The Clinical and Laboratory Standards Institute recently elected to lower the susceptibility breakpoint of piperacillin against Pseudomonas aeruginosa based largely on pharmacokinetic-pharmacodynamic modeling. We conducted a retrospective study to determine if lowering susceptibility breakpoints predicts improved clinical outcomes in children.
Abstract
Background. The Clinical and Laboratory Standards Institute (CLSI) recently elected to adjust the previous piperacillin susceptibility breakpoint of ≤64 µg/mL against Pseudomonas aeruginosa to ≤16 µg/mL, based largely on pharmacokinetic–pharmacodynamic (PK–PD) modeling studies. Data on whether PK–PD modeling correlates with clinical outcomes in children are needed before resorting to broader classes of antibiotics to treat P. aeruginosa.
Methods. We performed a retrospective cohort study of children with P. aeruginosa bacteremia between 2001 and 2010 who were prescribed piperacillin. Baseline characteristics and clinical outcomes of children with piperacillin minimum inhibitory concentrations (MICs) of ≤16 µg/mL and of 32–64 µg/mL were compared. The primary outcome was 30-day mortality.
Results. There were 170 children with P. aeruginosa bacteremia receiving piperacillin therapy who met inclusion criteria. One hundred twenty-four (72%) children had piperacillin MICs of ≤16 µg/mL and 46 (28%) children had piperacillin MICs of 32–64 µg/mL. There was no significant difference in baseline characteristics between the 2 groups. Thirty-day mortality was 9% and 24% in children with a piperacillin MIC of ≤16 µg/mL and of 32–64 µg/mL, respectively. Using multivariable logistic regression, children with elevated MICs had increased odds of mortality compared with children with lower MICs (odds ratio, 3.21; 95% confidence interval, 1.26–8.16).
Conclusions. Our finding that elevated piperacillin MICs are associated with higher mortality in children supports the recent CLSI recommendation to lower the breakpoint of piperacillin against P. aeruginosa to ≤16 µg/mL. Alternate therapeutic choices should be considered when piperacillin MICs against P. aeruginosa are ≥32 µg/mL.
In June 2011, the Clinical and Laboratory Standards Institute (CLSI) elected to adjust susceptibility breakpoints of piperacillin against Pseudomonas aeruginosa, with the susceptible range being defined as a piperacillin minimum inhibitory concentration (MIC) of ≤16 µg/mL, in contrast with the previously defined breakpoint of ≤64 µg/mL [1]. The previous breakpoint was largely guided by in vitro studies demonstrating a 2-fold decrease in the MIC against P. aeruginosa with the combination of an antipseudomonal penicillin and aminoglycoside; an assumption was made that patients would be treated with both classes of antibiotics concurrently [2–6]. Since then, the practice of combination therapy with an aminoglycoside has largely fallen out of favor because of the toxicity of the later agent, calling into question the rationale for maintaining the breakpoint at 64 µg/mL [7–9]. In addition, pharmacokinetic–pharmacodynamic (PK–PD) modeling employing Monte Carlo simulation techniques has suggested a low probability of attaining optimal pharmacodynamic targets with MICs of ≥32 µg/mL [10]. There have been no studies assessing the relationship between piperacillin MICs and clinical outcomes of children with pseudomonal infections, and it is unclear if Monte Carlo simulations reflect observed clinical outcomes in children. Bloodstream infections due to P. aeruginosa result in high mortality rates in children, making it essential that optimal antimicrobial therapy be prescribed for P. aeruginosa infections [11].
Piperacillin is commonly used as empiric therapy for children at risk for resistant organisms because of its broad spectrum of activity and its safety profile [12, 13]. With implementation of the new CLSI recommendations, a greater proportion of P. aeruginosa organisms once considered susceptible to piperacillin will now fall into the intermediate category, and therapeutic options for P. aeruginosa need to be reconsidered. Clinicians will increasingly need to resort to broader classes of antibiotics, such as carbapenems, for P. aeruginosa infections. This is concerning given the limited number of broad-spectrum agents targeting resistant P. aeruginosa in the pipelines of the pharmaceutical industry [14]. Consequently, determining whether piperacillin MICs of 32–64 µg/mL are associated with poor outcomes for children with P. aeruginosa infections is clinically important. We conducted a retrospective cohort study to determine if pediatric bacteremia caused by P. aeruginosa strains with reduced susceptibility to piperacillin is associated with an increased risk of mortality and microbiological failure.
