Enterococcal bloodstream infections are associated with an increased risk of mortality during the first year after hematopoietic stem cell transplantation, especially in patients with vancomycin-resistant enterococci (VRE) strains. Colonization with VRE and delayed engraftment are significant risk factors for VRE bacteremia.
Abstract
Background. Enterococci are an important cause of healthcare-associated infections. We retrospectively analyzed risk factors and outcome of vancomycin-resistant enterococci (VRE) and vancomycin-sensitive enterococci (VSE) infections.
Methods. Seven hundred fifty-two patients who received hematopoietic stem cell transplants from 2004 through 2008 at the University of Minnesota were included.
Results. Ninety-three patients had enterococcal bloodstream infection (BSI) during the first year after transplant. Vancomycin resistance was observed in 66% and 31% of isolates in adults and children, respectively. Cumulative incidence of VRE and VSE bacteremia was 6.6% (95% confidence interval [CI], 4.8%–8.4%) and 5.7% (95% CI, 4.0%–7.4%), respectively. Colonization with VRE before or after transplant was a risk factor for VRE bacteremia (odds ratio [OR], 3.3 [95% CI, 1.3–8.3] and 7.0 [95% CI, 4.0–14.8], respectively). Delay in engraftment increased the incidence of VRE bacteremia from 4.5% (95% CI, 2.9–6.6) if engrafted before day 21 and to 15% (95% CI, 3.2%–38%) if engrafted between days 36 and 42. In adults, mortality 30 days after infection was 38% for both VRE (95% CI, 25%–54%) and VSE cases (95% CI, 21%–62%). The hazard ratio for all-cause mortality up to 1 year after transplant was 4.2 (95% CI, 3.1–6.9) and 2.7 (95% CI, 1.4–5.1) for patients with VRE and VSE BSIs, respectively, compared to patients without enterococcal BSI. In pediatric patients, mortality 30 days after VRE and VSE bacteremia was 20% (95% CI, 5.4%–59%) and 4.5% (95% CI, .6%–28%), respectively.
Conclusion. High rates of vancomycin resistance and association of enterococcal infections with significant mortality warrant further efforts to optimize prevention and management of these infections.
Enterococci are a significant component of the normal bacterial flora of the lower gastrointestinal tract and other mucocutaneous surfaces. Although they are of low virulence in healthy immunocompetent individuals, enterococci are the third leading cause of healthcare-associated infections in the United States, with 29% of isolates being resistant to vancomycin [1]. Vancomycin-resistant enterococci (VRE) are commonly isolated from blood and other sites in recipients of hematopoietic stem cell transplantation (HSCT) [2–7], with directly attributable mortality of preengraftment VRE bloodstream infections (BSIs) recently reported at 9% [6]. Given the complex medical condition of recipients of allogeneic HSCT, it is often difficult to attribute mortality between several coexistent causes. VRE infection may actually serve as a marker of concomitant severe illness [4]. We retrospectively reviewed cases of enterococcal bloodstream infections among allogeneic HSCT recipients at the University of Minnesota and analyzed risk factors for enterococcal BSI and the association of these infections with outcomes.
METHODS
Patients and Data Collection
Patients receiving their first allogeneic HSCT at the University of Minnesota between 1 January 2004 and 31 December 2008 were included in the study. Demographic information, transplant characteristics, infection dates, and outcomes were obtained from the University of Minnesota Blood and Marrow Transplant Database, which contains prospectively collected data on all patients receiving transplantation at our center including episodes of clinically significant infections treated at the University of Minnesota and outside facilities. Patients' electronic medical records were reviewed to verify the information on infection episodes, and sensitivity to vancomycin was recorded. Data were censored at relapse of underlying disease or second allogeneic transplant. Survival status was known for all patients included in the study. The study was reviewed and approved by the University of Minnesota Institutional Review Board.
VRE Screening
All patients admitted to the blood and marrow transplant unit were screened at admission for VRE by culture using perirectal swabs. Surveillance cultures were then performed periodically, generally weekly, throughout the initial hospitalization. The ongoing VRE surveillance included only hospitalized patients, except as clinically indicated. Patients who were found to be colonized or infected with VRE were placed under contact isolation.
Blood Cultures
Blood cultures were drawn through a tunneled central venous line or peripherally inserted central catheter in all patients with new onset of fever or with other signs of infection, at the discretion of treating physicians. After the initial positive blood culture, blood cultures were generally drawn daily until 2 consecutive cultures were negative and the patient had defervesced.
