characterize mature endothelial cells 14. As shown in Figure 2b, these VE-cadherin+/eGFP+ cells have avid metabolism of Dil-ac-LDL. The characteristics of ESC-ECs were also assessed by culturing on matrigel, an extracellular matrix basement membrane that can be used to promote vascular morphogenesis of endothelial cells 13. In contrast to undifferentiated ES cells, ESC-ECs were able to spontaneously reorganize into cord-like structures (Figure 2c). To understand the temporal kinetics of endothelial marker expression in these cells, we performed RT-PCR analysis of common endothelial markers. As shown in Figure 2d, the levels of Flk-1, eNOS, and vWF increased during ES cell differentiation from day 0 to day 4 (Flk-1+ sort) to day 8 (VE-cadherin+/eGFP+ sort). The ES cell marker Oct-4 was expressed in high levels at day 0, but progressively decreased upon ES cell differentiation into ESC-ECs at days 4 and 8. Adult mouse microvascular endothelial cells showed robust endothelial marker expression but no Oct 4 expression as expected. Finally, for both FACS and RT-PCR analysis, the CD31 expression was present in both undifferentiated mouse ES cells and their differentiated derivatives, a pattern described by others 18. Thus, CD31 alone can not be used as a marker for distinguishing mouse ES cells from ECs.
Figure 2.
In vitro characterization of ESC-ECs. (a) FACS analysis of common endothelial markers in undifferentiated ES cells and differentiated ESC-ECs. (b) Uptake of Dil-ac-LDL by ESC-ECs. (c) Vasculogenic formation by ESC-ECs after 24 and 48 hours of plating. Undifferentiated ES cells do not form cord-like structures (100x magnification). (d) Expression profile of endothelial cell-specific genes at different time points: undifferentiated ES cells (day 0), Flk-1+ cells (day 4), and VE-cadherin+/eGFP+ cells (day 8). The positive control is adult mouse endothelial cells (mEC) from the lung microvasculature. The negative control has no PCR templates.
Expression of novel double fusion (DF) reporter genes
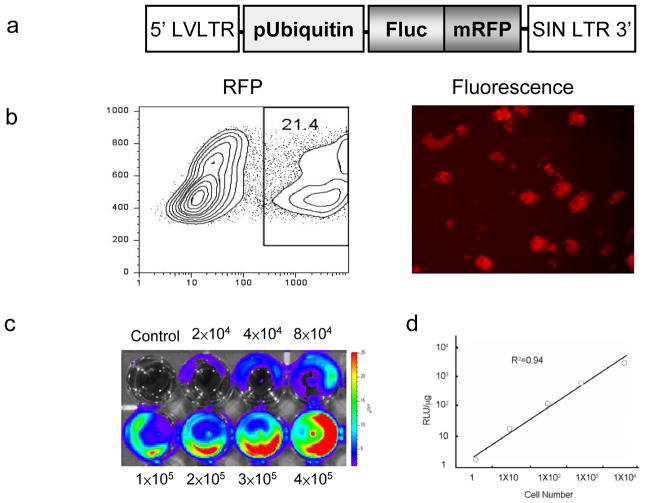
To develop an imaging assay for tracking transplanted ESC-ECs, we used a DF reporter gene consisting of Fluc-mRFP (Figure 3a). The efficiency of lentiviral transduction of mouse ES cell was ~21% (Figure 3b). These cells retained their ability to differentiate into Flk-1+ (on day 4) and VE-cadherin+/eGFP+ (day 8) endothelial-like cells (data not shown). In addition, previous studies also documented that these reporter genes do not adversely affect ES cell survival, proliferation, and differentiation 16,19. To confirm that level of Fluc expression correlated with cell numbers, ES cells were assayed ex vivo using the Xenogen IVIS system, producing a strong linear correlation (r2=0.98) (Figure 2c). Similarly, there was also a strong correlation between Fluc activity and cell numbers (r2=0.94) based on in vitro enzyme assays measured by luminometer (Figure 2d).
Figure 3.
