Abstract
Background
Cardiac stem cell therapy remains hampered by acute donor cell death post transplantation and the lack of reliable methods for tracking cell survival in vivo. We hypothesize that cells transfected with inducible vascular endothelial growth factor 165 (VEGF165) can improve their survival as monitored by novel molecular imaging techniques.
Methods and Results
Mouse embryonic stem (ES) cells were transfected with an inducible, bi-directional tetracycline (Bi-Tet) promoter driving VEGF165 and renilla luciferase (Rluc). Addition of doxycycline induced Bi-Tet expression of VEGF165 and Rluc significantly compared to baseline (P<0.05). Expression of VEGF165 enhanced ES cell proliferation and inhibited apoptosis as determined by Annexin-V staining. For noninvasive imaging, ES cells were transduced with a double fusion (DF) reporter gene consisting of firefly luciferase and enhanced green fluorescence protein (Fluc-eGFP). There was a robust correlation between cell number and Fluc activity (R2=0.99). Analysis by immunostaining, histology, and RT-PCR confirmed that expression of Bi-Tet and DF systems did not affect ES cell self-renewal or pluripotency. ES cells were differentiated into beating embryoid bodies expressing cardiac markers such as troponin, Nkx2.5, and β-MHC. Afterwards, 5×105 cells obtained from these beating embryoid bodies or saline were injected into the myocardium of SV129 mice (n=36) following ligation of the left anterior descending (LAD) artery. Bioluminescence imaging (BLI) and echocardiography showed that VEGF165 induction led to significant improvements in both transplanted cell survival and cardiac function (P<0.05).
Conclusion
This is the first study to demonstrate imaging of embryonic stem cell mediated gene therapy targeting cardiovascular disease. With further validation, this platform may have broad applications for current basic research and future clinical studies.
Keywords: embryonic stem cell, gene therapy, molecular imaging, Tet-on system, vascular endothelial growth factor, myocardial infarction
INTRODUCTION
Ischemic heart disease (IHD) remains the leading cause of morbidity and mortality in the US. Despite advances in pharmacotherapy and coronary artery bypass surgery, a significant proportion of patients continue to develop refractory symptoms. In recent years, stem cell transplantation has become arguably the most promising approach for the treatment of IHD. Several studies have shown that transplantation of bone marrow cells (Orlic et al., 2001), endothelial progenitor cells (Takahashi et al., 1999), and skeletal myoblasts (Murry et al., 1996) can help accelerate the myocardial regenerative process. Likewise, transplantation of undifferentiated mouse embryonic stem (ES) cells (Hodgson et al., 2004) or ES cell-derived beating embryoid bodies (Behfar et al., 2002; Min et al., 2002; Min et al., 2003) can prevent adverse ventricular remodeling.
At present, a critical concern in stem cell transplantation remains acute donor cell death. For example, neonatal cardiomyocytes transplanted into the myocardium suffer >70% cell loss within the first 24 hours (Muller-Ehmsen et al., 2002). This phenomenon of early cell death is also thought to be the primary reason why cell-based therapy has been unsuccessful to date in a similar related field—treatment of patients with Duchenne’s muscular dystrophy (DMD) (Smythe et al., 2000). Another concern underlying stem cell therapy is the lack of available technology for monitoring delivered cell fate in vivo. It is well known that traditional postmortem histology requires animals to be sacrificed at different time points and does not shed light upon the longitudinal cell survival and biodistribution patterns within individual subject. Thus, the ability to track cell fate noninvasively would, therefore, represent an important first step toward better understanding of the molecular and cellular mechanisms governing stem cell biology in vivo.
In this study, we hypothesize that inducible VEGF expression may improve the survival and proliferation of transplanted cells in vivo and that these processes can be monitored by non-invasive molecular imaging studies.
EXPERIMENTAL PROCEDURES
Preparation of mouse ES cells
Mouse ES-D3 (CRL-1934) line was obtained from the American Type Culture Collection (Manassas, VA) and maintained with DMEM on mitotically inactive mouse embryonic fibroblast (MEF) feeder cells. The medium was supplemented with 15% fetal bovine serum, 0.1 mM β-mercaptoethanol, and 103 units/ml of leukemia inhibitory factor (Chemicon, Temecula, CA) to suppress ES cell differentiation as described (Boheler et al., 2002).
Cloning and Bi-Tet transfection
The regulator plasmid pTet-On (Clontech, Mountain View, CA) was transfected into mouse ES cells by electroporation at 240V and 500μF (Gene Pulser Apparatus, BioRad). For the response plasmid pBi-Tet, both human VEGF165 and renilla luciferase were cloned into the bi-directional cassettes. Afterwards, pBi-Tet expressing VEGF165-TRE-renilla was co-transfected with a selection plasmid pTK-hygromycin at a ratio of 4:1. Positive colonies were selected with 200 μg/ml of Hygromycin B. To prevent baseline leaky expression, FBS was replaced by Tet System Approved FBS (BD, Palo Alto, CA).
Transduction with double fusion reporter genes
Mouse ES cells were also transduced with self-inactivating lentiviral vector carrying an ubiquitin promoter that drives firefly luciferase and enhanced green fluorescence protein (LV-pUb-Fluc-eGFP) at a multiplicity of infection (MOI) of 10. The LV-pUb-Fluc-eGFP vector is a gift from Dr. Sanjiv Gambhir (Stanford University). Stable clones were isolated using fluorescence activated cell sorter (FACS) for eGFP expression.