Definitions
Enterococcal bloodstream infection was defined as any blood culture growing Enterococcus species [8]. For this analysis, an Enterococcus isolate was considered vancomycin resistant if it had a minimum inhibitory concentration (MIC) of ≥32 µg/mL. The isolates were considered vancomycin sensitive if they had an MIC of ≤8 µg/mL.
Patients who developed a VRE BSI at any time during the first 365 days after transplant were included in the “VRE BSI” group. Patients who developed a BSI with vancomycin-sensitive enterococci (VSE), but never developed a VRE BSI in the first year, were placed in the “VSE BSI” group. Patients who never had an enterococcal BSI were included in the “No enterococcal BSI” group. Patients who had VRE isolated from a surveillance culture or from any other nonsterile body site were considered to be VRE colonized.
Patients without neutrophil recovery to >500/μL by day 42 were considered to have graft failure.
Statistical Methods
For descriptive tables, frequencies and incidence rates were calculated. Comparisons among groups were made using the χ2 test, Fisher exact test, or Wilcoxon test. Kaplan-Meier and cumulative incidence estimates were made for overall survival and nonrelapse mortality, respectively. Analyses used a follow-up period of 1 year and no patients were lost to follow-up. Multivariate Cox regression was used to determine risk factors for the cause-specific hazards of VRE and VSE BSI, using time-dependent covariates for colonization, graft-vs-host disease (GVHD), and engraftment. This method has been used in other studies [9, 10] to analyze time-dependent risk factors in the presence of a competing hazard of death. An alternative method, Fine and Gray regression, which does not allow for time-dependent risk factors, was also tested and showed good agreement with the Cox model for non-time-dependent factors (not reported). Multivariate Cox regression was used to test the association of BSI with 1-year overall survival, using time-dependent covariates for VRE BSI, VSE BSI, GVHD, engraftment, and colonization [11–13]. Multivariate logistic regression was used to analyze risk factors for colonization either before or after transplant.
RESULTS
Seven hundred fifty-two patients underwent their first allogeneic transplant within this 5-year timeframe. The median follow-up was 47 months, with a minimum follow-up of 1 year. Patient and transplant characteristics are shown in Table 1.
Table 1.
Patient and Transplant Characteristics
| Factors | No Enterococcal BSI Within 1 Year, No. (%) | VRE BSI, No. (%) | VSE BSI, No. (%) |
|---|---|---|---|
| Total | 659 | 50 | 43 |
| Year of transplant | |||
| 2004 | 120 (18) | 8 (16) | 6 (14) |
| 2005 | 143 (22) | 12 (24) | 10 (23) |
| 2006 | 127 (19) | 13 (26) | 8 (19) |
| 2007 | 130 (20) | 8 (16) | 12 (28) |
| 2008 | 139 (21) | 9 (18) | 7 (16) |
| Age at transplant | |||
| Median (range), years | 36 (0–74) | 35 (0–68) | 15 (0–67) |
| ≥18 years | 430 (65) | 40 (80) | 21 (49) |
| Sex (male) | 390 (59) | 36 (72) | 26 (60) |
| Diagnosis | |||
| Leukemia | 309 (47) | 31 (62) | 17 (40) |
| Lymphoma | 106 (16) | 5 (10) | 5 (12) |
| MDS | 48 (7) | 3 (6) | 4 (9) |
| Nonmalignancy | 162 (25) | 7 (14) | 14 (33) |
| Other malignancy | 34 (5) | 4 (8) | 3 (7) |
| Donor type | |||
| Related | 221 (34) | 13 (26) | 12 (28) |
| Unrelated (PBSC or marrow) | 69 (10) | 5 (10) | 7 (16) |
| Unrelated (UCB) | 368 (56) | 32 (64) | 24 (56) |
| Preparative regimen | |||
| Myeloablative | 362 (55) | 32 (64) | 27 (63) |
| No radiation | 104 (16) | 7 (14) | 11 (26) |
| Radiation | 258 (39) | 25 (50) | 16 (37) |
| Reduced intensity | 294 (45) | 18 (36) | 16 (37) |
| GVHD prophylaxis | |||
| CSA/MTX | 125 (19) | 11 (22) | 8 (19) |
| CSA/MMF | 442 (67) | 35 (70) | 25 (58) |
| Other | 92 (14) | 4 (8) | 10 (23) |
| Recipient/donor CMV serostatus | |||
| Recipient + | 351 (53) | 31 (62) | 26 (60) |
| Recipient −/Donor − | 262 (40) | 17 (34) | 15 (35) |
| Recipient −/Donor + | 42 (6) | 2 (4) | 2 (5) |
| Karnofsky/Lansky score at transplant | |||
| <90 | 77 (12) | 3 (6) | 5 (12) |
| 90–100 | 513 (78) | 41 (82) | 35 (81) |
Abbreviations: BSI, bloodstream infection; CMV, cytomegalovirus; CSA, cyclosporine; GVHD, graft-vs-host disease; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MTX, methotrexate; PBSC, peripheral blood stem cells; UCB, umbilical cord blood; VRE, vancomycin-resistant enterococcus; VSE, vancomycin-sensitive enterococcus.