Transduction of ES cells with double fusion reporter genes. (a) Schema of the DF reporter gene with ubiquitin promoter driving Fluc and mRFP joined by a 20-amino acid linker. (b) FACS analysis showing lentiviral transduction efficiency (~21.4%) after 48 hours. Isolated cells are strongly positive for mRFP on fluorescence microscopy. (c) Ex vivo imaging analysis of transduced cells show increasing bioluminescence signals with cell numbers (r2=0.98). (d) In vitro enzyme assay show increasing Fluc enzyme activity with cell numbers (r2=0.94).
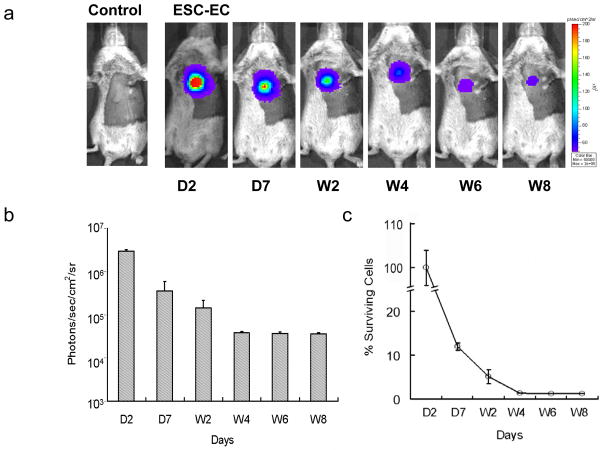
Molecular imaging of ESC-EC survival in living animals
To non-invasively assess the engraftment of ESC-ECs, bioluminescence imaging was performed for 8 weeks (Figure 4a). Cell signal was most robust immediately after transplantation, which gradually decreased from day 2 (2.95x106±1.21x105 photons/sec/cm2/sr) to day 7 (3.56x105±2.36x104) to week 2 (1.45x105±4.94x104) to week 4 (3.87x104±4.50x102) to week 6 (3.68x104±3.65x102) and to week 8 (3.60x104±2.57x103) (P<0.01 vs. control for all time points) (Figure 4b). Control animals injected with PBS showed no imaging signals as expected. When cell signals were normalized to day 2, the quantified cell survival activity was 12.2±0.8% at day 7, 5.1±1.6% at week 2, 1.3±0.1% at week 4, 1.2±0.1% at week 6, and 1.1±0.1% at week 8 (Figure 4c).
Figure 4.
Molecular imaging of ESC-EC fate after transplantation. (a) A representative animal injected with 5x105 ESC-ECs shows significant bioluminescence activity at day 2 which decreases progressively over the following 8 weeks. Control animal injected with PBS show no imaging signals as expected. (b) Detailed quantitative analysis of signals from all animals transplanted with ESC-ECs (signal activity is expressed as photons/sec/cm2/sr). (c) Donor cell survival plotted as % signal activity from day 2 to week 8.
Transplanted ESC-ECs can improve left ventricular function
Echocardiography performed 2 days before and 2 days after the LAD ligation showed comparable FS and LVEF between the two groups. However, 8 weeks after LAD ligation, the ESC-EC group had significantly higher FS compared to the control group (P<0.05) (Figure 5a). At week 8, myocardial neovascularization as assessed by capillary density was also enhanced significantly in mice receiving ESC-EC transplantation compared to PBS (Figure 5b). But relatively few surviving cells surrounding existing vasculature in the myocardium were identified, consistent with the in vivo imaging data showing weak cell signal activity at week 8.
Figure 5.
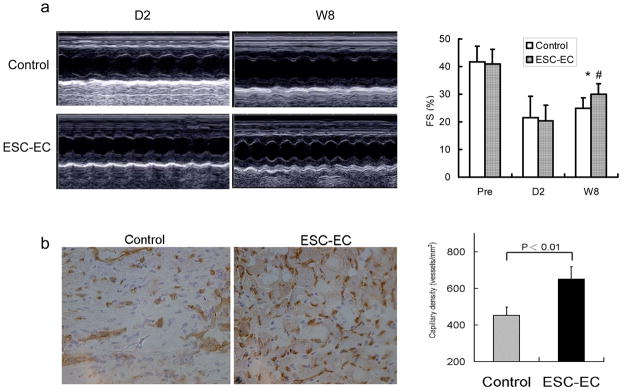
Functional evaluation of transplanted ESC-ECs. (a) Representative M-mode still frames of control and ESC-EC animals at day 2 and week 8 after LAD ligation. Echocardiographic data comparing fractional shortening (FS) between the two groups 2 days before (pre), 2 days after, and 8 weeks after LD ligation. *P<0.05 compared with control; #P<0.01 compared with day 2 (n=10 in all group). (b) Quantitative analysis of capillary density was significantly higher in the ESC-EC group compared to the control group (200x magnification).