METHODS
Setting and Participants
Our study was conducted at the Johns Hopkins Hospital (JHH) Children's Center. The JHH Children's Center is a 186-bed tertiary care pediatric hospital serving Maryland and surrounding states. Children aged ≤18 years who were admitted to JHH from 1 January 2001 through 31 December 2010 with a positive blood culture for P. aeruginosa were included in our study. Children who were prescribed an antipseudomonal β-lactam other than piperacillin, those who did not receive antipseudomonal therapy within 24 hours of the first positive blood culture, those with a piperacillin MIC against P. aeruginosa ≥128 µg/mL, and those with polymicrobial bloodstream infections were excluded. Additionally, children who died within 48 hours of the first positive blood culture for P. aeruginosa were also excluded because mortality was likely independent of their definitive antimicrobial therapy [15].
Data Collection
Laboratory databases were queried to identify all blood cultures from which P. aeruginosa was isolated during the study period. Patient characteristics of children with P. aeruginosa were extracted from medical records. Pertinent data that were retrieved from electronic and medical records included demographic characteristics, absolute neutrophil count at the time of first positive blood culture, preexisting medical conditions, presence of a central line, and other body sites where P. aeruginosa was recovered. Severity of illness at the time of the first positive blood culture was assessed using the pediatric risk of mortality (PRISM) score [16]. Data were also collected on the use of combination antibiotic therapy (piperacillin + aminoglycoside or fluoroquinolone) and whether a central line was removed within 48 hours from the time P. aeruginosa was first cultured from the bloodstream because these exposures were hypothesized to be associated with both the piperacillin MIC and patient outcomes.
The primary exposure of interest was piperacillin MIC using the revised CLSI breakpoint. Of note, although we refer to piperacillin in this manuscript, 98% of piperacillin prescribed to children included in this study was used in combination with tazobactam. The MICs were categorized as dichotomous variables with ≤16 µg/mL treated as susceptible and MICs between 32 and 64 µg/mL treated as intermediate. The primary outcome was 30-day all-cause mortality from the first day of bacteremia. We made the assumption that children who were alive at the time of discharge, if <30 days from the time of the initial positive blood culture, were alive at 30 days. This was confirmed when all included children who were alive at the time of hospital discharge had subsequent medical visits documented on a query of their medical records. The secondary outcome was 30-day microbiological failure. Microbiological failure was defined using criteria for clinically significant isolates (an organism isolated from a sterile body site or a significant quantity of growth from nonsterile sites, such as urine [if a catheter was in place], wounds, or tracheal aspirates) [17]. The study was approved by the Johns Hopkins University School of Medicine Review Board, with a waiver of informed consent.
Statistical Analysis
Summary statistics were constructed using frequencies and proportions for categorical data and medians and interquartile ranges for continuous variables. Pearson χ2 and Fisher exact tests were used for unadjusted comparisons of categorical baseline characteristics and clinical outcomes of children with MICs in the susceptible and intermediate ranges. Both univariate and multivariable logistic regression were conducted to determine independent predictors of mortality and microbiological relapse. Relationships between factors hypothesized to influence clinical and microbiological outcome and MIC category were explored using scatterplots and univariate analysis. Univariate analysis was performed separately for each of the potential explanatory variables to ascertain odds ratios (ORs) and 95% confidence intervals (CIs). Covariates with P values <.20 in the unadjusted model were included in the adjusted model. Additionally, any variable specified as a potential confounder a priori based on previous literature or biological plausibility (eg, combination therapy and PRISM score) was also included in the model. Variables not included in the final model changed the point estimate for the effect of combination therapy by <10%. Because the effect of combination therapy on mortality may differ for children with MICs of ≤16 µg/mL and MICs of 32–64 µg/mL, an interaction term was included in our model to test for effect modification. The MICs from January 2000 to August 2006 were determined using agar dilution as the susceptibility testing method, and the MICs after August 2006 were determined using the BD Phoenix Automated Microbiology System. To explore the possibility of a change in testing leading to misclassification bias, an analysis was conducted with data stratified based on the method of susceptibility testing.
We also evaluated the MIC–mortality relationship after matching children on their probability of having higher MICs. We generated propensity scores for the probability of having an elevated MIC by regressing MIC on age, gender, PRISM score, length of hospital stay prior to bacteremia, intensive care unit admission, use of combination therapy, number of preexisting medical conditions, absolute neutrophil count categorization, pressor requirement, mechanical ventilation status, and central line status. We then used nonparametic, 1:1 nearest neighbor matching to match patients based on propensity score and conducted a matched-pair analysis. Patients who could not be matched to a patient with a propensity score within 0.25 standard deviations were excluded from analysis. For all statistical tests, 2-sided P values of <.05 were considered to be statistically significant. Data were analyzed using Stata, version 11.1 (StataCorp) and the MatchIt package for the R programming language.