Colonization
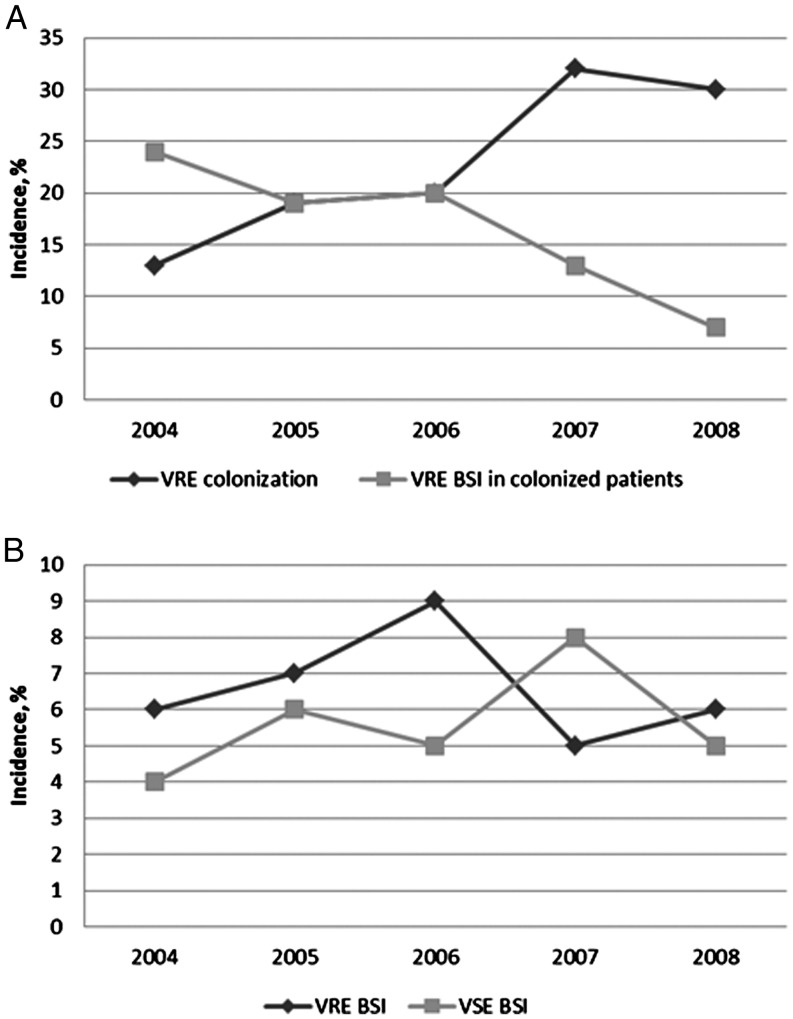
Overall, 173 (23%) of patients were colonized with VRE throughout the study period. Of these, 25% had been known to be VRE colonized prior to their transplant. Patients with an underlying diagnosis of leukemia or myelodysplastic syndrome were more likely to be colonized compared to patients with lymphoma or nonmalignancy (odds ratio [OR], 2.4 [95% confidence interval {CI}, 1.6–3.6] for leukemia and 2.6 [95% CI, 1.3–5.0] for patients with myelodysplastic syndrome). Risk of colonization was greater in patients aged >60 years (OR, 2.0 [95% CI, 1.3–3.4]) compared to younger adults. The rate of VRE colonization increased during the study period from 13% in 2004 to 30% in 2008 (P < .01, Figure 1). We observed no trend in age or diagnoses over the 5 years of study to account for the increase in colonization.
Figure 1.
Vancomycin-resistant enterococci (VRE) colonization rates and incidence of VRE and vancomycin-sensitive enterococci (VSE) bacteremia. A, Incidence of VRE colonization and incidence of VRE bacteremia in colonized patients by year. B, Incidence of VRE and VSE bloodstream infections (BSIs) by year.