DISCUSSION
In this study, we describe the differentiation, survival, and function of ES cell-derived endothelial cells for cardiovascular applications. The major findings can be summarized as follows: (1) ES cells can be differentiated into endothelial-like cells using a sequential combination of Flk-1+ (early EC marker) and VE-cadherin+ (late EC marker) sorting; (2) these ESC-ECs express typical endothelial markers (Flk-1, eNOS, vWF) and can uptake Dil-ac-LDL as well as participate in vasculogenesis on matrigel assay; (3) transplanted ESC-ECs can improve ventricular function and enhance neoangiogenesis in a mouse model of myocardial infarction; (4) importantly, transplanted cell fate can be monitored noninvasively within the same animals for up to 8 weeks using bioluminescence imaging; (5) however, drastic decrease of cell signal activity was observed within this time period, indicating significant donor cell loss.
In recent years, several animal studies have shown that transplantation of endothelial progenitor cells isolated from the peripheral blood or bone marrow can improve cardiac function 3–6. But whether these cells will ultimately play a role in human application requires further evaluation. For example, initial results from the TOPCARE-AMI trial showed that patients who received circulating endothelial progenitor cells had improvement in LVEF from 51±10% to 59±10% at 4 months (P<0.001) 7. However, a recent larger study from the same group showed that patients who received circulating endothelial progenitor cells had no improvement in LVEF at 3 months (−0.4±2.2; P=0.60) 10. Although the study design and patient population are different between the two trials, the discrepancy suggests that a detailed mechanistic understanding of how these transplanted cells can improve cardiac function is still lacking. It is possible that patients who have CAD as well as other comorbidities such as diabetes, hypertension, and hypercholesterolemia may posses poorly functioning endothelial cells to begin with, which may not elicit much long-term functional improvement 8,9. In this regard, positive beneficial results shown in pre-clinical studies that are based predominantly on transplanting stem cells from young healthy adult animals may not be representative of the routine clinical scenario involving older patients 6,8,9. Therefore, therapies utilizing alternative sources of cells possessing limitless potential for self-renewal may be an attractive option.
Compared to adult stem cells, ES cells are unique in their ability to differentiate into virtually all cell types, including neurons, cardiomyocytes, hepatocytes, islet cells, skeletal muscle cells, and endothelial cells 20. To date, however, the main obstacle remains a lack of reliable methodology to purify cells of interest from other unwanted cell populations. One common approach is to use cell lineage-specific promoters driving GFP or drug-resistant genes. In the case of endothelial cell isolation, most available promoters are still plagued by non-specific expression in other cell types. For instance, the vWF promoter is active in megakaryocytes, the PECAM-1 promoter is active in hematopoietic cells, and the VEGF receptor 2 promoter is active in undifferentiated ES cells 15. By contrast, the VE-cadherin promoter is known to be constitutively expressed specifically by endothelial cells 21. Building upon the experience from these studies, we used a combinatorial approach of both early (Flk-1+) 12 and late EC marker (VE-cadherin+) 18. We were able to differentiate mouse EC cells into endothelial-like cells that express surface markers similar to adult mouse endothelial cells. Upon isolation, these ESC-ECs can form tube-like structures when cultured on matrigel and can uptake Dil-ac-LDL that is a typical phenotype of mature endothelial cells. Our data also concur with other studies describing the functionality of endothelial cells isolated from mouse 12, monkey 22, and human ES cells 11.
In this study, we determined the longitudinal cell survival kinetics within the same cohort of animals animal using a novel imaging technique, avoiding the sampling biases and errors that may occur when different groups of animals are sacrificed at different time points 23. Our imaging data suggest that by week 8, <2% of the transplanted ESC-ECs are still alive. This observation conforms with other studies showing poor donor cell survival using serial histology, TUNEL apoptosis assay, or Taqman Sry PCR techniques 24. Indeed, our imaging and histologic analysis provide no definitive proof that donor derived cardiac myocytes or vasculature are being regenerated after transplantation with ESC-ECs. These findings indicate that other mechanisms such as activation of paracrine pathways may play a role 2, but additional studies will be needed in the future to test this hypothesis. Interestingly, Levenberg et al. showed that ESC-ECs, when seeded in biodegradable polymer scaffolds, can lead to long-term engraftment and formation of blood-carrying microvessels 11. Thus, tissue engineering techniques, rather than direct stem cell transplantation, may prove to be a more viable approach in the future 25.