RESULTS
Baseline Characteristics
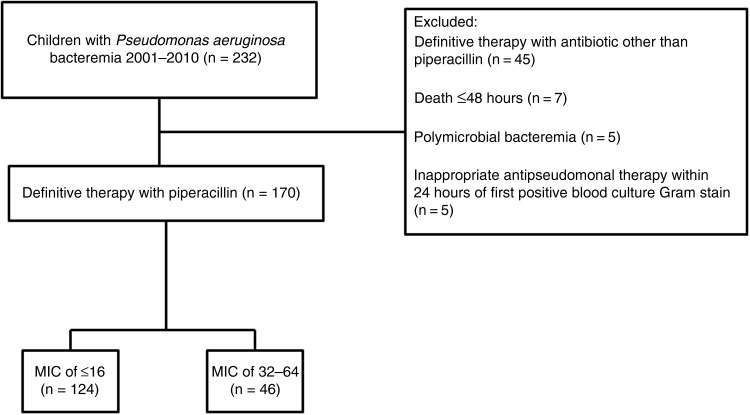
A total of 232 children with positive blood cultures for P. aeruginosa were identified. There were 170 children who met eligibility criteria (Figure 1). There were no children with P. aeruginosa bacteremia with piperacillin MICs against P. aeruginosa of ≥128 µg/mL who received piperacillin therapy. One hundred twenty-four (72%) children with P. aeruginosa bacteremia had isolates with an MIC of ≤16 µg/mL and 46 (28%) had an MIC of 32–64 µg/mL (Table 1). There were no significant differences in baseline characteristics between the two groups. Forty-three (25%) children with bacteremia had P. aeruginosa recovered from other body sites, with the lungs (8%) and urinary tract (8%) being the most common additional sites. Thirty-two children received 400 mg/kg/day of piperacillin, and the remainder received 300 mg/kg/day (doses rounded). Dosages were equally distributed between the two MIC categories and were appropriately adjusted for all children with renal impairment. None of the children were prescribed extended infusion or continuous infusion piperacillin. Combination therapy was administered to 90 (73%) and 27 (59%) children in the low and elevated MIC categories, respectively. Combination therapy consisted of piperacillin and an aminoglycoside in all but 1 child who received piperacillin and ciprofloxacin. Eighty-five (50%) children had a diagnosis of a malignancy at the time of P. aeruginosa bacteremia.
Figure 1.
Study design of Pseudomonas aeruginosa bacteremia in children prescribed piperacillin using revised Clinical and Laboratory Standards Institute breakpoints of piperacillin for P. aeruginosa between 2001 and 2010. Abbreviation: MIC, minimum inhibitory concentration in µg/mL.
Table 1.
Baseline Data of 170 Children Prescribed Piperacillin as Therapy for Pseudomonas aeruginosa Bacteremia Comparing Piperacillin Minimum Inhibitory Concentrations of ≤16 µg/mL and 32–64 µg/mL
| Characteristics | MIC of ≤16 µg/mL (n = 124; 72%) | MIC of 32–64 µg/mL (n = 46; 28%) | P Value |
|---|---|---|---|
| Age, median (IQR) | 3 (1–10) | 5.5 (1–15) | .06 |
| Number of preexisting conditions, median (IQR) | 1 (1–2) | 1 (1–2) | .42 |
| Preexisting medical conditions | |||
| Neuromuscular | 7 (5.6) | 5 (10.9) | .31 |
| Cardiovascular | 7 (5.6) | 2 (4.3) | .54 |
| Respiratory | 8 (6.5) | 2 (4.3) | .46 |
| Renal | 13 (10.5) | 6 (13) | .41 |
| Gastrointestinal | 11 (8.9) | 7 (15.2) | .18 |
| Hematologic | 7 (5.6) | 4 (8.7) | .34 |
| Malignancy | 66 (53.2) | 19 (41.3) | .23 |
| PRISM scores, median (IQR) | 6 (4–14) | 8 (4–20) | .41 |
| Immunocompromised status | 76 (61) | 23 (50) | .19 |
| Intensive care unit admission during time of bacteremia | 39 (31) | 17 (37) | .50 |
| Mechanical ventilation during time of bacteremia | 18 (15) | 3 (7) | .16 |
| Duration of hospitalization prior to bacteremia, median (IQR) | 1 (1–6) | 1 (1–14) | .11 |
| Absolute neutrophil count of <500 | 39 (32) | 14 (33) | .90 |
| β-lactam + aminoglycoside definitive combination therapy | 90 (73) | 27 (59) | .08 |
| 400 mg/kg/day of piperacillin prescribed | 26 (21) | 6 (14) | .37 |
| Other body sites where Pseudomonas spp. were recovereda | |||
| Urine | 8 (6.5) | 6 (13) | .21 |
| Sputum | 4 (3.2) | … | |
| Pleural or BAL fluid | 9 (7.3) | 5 (10.9) | .53 |
| Bone or joint specimens | 4 (3.2) | 2 (4.3) | .66 |
| Abdominal abscess | 3 (2.4) | … | |
| Soft tissue | … | 2 (4.3) |
Data are no. (%) unless otherwise noted.