Incidence of and Risk Factors for Enterococcal Bloodstream Infections
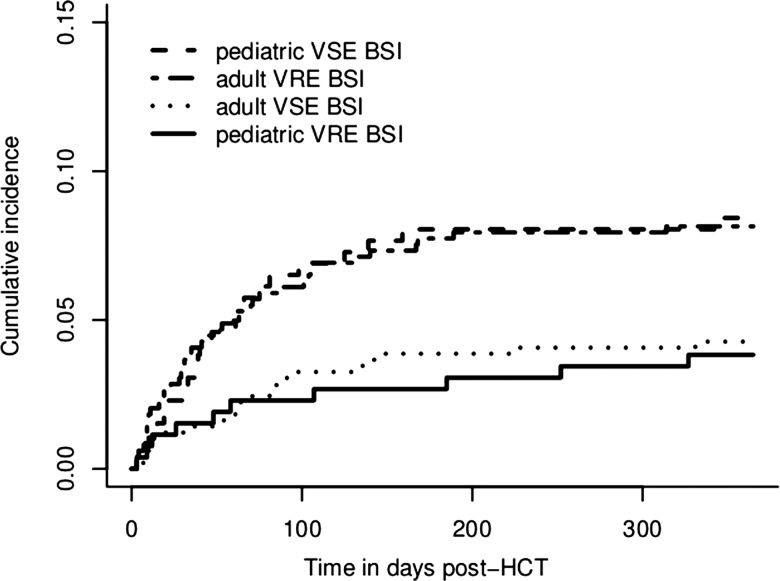
Ninety-three patients had enterococcal BSIs during the first year following transplantation. Overall, 14% (95% CI, 9.6%–21%) of patients colonized with VRE developed VRE BSI, whereas the incidence of VRE bacteremia was only 4% (95% CI, 2.8%–6.3%) in the noncolonized group. The cumulative incidence of first enterococcal BSI in the presence of the competing risk of death was 6.6% (95% CI, 4.8%–8.4%) and 5.7% (4.0%–7.4%) for VRE and VSE BSI, respectively, during the first year after transplantation. Most of the infections occurred in the first 60 days as shown in Figure 2. None of the patients with VSE bacteremia subsequently developed a VRE bacteremia. Although the rates of VRE colonization were increasing throughout the study period, the incidence of VRE BSI among colonized patients decreased significantly, resulting in similar rates of VRE BSI incidence (Figure 1). Rates of vancomycin resistance among bloodstream isolates were 66% and 31% in adults and children, respectively.
Figure 2.
Cumulative incidence of enterococcal bloodstream infections adjusted for the competing risk of death. BSI, bloodstream infection; HCT, hematopoetic cell transplantation; VRE, vancomycin-resistant enterococci; VSE, vancomycin-sensitive enterococci.
Risk factors for acquiring a VRE BSI are presented in Table 2. Colonization with VRE, delay in engraftment, and severe acute GVHD were associated with an increased risk of VRE bacteremia. Among patients who engrafted by day 42, the percentage who ever contracted VRE BSI was 4.5% (23 of 516) if engraftment occurred by day 21, 6.3% (8 of 127) if engraftment occurred during days 22–28, 11.9% (5 of 42) if engraftment occurred during days 29–35, and 15.0% (3 of 20) if engraftment occurred during days 36–42. The VRE BSI incidence was 25% (10 of 40) for patients who were alive and not engrafted by day 42. None of the risk factors tested was associated significantly with VSE bacteremia, including acute and chronic GVHD, time to engraftment, age, underlying diagnosis, and conditioning regimen.
Table 2.
Risk Factors for Vancomycin-Resistant Enterococcal Bloodstream Infection
| Factor | Risk Ratio Estimate (95% CI) | P Value |
|---|---|---|
| Colonized before HSCT | 3.3 (1.3–8.3) | .01 |
| Colonized after HSCTa | 7.7 (4.0–14.8) | <.01 |
| aGVHD (grade 3–4)a | 3.5 (1.7–7.0) | <.01 |
| cGVHDa | 1.3 (.3–5.2) | .68 |
| Engrafted by day 42a,b | 0.1 (.0–.5) | <.01 |
| Age | ||
| Age <18 years | 0.4 (.2–.9) | .03 |
| Age 18–59 years | Ref | |
| Age ≥60 years | 0.9 (.3–2.6) | .83 |
| Diagnosis | ||
| Leukemia | 1.0 (.5–1.9) | .92 |
| MDS | 0.5 (.1–1.8) | .29 |
| Other | Ref | |
| Conditioning | ||
| RIC | 0.7 (.3–1.3) | .26 |
| Myeloablative | Ref |
Abbreviations: aGVHD, acute graft-vs-host disease; cGVHD, chronic graft-vs-host disease; CI, confidence interval; HSCT, hematopoietic stem cell transplantation; MDS, myelodysplastic syndrome; Ref, reference; RIC, reduced-intensity conditioning.
a Time-dependent covariate.
b Relative risk of event for a patient who has engrafted compared to a patient who has not engrafted up to day 42 after transplant.