In summary, stem cell therapy is an exciting area of investigation. With further validation, the ESC-ECs described here could provide a continual source of endothelial cells for treatment of myocardial ischemia and peripheral vascular disease. Furthermore, we believe molecular imaging will likely play a critical “watchdog” role to monitor the viability of these transplanted cells (or engineered tissues) for cardiovascular diseases and others alike. The in vivo information gathered will provide greater insight into stem cell physiology in living subjects and lay the framework for more complex, refined studies in the future.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NHLBI (JCW, JPC); AHA, ACCF-GE (JCW) as well as the Stanford Dean’s Fellowship (ZL).
Footnotes
CONFLICT OF INTERESTS: The authors declare that they have no conflicts to disclose.
References
- 1.Laflamme MA, Murry CE. Regenerating the heart. Nat Biotechnol. 2005;23:845–56. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 2.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ Res. 2005;96:151–63. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 3.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–6. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–53. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 7.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, Abolmaali ND, Vogl TJ, Hofmann WK, Martin H, Dimmeler S, Zeiher AM. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 9.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 10.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–32. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 11.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–6. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- 13.Doetschman T, Shull M, Kier A, Coffin JD. Embryonic stem cell model systems for vascular morphogenesis and cardiac disorders. Hypertension. 1993;22:618–29. doi: 10.1161/01.hyp.22.4.618. [DOI] [PubMed] [Google Scholar]
- 14.Voyta JC, Via DP, Butterfield CE, Zetter BR. Identification and isolation of endothelial cells based on their increased uptake of acetylated-low density lipoprotein. J Cell Biol. 1984;99:2034–40. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hisatsune H, Matsumura K, Ogawa M, Uemura A, Kondo N, Yamashita JK, Katsuta H, Nishikawa S, Chiba T, Nishikawa S. High level of endothelial cell-specific gene expression by a combination of the 5′ flanking region and the 5′ half of the first intron of the VE-cadherin gene. Blood. 2005;105:4657–63. doi: 10.1182/blood-2004-09-3554. [DOI] [PubMed] [Google Scholar]
- 16.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–14. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–39. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- 18.Vittet D, Prandini MH, Berthier R, Schweitzer A, Martin–Sisteron H, Uzan G, Dejana E. Embryonic stem cells differentiate in vitro to endothelial cells through successive maturation steps. Blood. 1996;88:3424–31. [PubMed] [Google Scholar]
- 19.Wu JC, Spin JM, Cao F, Lin S, Xie X, Gheysens O, Chen IY, Sheikh AY, Robbins RC, Tsalenko A, Gambhir SS, Quertermous T. Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol Genomics. 2006;25:29–38. doi: 10.1152/physiolgenomics.00254.2005. [DOI] [PubMed] [Google Scholar]
- 20.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–78. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 21.Gory S, Vernet M, Laurent M, Dejana E, Dalmon J, Huber P. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood. 1999;93:184–92. [PubMed] [Google Scholar]
- 22.Kaufman DS, Lewis RL, Hanson ET, Auerbach R, Plendl J, Thomson JA. Functional endothelial cells derived from rhesus monkey embryonic stem cells. Blood. 2004;103:1325–32. doi: 10.1182/blood-2003-03-0799. [DOI] [PubMed] [Google Scholar]
- 23.Chang GY, Xie X, Wu JC. Overview of stem cells and imaging modalities for cardiovascular diseases. J Nucl Cardiol. 2006;13:554–69. doi: 10.1016/j.nuclcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Reinecke H, Murry CE. Taking the death toll after cardiomyocyte grafting: a reminder of the importance of quantitative biology. J Mol Cell Cardiol. 2002;34:251–3. doi: 10.1006/jmcc.2001.1494. [DOI] [PubMed] [Google Scholar]
- 25.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.