Abbreviations: BAL, bronchoalveolar lavage; IQR, interquartile range; MIC, minimum inhibitory concentration; PRISM, pediatric risk of mortality.
a Using criteria for clinically significant isolates (an organism isolated from a sterile body site or a significant quantity of growth from nonsterile sites, such as urine [if a catheter is in place], wounds, or tracheal aspirates [17].
Percent Susceptibilities
When assessing all 232 positive blood cultures for P. aeruginosa bacteremia over the 10-year study period, approximately 97% of isolates were susceptible to piperacillin using previous CLSI criteria. After implementation of the revised breakpoints, approximately 28% of isolates were classified as intermediate and 72% as susceptible to piperacillin (Table 2).
Table 2.
Percentage of Pseudomonas aeruginosa Isolates Susceptible to Piperacillin Prior to and After Implementation of Clinical and Laboratory Standards Institute 2012 Changes, Using All First Positive Blood Culture Isolates in Children From 2001 to 2010 at Johns Hopkins Hospital
| Prior to Revised Breakpoints | After Implementation of Revised Breakpoints | Percent Change | |
|---|---|---|---|
| Susceptible | 225 | 163 | ↓ 27.6% |
| Intermediate | …a | 62 | … |
| Resistant | 7 | 7 | … |
a No intermediate range defined for piperacillin prior to breakpoint revision.
Mortality
Twenty-nine (12.5%) of the 232 children with P. aeruginosa from 2001 to 2010 died, regardless of the antibiotic therapy prescribed. There were 22 (13%) children meeting eligibility criteria who died >48 hours after the initial positive blood culture was obtained. Thirty-day mortality was 9% and 24% in children with a piperacillin MIC of ≤16 µg/mL and of 32–64 µg/mL, respectively. The use of piperacillin monotherapy or piperacillin dosed at 300 mg/kg/day was not associated with an increased risk of mortality. Children with elevated MICs had increased unadjusted odds of mortality compared with children with lower MICs (OR, 3.23; 95% CI, 1.30–8.08). After adjusting for PRISM score, receipt of combination therapy, and failure to remove the central line, the odds of mortality remained increased in those children with higher MICs (OR, 3.21; 95% CI, 1.26–8.16; Table 3). The effect of combination therapy on mortality for children with P. aeruginosa bacteremia was no different for children with MICs of 32–64 µg/mL and of ≤16 µg/mL (OR, 2.08; 95% CI, .31–13.99 vs OR: 2.00; 95% CI, .44–9.13). There was <10% difference in the OR estimates for mortality between the 2 MIC categories comparing disc diffusion and automated testing methods.
Table 3.
Mortality of 170 Children With Pseudomonas aeruginosa Bacteremia Receiving Piperacillin (2001–2010)
| Unadjusted Odds Ratio | 95% Confidence Interval | P Value | Adjusteda Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|---|
| Age | 1.03 | .96–1.10 | .50 | |||
| Minimum inhibitory concentration of 32–64 µg/mL | 3.23 | 1.30–8.08 | .01 | 3.21 | 1.26–8.16 | .01 |
| Piperacillin + aminoglycoside | 0.76 | .30–1.95 | .57 | 0.77 | .29–2.09 | .62 |
| Pediatric risk of mortality score | 1.00 | .96–1.04 | .99 | 1.00 | .96–1.03 | 1.03 |
| Failure to remove central line | 1.85 | .74–4.67 | .20 | 2.00 | .76–5.27 | .16 |
| Absolute neutrophil count of <500 cells/mL | 1.24 | .46–3.37 | .67 | |||
| Number of preexisting medical conditions | 0.91 | .45–1.86 | .80 |
a Model includes minimum inhibitory concentration of 32–64 µg/mL, piperacillin + aminoglycoside therapy, pediatric risk of mortality score, and failure to remove central line.