Outcome of Patients With Enterococcal Infections
In adult patients, the all-cause mortality 30 days after the onset of BSI was 38% for both VRE (95% CI, 25%–54%) and VSE (95% CI, 21%–62%) bacteremia. In pediatric patients, the 30-day mortality was 20% (95% CI, 5.4%–59%) and 4.5% (95% CI, .6%–28%) for VRE and VSE BSI, respectively (P = .22). Median time to resolution of BSI (first of the 2 consecutively negative blood cultures after infection) was 3 (range, 1–14) and 1 (range, 1–10) days in VRE and VSE BSI, respectively (P = .001). Overall survival at 1 year after onset of BSI in adults was 20% (8 of 40) and 48% (10 of 21) for VRE and VSE BSI, respectively (P = .04). In pediatric patients, 1-year survival was 60% (6 of 10) and 86% (19 of 22) for VRE and VSE BSI, respectively (P = .17). We did not observe a statistically significant difference in overall survival 1 year after infection between VRE BSI occurring before and after day 60 (25% and 13% for early and late infections, respectively, P = .43). Overall survival and nonrelapse mortality at 1 year after transplant are summarized in Table 3 and were inferior among patients who acquired VRE BSI compared to the other groups. In a multivariate analysis of survival during first year after transplant, VRE and VSE bloodstream infections were associated with relative increase in the mortality rate of 4.2 (95% CI, 3.1–6.9) and 2.3 (95% CI, 1.4–5.1), P < .01), respectively. Enterococcal bacteremia and time to engraftment (in 1-week intervals) were the only other variables significantly associated with survival in this analysis (Table 4).
Table 3.
Comparison of Overall Outcomes: Vancomycin-Resistant Enterococcal Bloodstream Infection, Vancomycin-Sensitive Enterococcal Bloodstream Infection, and No Enterococcal Bloodstream Infection Groups
| Outcome | VRE BSI | VSE BSI | No Enterococcal BSI |
|---|---|---|---|
| Pediatric patients, no. | 10 | 22 | 229 |
| 1-y nonrelapse mortality (95% CI) | 30% (3–57) | 9% (0–21) | 15% (10–20) |
| 1-y OS (95% CI) | 70% (33–89) | 86% (63–95) | 80% (74–84) |
| Adult patients, no. | 40 | 21 | 430 |
| 1-y nonrelapse mortality (95% CI) | 53% (35–70) | 33% (13–53) | 22% (18–26) |
| 1-y OS (95% CI) | 23% (11–36) | 48% (26–67) | 63% (58–67) |
Abbreviations: BSI, bloodstream infection; CI, confidence interval; OS, overall survival; VRE, vancomycin-resistant enterococci; VSE, vancomycin-sensitive enterococci.
Table 4.
Risk Factors for All-Cause Mortality up to 1 Year Following Transplantation, Among Adult Patients
| Factor | HR (95% CI) | P Value |
|---|---|---|
| VRE BSIa | 4.6 (3.1–6.9) | <.01 |
| VSE BSIa | 2.7 (1.4–5.1) | <.01 |
| aGVHD (grade 3–4)a | 1.3 (.9–1.8) | .21 |
| cGVHDa | 0.8 (.5–1.3) | .38 |
| Engrafted by day 42a,b | 0.3 (.1–.6) | <.01 |
| Age, years | ||
| 18–49 | Ref | |
| ≥60 | 1.1 (.7–1.7) | .61 |
| Sex | ||
| Male | 0.9 (.7–1.2) | .54 |
| Female | Ref | |
| Diagnosis | ||
| Leukemia | Ref | |
| Lymphoma | 1.0 (.7–1.4) | .84 |
| MDS | 1.2 (.7–1.9) | .49 |
| Nonmalignancy | 0.9 (.5–1.9) | .87 |
| Other malignancy | 1.7 (1.1–2.8) | .02 |
| CMV | ||
| CMV R − /D − | 1.1 (.8–1.4) | .68 |
| CMV R − /D + | 1.0 (.5–2.0) | .96 |
| CMV R + | Ref | |
| Donor type | ||
| MRD | Ref | |
| MUD | 1.1 (.6–2.1) | .80 |
| UCB | 1.0 (.7–1.4) | .87 |
| KPS | ||
| KPS <90 | 1.4 (.9–2.1) | .11 |
| KPS 90–100 | Ref |
Abbreviations: aGVHD, acute graft-vs-host disease; BSI, bloodstream infection; cGVHD, chronic graft-vs-host disease; CI, confidence interval; CMV, cytomegalovirus; D, donor; HR, hazard ratio; KPS, Karnofsky performance score; MDS, myelodysplastic syndrome; MRD, matched related donor; MUD, matched unrelated donor; R, recipient; Ref, reference; UCB, umbilical cord blood; VRE, vancomycin-resistant enterococci; VSE, vancomycin-sensitive enterococci.
a Time-dependent covariate.
b Relative risk of event for a patient who has engrafted compared to a patient who has not engrafted up to day 42 after transplant.
DISCUSSION
This study describes a large cohort of HSCT patients with enterococcal BSI. Several observations have emerged from this study. First, VRE colonized patients had a higher risk of developing VRE bloodstream infection than patients not colonized, in line with previous reports in patients with cancer and other conditions, reviewed in [14]. Second, the risk of developing a VRE bacteremia increased with each week's delay in engraftment, and was highest for those not engrafted by day 42. Third, the rate of resistance was substantially higher for adult patients than pediatric patients. Fourth, VRE BSI was associated with inferior overall survival at 1 year after transplant. And finally, both VRE and VSE bloodstream infections occurred at similar rates throughout the first year after transplant.
Vancomycin-resistant enterococci have emerged as important pathogens in healthcare-associated infections over the last 2 decades [14–17], with reported rates of vancomycin resistance among invasive isolates of Enterococcus faecium varying internationally from 0% to 35% in 2009 [1, 18]. This cohort had a 7% rate of VRE bacteremia in the first year following HSCT, although VRE-colonized patients developed BSI at a rate of 14%. Most of these infections occurred during the preengraftment period at a rate similar to previous reports [4, 6]; the high-risk period in our cohort extended to approximately day 100 after HSCT.
The rate of vancomycin resistance for enterococcal bloodstream isolates among our adult patients was 66%, whereas the isolates from pediatric patients had a lower resistance rate of 38%. During the period of study, these patient groups were housed 1 floor apart. The hospital has an antimicrobial stewardship program that restricts vancomycin use for both adult and pediatric patients after 48–72 hours of use. While restriction of vancomycin use has been suggested as a means of reducing the rate of VRE infections, a systematic review [19] was not able to confirm this effect. In our patient cohort, 23% of allogeneic HSCT patients were colonized with VRE, with 25% of those having confirmed colonization prior to their transplant based on either admission cultures or cultures performed during previous hospitalizations at our center. This is in agreement with previously reported rates of colonization in HSCT patients [6, 20, 21] and other medical or surgical patients in the United States [16, 22]. Barrier precautions and contact isolation have not been clearly shown previously to be effective in preventing VRE colonization [23, 24] but are widely used in HSCT recipients.
We found equivalent, but disturbingly high, all-cause mortality 30 days after infection in patients with VRE and VSE bacteremia. We speculate that this mortality is not directly attributable to enterococcal bacteremia per se, but rather that the enterococcal BSIs preferentially occur in patients with delayed engraftment and other significant medical conditions. During the management of fever at our center, standard procedure is to use vancomycin, ceftazidime, and often tobramycin as empirical antimicrobial therapy until results of blood, urine, and sputum cultures are obtained. VRE therapy will be added after VRE is isolated from culture, and therapy is refined once the sensitivity profile is available. In patients known to be colonized with VRE, VRE-specific therapy will be added once a culture is identified as containing gram-positive cocci in chains. The number of events in our study is not high enough to detect a potential difference in outcome caused by the later addition of VRE therapy at the time of identification of a VRE pathogen compared with VSE, which are covered by the empirical antimicrobial therapy. A meta-analysis of studies of mortality associated with VRE vs VSE infections, which controlled for underlying severity of illness, found a higher risk of mortality in patients with VRE infections [25, 26]. However, most of the studies included in the metaanalysis were published before widespread availability of antibiotics active against VRE. A recently published analysis of outcome of VRE BSI in a mixed patient population from a tertiary care institution showed a short-term mortality rate similar to our cohort [27].