There were 45 children with P. aeruginosa bacteremia who were excluded from the analysis because they received an antibiotic other than piperacillin. Thirty children who received an antibiotic other than piperacillin had MICs of ≤16 µg/mL, and 10 children had MICs of 32–64 µg/mL. There was 1 death (10%) in children in the 32–64 µg/mL MIC category who received an antibiotic other than piperacillin, which was not significantly different from the 11 (24%) deaths in children in the 32–64 µg/mL MIC category who received piperacillin (P = .67).
Incorporating propensity score analysis, all patients with an elevated MIC and a retained central line were matched to patients with a low MIC and a retained central line. The relative odds of mortality for children with intermediate MICs remained elevated and statistically significant, even though these matched pairs constituted less than half of the original sample (n = 39; OR, 6.28; 95% CI, 1.47–43.95; P = .03).
Microbiological Failure
There were 30 children who had microbiological failure within 30 days (17.6%), with a median time to relapse of approximately 18 days. Eighteen (14.9%) of the children with an MIC of ≤16 μg/mL met criteria for microbiological failure within 30 days compared with 12 (26%) of the children who had a piperacillin MIC of 32–64 µg/mL μg/mL (Table 4). There was a trend toward an increased odds of microbiological failure in children who had elevated MICs, and this was unchanged after adjusting for receipt of combination therapy, PRISM score, and failure to remove the central line (OR, 2.28; 95% CI, .95–5.50). One hundred thirty-three (78%) children in the cohort had a central line in place during the first day of bacteremia, and 56% of these children had a central line removed ≤48 hours from the time of positive blood culture. Children with P. aeruginosa bacteremia who did not have their central line removed during the time they were bacteremic had >4 times the odds of microbiological failure compared with children who underwent central line removal (OR, 4.09; 95% CI, 1.59–10.50). Fifteen (50%) of the children with microbiological failure within 30 days of their initial positive blood culture had a ≥4-fold increase (or attained an MIC of ≥128 µg/mL) in piperacillin MIC against P. aeruginosa on the culture recovered at the time of relapse, with no difference observed between the ≤16 µg/mL and 32–64 µg/mL MIC categories or the monotherapy and combination therapy groups (data not shown).
Table 4.
Microbiological Failure of 170 Children With Pseudomonas aeruginosa Bacteremia Receiving Piperacillin (2001–2010)
| Unadjusted Odds Ratio | 95% Confidence Interval | P Value | Adjusteda Odds Ratio | 95% Confidence Interval | P Value | |
|---|---|---|---|---|---|---|
| Age | 1.01 | .95–1.07 | .75 | |||
| Minimum inhibitory concentration of 32–64 µg/mL | 2.08 | .90–4.75 | .08 | 2.28 | .95–5.50 | .07 |
| Piperacillin + aminoglycoside | 1.61 | .64–4.02 | .31 | 1.48 | .56–3.90 | .44 |
| Pediatric risk of mortality score | 1.02 | .99–1.05 | .16 | 1.03 | .99–1.06 | .12 |
| Failure to remove central line | 4.01 | 1.61–9.97 | <.01 | 4.09 | 1.59–10.50 | <.01 |
| Absolute neutrophil count of <500 cells/mL | 1.07 | .45–2.52 | .88 | |||
| Number of preexisting medical conditions | 0.79 | .41–1.51 | .49 |
a Model includes minimum inhibitory concentration of 32–64 µg/mL, piperacillin + aminoglycoside therapy, pediatric risk of mortality score, and failure to remove central line.
DISCUSSION
The results of our study support the recent CLSI recommendation to lower the breakpoint of piperacillin against P. aeruginosa to ≤16 µg/mL. Our results indicate that children with piperacillin MICs of ≥32 µg/mL have >3 times the odds of death within 30 days of their first positive blood culture compared with children with more susceptible piperacillin MICs against P. aeruginosa. Additionally, children with elevated MICs against P. aeruginosa receiving piperacillin therapy have a trend toward an increased risk of microbiological relapse compared to children with lower MICs.