We did not evaluate directly attributable mortality caused by enterococcal BSI because of high prevalence of concomitant medical conditions, which make such attribution in HSCT patients subjective and arbitrary. While the extent of direct contribution of enterococcal infections to negative outcome has been a matter of debate, the association of these infections with morbidity and mortality has been demonstrated previously in HSCT and hematologic malignancies [20, 26, 28, 29], solid organ transplantation [30], and other patients [16, 17, 31–35]. Our findings of similar short-term mortality of VSE and VRE bacteremia, but increased nonrelapse mortality at 1 year after HSCT of the VRE vs VSE vs “no enterococcal bacteremia” groups, support the opinion that VRE infections may serve as a marker of severity of underlying medical condition. In contrast to nonopportunistic bacterial pathogens, enterococcal infections rarely present with shock, pneumonia, or other end-organ damage clearly linked with morbidity and mortality. Further experimental and clinical studies are needed to elucidate pathogenesis of enterococcal infections and their contribution to outcome in light of mortality that is similar to that of bacteremia caused by organisms known to be more virulent. In summary, we have observed a high rate of vancomycin resistance among bloodstream enterococcal isolates, which underscores a need for development of new strategies to combat VRE infections of sterile sites. Periodic surveillance for VRE colonization can indicate patients for whom anti-VRE antibiotics should be included in their empiric therapy or neutropenic fever, or at least for prompt initiation on identification of gram-positive cocci in cultures taken from an otherwise sterile site. The clinical utility of such an approach needs further investigation.
Notes
Financial support. This work was supported in part by the National Institutes of Health (NIH P30 CA77598) utilizing the Biostatistics and Bioinformatics shared resource at the University of Minnesota Masonic Cancer Center and by the Proshek-Fulbright scholarship (to J. V.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 2.Koc Y, Snydman DR, Schenkein DS, Miller KB. Vancomycin-resistant enterococcal infections in bone marrow transplant recipients. Bone Marrow Transplant. 1998;22:207–9. doi: 10.1038/sj.bmt.1701303. [DOI] [PubMed] [Google Scholar]
- 3.Kirkpatrick BD, Harrington SM, Smith D, et al. An outbreak of vancomycin-dependent Enterococcus faecium in a bone marrow transplant unit. Clin Infect Dis. 1999;29:1268–73. doi: 10.1086/313456. [DOI] [PubMed] [Google Scholar]
- 4.Avery R, Kalaycio M, Pohlman B, et al. Early vancomycin-resistant enterococcus (VRE) bacteremia after allogeneic bone marrow transplantation is associated with a rapidly deteriorating clinical course. Bone Marrow Transplant. 2005;35:497–9. doi: 10.1038/sj.bmt.1704821. [DOI] [PubMed] [Google Scholar]
- 5.Bossaer JB, Hall PD, Garrett-Mayer E. Incidence of vancomycin-resistant enterococci (VRE) infection in high-risk febrile neutropenic patients colonized with VRE. Support Care Cancer. 2010;19:231–7. doi: 10.1007/s00520-009-0808-y. [DOI] [PubMed] [Google Scholar]
- 6.Kamboj M, Chung D, Seo SK, et al. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant. 2010;16:1576–81. doi: 10.1016/j.bbmt.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peel T, Cheng AC, Spelman T, Huysmans M, Spelman D. Differing risk factors for vancomycin-resistant and vancomycin-sensitive enterococcal bacteraemia. Clin Microbiol Infect. 2011;18:1–7. doi: 10.1111/j.1469-0691.2011.03591.x. [DOI] [PubMed] [Google Scholar]
- 8.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Wolkewitz M, Vonberg RP, Grundmann H, et al. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: application of competing risks models. Crit Care. 2008;12:R44. doi: 10.1186/cc6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 11.Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 13.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 14.Salgado CD. The risk of developing a vancomycin-resistant Enterococcus bloodstream infection for colonized patients. Am J Infect Control. 2008;36:S175.e5–8. doi: 10.1016/j.ajic.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Wells CL, Juni BA, Cameron SB, et al. Stool carriage, clinical isolation, and mortality during an outbreak of vancomycin-resistant enterococci in hospitalized medical and/or surgical patients. Clin Infect Dis. 1995;21:45–50. doi: 10.1093/clinids/21.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Goetz AM, Rihs JD, Wagener MM, Muder RR. Infection and colonization with vancomycin-resistant Enterococcus faecium in an acute care Veterans Affairs Medical Center: a 2-year survey. Am J Infect Control. 1998;26:558–62. doi: 10.1053/ic.1998.v26.a86286. [DOI] [PubMed] [Google Scholar]
- 17.Chavers LS, Moser SA, Benjamin WH, et al. Vancomycin-resistant enterococci: 15 years and counting. J Hosp Infect. 2003;53:159–71. doi: 10.1053/jhin.2002.1375. [DOI] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control website. http://www.ecdc.europa.eu/en/activities/surveillance/EARS-Net/database/Pages/database.aspx . Accessed 20 September 2011. [Google Scholar]
- 19.De Bruin MA, Riley LW. Does vancomycin prescribing intervention affect vancomycin-resistant enterococcus infection and colonization in hospitals? A systematic review. BMC Infect Dis. 2007;7:24. doi: 10.1186/1471-2334-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstock DM, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13:615–21. doi: 10.1016/j.bbmt.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 21.Zirakzadeh A, Gastineau DA, Mandrekar JN, Burke JP, Johnston PB, Patel R. Vancomycin-resistant enterococcal colonization appears associated with increased mortality among allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41:385–92. doi: 10.1038/sj.bmt.1705912. [DOI] [PubMed] [Google Scholar]
- 22.Nourse C, Murphy H, Byrne C, et al. Control of a nosocomial outbreak of vancomycin resistant Enterococcus faecium in a paediatric oncology unit: risk factors for colonisation. Eur J Pediatr. 1998;157:20–7. doi: 10.1007/s004310050760. [DOI] [PubMed] [Google Scholar]
- 23.Huskins WC, Huckabee CM, O'Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364:1407–18. doi: 10.1056/NEJMoa1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Almyroudis NG, Lesse AJ, Hahn T, et al. Molecular epidemiology and risk factors for colonization by vancomycin-resistant Enterococcus in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2011;32:490–6. doi: 10.1086/659408. [DOI] [PubMed] [Google Scholar]
- 25.DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41:327. doi: 10.1086/430909. [DOI] [PubMed] [Google Scholar]
- 26.DiazGranados CA, Jernigan JA. Impact of vancomycin resistance on mortality among patients with neutropenia and enterococcal bloodstream infection. J Infect Dis. 2005;191:588–95. doi: 10.1086/427512. [DOI] [PubMed] [Google Scholar]
- 27.McKinnell JA, Patel M, Shirley RM, Kunz DF, Moser SA, Baddley JW. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol Infect. 2011;139:1342–50. doi: 10.1017/S0950268810002475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todeschini G, Tecchio C, Borghero C, et al. Association between Enterococcus bacteraemia and death in neutropenic patients with haematological malignancies. Infection. 2006;53:266–73. doi: 10.1016/j.jinf.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Theilacker C, Jonas D, Huebner J, Bertz H, Kern WV. Outcomes of invasive infection due to vancomycin-resistant Enterococcus faecium during a recent outbreak. Infection. 2009;37:540–3. doi: 10.1007/s15010-009-9023-5. [DOI] [PubMed] [Google Scholar]
- 30.Papanicolaou GA, Meyers BR, Meyers J, et al. Nosocomial infections with vancomycin-resistant Enterococcus faecium in liver transplant recipients: risk factors for acquisition and mortality. Clin Infect Dis. 1996;23:760–6. doi: 10.1093/clinids/23.4.760. [DOI] [PubMed] [Google Scholar]
- 31.Fernández Fernández FJ, De La Fuente Aguado J, Rubianes González M, et al. Enterococcus faecalis bacteremia. Rev Clin Esp. 2004;204:244–50. doi: 10.1157/13061409. [DOI] [PubMed] [Google Scholar]
- 32.Graninger W, Ragette R. Nosocomial bacteremia due to Enterococcus faecalis without endocarditis. Clin Infect Dis. 1992;15:49–57. doi: 10.1093/clinids/15.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Noskin GA, Peterson LR, Warren JR. Enterococcus faecium and Enterococcus faecalis bacteremia: acquisition and outcome. Clin Infect Dis. 1995;20:296–301. doi: 10.1093/clinids/20.2.296. [DOI] [PubMed] [Google Scholar]
- 34.Suppola JP, Kuikka A, Vaara M, Valtonen VV. Comparison of risk factors and outcome in patients with Enterococcus faecalis vs Enterococcus faecium bacteraemia. Scand J Infect Dis. 1998;30:153–7. doi: 10.1080/003655498750003546. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee I, Dulhunty JM, Iredell J, et al. Predictors and outcome associated with an Enterococcus positive isolate during intensive care unit admission. Anaesth Intensive Care. 2009;37:976–82. doi: 10.1177/0310057X0903700610. [DOI] [PubMed] [Google Scholar]