Our results are in accord with previous PK–PD modeling studies employing Monte Carlo simulation to predict the microbiological success of varying piperacillin MICs against P. aeruginosa [18]. Previous PK–PD studies have suggested that there is a low probability of attaining the optimal pharmacodynamic target with MICs of piperacillin against P. aeruginosa that are >32 µg/mL [10, 19, 20]. Additionally, in a retrospective study of 17 adult patients with P. aeruginosa bacteremia treated with piperacillin, 100% microbiological efficacy was attained when the MIC was <16 µg/mL, but the efficacy decreased to 33% when the MIC was 32 µg/mL and decreased to 0% when the MIC was ≥64 µg/mL [21]. Ours is the first study evaluating clinical outcomes of children with P. aeruginosa bacteremia as a function of piperacillin MICs.
We cannot conclude from our study whether children with piperacillin MICs of ≥32 µg/mL would have had improved outcomes if they had received an alternate antipseudomonal agent. We performed an analysis comparing children with P. aeruginosa bacteremia with piperacillin MICs of ≥32 µg/mL with children who received a nonpiperacillin antipseudomonal β-lactam. Mortality was 24% in the former group, compared with 10% in the later group. Although mortality was higher for children who were prescribed piperacillin, it did not achieve statistical significance, and our study was not adequately powered to address this question. In a study of 34 adult patients with P. aeruginosa bacteremia and a piperacillin MIC of ≥32 µg/mL, the 30-day mortality rate was approximately 86% in the piperacillin group, compared with 22% in the nonpiperacillin group (P < .01) [22]. Future studies need to be conducted to determine the optimal β-lactam for P. aeruginosa isolates with elevated MICs.
The PK–PD modeling of piperacillin has demonstrated that the nonprotein bound drug concentration needs to exceed the MIC of P. aeruginosa at least 50% of the time to achieve optimal bactericidal activity [23]. It has also been shown that the probability of target attainment for piperacillin regimens dosed 300 mg/kg/day and 400 mg/kg/day for an MIC of 16 µg/mL is approximately 20% and 50%, respectively. In our cohort, only 20% of children were prescribed 400 mg/kg/day [24]. Until larger studies are conducted to evaluate optimal piperacillin dosing for Pseudomonas bacteremia in children, prescribing 400 mg/kg/day appears warranted based on existing PK–PD studies.
In the past when CLSI breakpoint changes have been instituted, there has been hesitation on the part of the medical community to adopt these changes. This is because CLSI recommendations are based largely on PK–PD modeling or theoretical concerns regarding insensitive techniques to identify organisms harboring β-lactamase resistance genes [1, 25, 26]. Clinical data supporting CLSI recommendations are often limited [27]. In 2010, the CLSI issued new breakpoints for carbapenems and several cephalosporins against Enterobacteriaceae in an effort to better detect the presence of β-lactamase–producing organisms [28]. However, a study following the CLSI changes found that nonsusceptibility to carbapenems using the updated breakpoints poorly predicted the presence of carbapenemase production (eg, 3.6% and 18.3% for Escherichia coli and Enterobacter spp., respectively), making some reluctant to incorporate the updated susceptibility breakpoints [29]. Our findings support the recent CLSI recommendations regarding piperacillin. Based on our institution's data, if the new breakpoints are not implemented, almost 28% of children receiving piperacillin may be receiving suboptimal therapy against P. aeruginosa, resulting in adverse patient outcomes.
Piperacillin is commonly used as a first-line agent for children at risk for resistant organisms because of its broad spectrum of activity. Data from 1997 to 2007 provided by the SENTRY Program demonstrated that piperacillin was the β-lactam with the greatest activity against P. aeruginosa [30]. Using P. aeruginosa breakpoints of ≤64 µg/mL, piperacillin had the broadest coverage, with an overall susceptibility percentage of 83.6%, followed by meropenem and imipenem at 83% and 79.7%, respectively. With incorporation of the new CLSI recommendations, however, the proportion of P. aeruginosa isolates susceptible to piperacillin will likely decrease, and institution-specific antibiograms should be inspected closely to determine if piperacillin remains an adequate agent for empiric coverage in children in whom P. aeruginosa infections are of concern.
There are several limitations to our study. First, as this is a single-institution study, inter-institution differences in antibiotic prescribing practices, antibiotic susceptibility patterns, and patient populations may affect the applicability of our results to other pediatric healthcare facilities. Second, when evaluating the outcome of mortality, we cannot assume that all mortality within 30 days was attributable to inadequate antimicrobial therapy due to elevated piperacillin MICs. It is possible that children with P. aeruginosa bacteremia had unmeasured attributes that affected their risk of death, such as their underlying medical conditions. However, using 30-day all-cause mortality as opposed to attributable mortality allowed for objective assessment of the mortality endpoint. Third, the focus of our study was definitive therapy against P. aeruginosa with piperacillin. Although we restricted inclusion to children who received an antipseudomonal agent within 24 hours of the first positive blood culture, we cannot make any conclusions regarding the various choices of antipseudomonal empirical regimens and how they may have impacted clinical outcomes.
Overall, our data support the assertion by the CLSI that the long-established breakpoints of piperacillin against P. aeruginosa needed to be reevaluated. Our data suggest that children with P. aeruginosa bacteremia with piperacillin MICs of ≥32 µg/mL against P. aeruginosa have >3 times the adjusted odds of death compared with children with lower MICs receiving piperacillin. Institution-specific clinical algorithms incorporating piperacillin as empiric therapy for suspected P. aeruginosa infections may need to be reconsidered if susceptibility proportions for piperacillin are unacceptably low using the revised breakpoints. When piperacillin MICs against P. aeruginosa are ≥32 µg/mL, alternate therapeutic choices or strategies of infusion should be considered.
Notes
Financial support. This work was supported by a National Institute of Health grant (KL2RR025006 to P. D. T.) and the Baurenschmidt Fellowship Award (to P. D. T.). S. E. C. is supported by a Centers for Disease Control and Prevention grant (R01 CI000616-01).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Clinical and Laboratory Standards Institute. Summary mutes: Subcommittee on Antimicrobial Susceptibility Testing. http://www.clsi.org/Content/NavigationMenu/Committees/Microbiology/AST/ArchiveofPreviousEvents/ASTJanuary2011.pdf. Accessed 8 January 2012. [Google Scholar]
- 2.Klastersky J, Zinner SH. Synergistic combinations of antibiotics in gram-negative bacillary infections. Rev Infect Dis. 1982;4:294–301. doi: 10.1093/clinids/4.2.294. [DOI] [PubMed] [Google Scholar]
- 3.Giamarellou H, Zissis NP, Tagari G, Bouzos J. In vitro synergistic activities of aminoglycosides and new beta-lactams against multiresistant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1984;25:534–6. doi: 10.1128/aac.25.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.den Hollander JG, Horrevorts AM, van Goor ML, Verbrugh HA, Mouton JW. Synergism between tobramycin and ceftazidime against a resistant Pseudomonas aeruginosa strain, tested in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1997;41:95–100. doi: 10.1128/aac.41.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouton JW. Combination therapy as a tool to prevent emergence of bacterial resistance. Infection. 1999;27(Suppl 2):S24–8. doi: 10.1007/BF02561666. [DOI] [PubMed] [Google Scholar]
- 6.Wu YL, Scott EM, Po AL, Tariq VN. Ability of azlocillin and tobramycin in combination to delay or prevent resistance development in Pseudomonas aeruginosa. J Antimicrob Chemother. 1999;44:389–92. doi: 10.1093/jac/44.3.389. [DOI] [PubMed] [Google Scholar]
- 7.Paul M, Silbiger I, Grozinsky S, Soares-Weiser K, Leibovici L. Beta lactam antibiotic monotherapy versus beta lactam-aminoglycoside antibiotic combination therapy for sepsis. Cochrane Database Syst Rev. 2006;1:CD003344. doi: 10.1002/14651858.CD003344.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Paul M, Leibovici L. Combination antibiotic therapy for Pseudomonas aeruginosa bacteraemia. Lancet Infect Dis. 2005;5:192–3. doi: 10.1016/S1473-3099(05)70030-X. discussion 3–4. [DOI] [PubMed] [Google Scholar]
- 9.Marcus R, Paul M, Elphick H, Leibovici L. Clinical implications of beta-lactam-aminoglycoside synergism: systematic review of randomised trials. Int J Antimicrob Agents. 2011;37:491–503. doi: 10.1016/j.ijantimicag.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Lodise TP, Jr, Lomaestro B, Rodvold KA, Danziger LH, Drusano GL. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation. Antimicrob Agents Chemother. 2004;48:4718–24. doi: 10.1128/AAC.48.12.4718-4724.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang MA, Lee J, Choi EH, Lee HJ. Pseudomonas aeruginosa bacteremia in children over ten consecutive years: analysis of clinical characteristics, risk factors of multi-drug resistance and clinical outcomes. J Korean Med Sci. 2011;26:612–8. doi: 10.3346/jkms.2011.26.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:e56–93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 13.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–64. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 14.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 15.van Delden C. Pseudomonas aeruginosa bloodstream infections: how should we treat them? Int J Antimicrob Agents. 2007;30(Suppl 1):S71–5. doi: 10.1016/j.ijantimicag.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Costa GA, Delgado AF, Ferraro A, Okay TS. Application of the pediatric risk of mortality (PRISM) score and determination of mortality risk factors in a tertiary pediatric intensive care unit. Clinics (Sao Paulo) 2010;65:1087–92. doi: 10.1590/S1807-59322010001100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia L. Clinical microbiology procedures handbook. 3rd ed. Washington, DC: American Society for Microbiology; 2010. [Google Scholar]
- 18.Kuti JL, Nightingale CH, Quintiliani R, Nicolau DP. Pharmacodynamic profiling of continuously infused piperacillin/tazobactam against Pseudomonas aeruginosa using Monte Carlo analysis. Diagn Microbiol Infect Dis. 2002;44:51–7. doi: 10.1016/s0732-8893(02)00416-9. [DOI] [PubMed] [Google Scholar]
- 19.DeRyke CA, Kuti JL, Nicolau DP. Reevaluation of current susceptibility breakpoints for Gram-negative rods based on pharmacodynamic assessment. Diagn Microbiol Infect Dis. 2007;58:337–44. doi: 10.1016/j.diagmicrobio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Ellis JM, Kuti JL, Nicolau DP. Use of Monte Carlo simulation to assess the pharmacodynamics of beta-lactams against Pseudomonas aeruginosa infections in children: a report from the OPTAMA program. Clin Ther. 2005;27:1820–30. doi: 10.1016/j.clinthera.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Yamagishi Y, Terada M, Ohki E, Miura Y, Umemura T, Mikamo H. Investigation of the clinical breakpoints of piperacillin-tazobactam against infections caused by Pseudomonas aeruginosa. J Infect Chemother. 2012;18:127–9. doi: 10.1007/s10156-011-0285-3. [DOI] [PubMed] [Google Scholar]
- 22.Tam VH, Gamez EA, Weston JS, et al. Outcomes of bacteremia due to Pseudomonas aeruginosa with reduced susceptibility to piperacillin-tazobactam: implications on the appropriateness of the resistance breakpoint. Clin Infect Dis. 2008;46:862–7. doi: 10.1086/528712. [DOI] [PubMed] [Google Scholar]
- 23.Drusano GL. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.”. Nat Rev Microbiol. 2004;2:289–300. doi: 10.1038/nrmicro862. [DOI] [PubMed] [Google Scholar]
- 24.Courter JD, Kuti JL, Girotto JE, Nicolau DP. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr Blood Cancer. 2009;53:379–85. doi: 10.1002/pbc.22051. [DOI] [PubMed] [Google Scholar]
- 25.Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol. 2007;45:2723–5. doi: 10.1128/JCM.00015-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGettigan SE, Andreacchio K, Edelstein PH. Specificity of ertapenem susceptibility screening for detection of Klebsiella pneumoniae carbapenemases. J Clin Microbiol. 2009;47:785–6. doi: 10.1128/JCM.02143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Bano J, Picon E, Navarro MD, Lopez-Cerero L, Pascual A. Impact of changes in CLSI and EUCAST breakpoints for susceptibility in bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli. Clin Microbiol Infect. 2011 doi: 10.1111/j.1469-0691.2011.03673.x. doi:10.1111/j.1469-0691.2011.03673. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 20th information supplement (June 2010 update). M100-S20. Wayne, PA: CLSI: 2010. [Google Scholar]
- 29.Landman D, Salamera J, Singh M, Quale J. Accuracy of carbapenem nonsusceptibility for identification of KPC-possessing Enterobacteriaceae by use of the revised CLSI breakpoints. J Clin Microbiol. 2011;49:3931–3. doi: 10.1128/JCM.01176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones RN, Stilwell MG, Rhomberg PR, Sader HS. Antipseudomonal activity of piperacillin/tazobactam: more than a decade of experience from the SENTRY Antimicrobial Surveillance Program (1997–2007) Diagn Microbiol Infect Dis. 2009;65:331–4. doi: 10.1016/j.diagmicrobio.2009.06.022. [DOI] [PubMed] [Google Scholar]