Doxycycline induction of VEGF165 and renilla enzyme activities
Increasing dosages of doxycycline (0–5,000 ng/ml) were added to the culture medium over 48 hours to determine the optimal dose-response relationship between doxycycline and renilla/VEGF165 production. Cell supernatants were collected and VEGF165 was determined by ELISA according to manufacturer’s protocol (Quantikine Human VEGF Immunoassay, R&D Systems) (Wu et al., 2004). Renilla activity was determined using colenterazine (50 μg/ml) as the reporter substrate, whereas enzyme activity was expressed as relative light unit per milligram protein (RLU/mg).
Cell proliferation and apoptosis assays
The CyQuant cell proliferation assay (Molecular Probes, Eugene, OR) was measured using a microplate spectrofluorometer (Gemini EM, Sunnyvale, CA) at 24, 48, and 72 hour time points. Eight samples were assayed and averaged. The Annexin-V-PE apoptosis detection kit (BD, San Diego, CA) and propidium iodide (PI) were used to assess ES cell apoptosis after exposure to hypoxia (1% O2) for 24 hours. Apoptotic cells were quantified by flow cytometry (BD LSR cell analyzer, San Jose, CA).
Differentiation of ES cells into beating embryoid bodies
ES cells were dispersed with trypsin, resuspended in differentiation medium, and cultured using the hanging drop method as described (Maltsev et al., 1994). Cells were then seeded onto 48-well gelatin-coated plates for additional 10 days. Spontaneously beating clusters were dissected with a sterile micropipette and re-cultured for 24 hours before injection into animal hearts.
RT-PCR analysis of embryonic and cardiac specific transcriptions
The expression of ES cell (Oct4), endoderm (AFP), mesoderm (Flk-1), ectoderm (Ncam), and cardiac specific markers (MLC2V, Nkx2.5 and β-MHC) were compared before and after ES cell differentiation at days 0 and 14. Primer sequences for these specific genes are listed in Supplementary Table 1.
Myocardial infarction and echocardiography
Mice were induced with 2–4% isoflurane and maintained with 1.5–3% isoflurane with a small animal ventilator. Ligation of the mid left anterior descending (LAD) artery was performed in adult, female SV129 mice (Charles River Laboratories) by a single experienced surgeon (AYS) as described (Swijnenburg et al., 2005). Myocardial infarction was confirmed by myocardial blanching and EKG changes. After waiting for 15 minutes, animals were randomized into 3 groups (n=12/each group): (1) saline injection as control, (2) 5×105 of Bi-Tet cells from beating clusters without doxycycline induction, and (3) 5×105 of Bi-Tet cells from beating clusters with doxycycline induction (1 mg/ml in drinking water for 2 weeks). In all groups, the volume of injection was 25 μl using a 31-gauge Hamilton syringe. The site of injection is near the peri-infarct zone as indicated by myocardial tissue blanching. Echocardiography was performed using the Siemens-Acuson Sequoia ultrasound equipped with a 8–14-MHz transducer by an investigator (FC) blinded to group designation. Left ventricular ejection fraction was calculated as follows: LVEF= (S2−D2)/D2×100%, where S is the systolic and D the diastolic dimension of the left ventricle (Collins et al., 2003). All animal procedures were performed in accordance with the Stanford Animal Research Committee.
Bioluminescence imaging of cell survival and histologic analysis
After intraperitoneal injection of the reporter probe D-Luciferin (125 mg/kg body weight), animals were imaged for 30 min with 1-min acquisition intervals using the Xenogen In Vivo Imaging System (Alameda, CA). The same mice were scanned repetitively for 3 to 6 weeks. Bioluminescence was quantified in units of maximum photons per second per centimeter square per steridian (p/s/cm2/sr) as previously described (Wu et al., 2006). After imaging, animals were sacrificed and explanted hearts processed, embedded, and sectioned for hematoxylin and eosin (H&E) staining and immunofluorescence histology. Primary antibodies selected in this study were anti-troponin-T, connexin-43, Von Willebrand factor (vWF), and GFP derived from rabbit. Secondary antibodies were FITC or TRITC conjugated goat anti-rabbit IgG (Millipore, CA). Nuclei were shown by DAPI (Invitrogen, OR) staining. Slides were interpreted by an expert cardiac pathologist blinded to the study condition (AJC).
Statistical analysis
Data are given as mean ± standard deviation. For statistical analysis, the ANOVA and repeated measures ANOVA with post-hoc testing and two-tailed Student’s t –test were used. Differences were considered significant at P-values of <0.05.
RESULTS
Inducible expression of VEGF165 in mouse ES cells
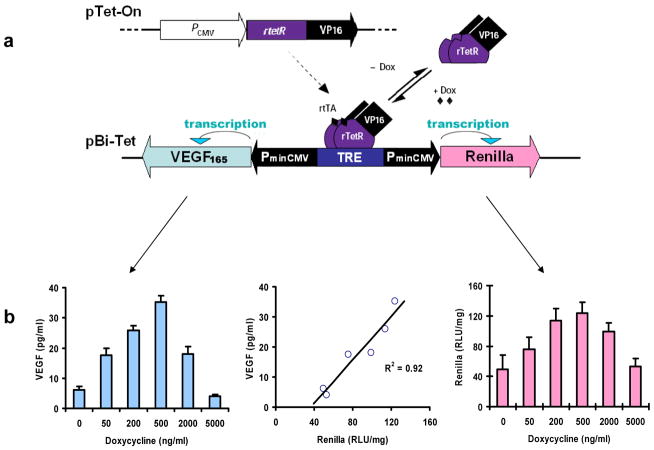
The tetracycline-responsive promoter is a powerful approach for tight control of gene expression in mammalian cells as first described by Gossen and Bujard (Gossen and Bujard, 1992). In the presence of doxycycline (Dox), the fusion protein of reverse Tet repressor protein and virion protein 16 activation domain (rTetR-VP16) binds to the tetracycline-responsive element (TRE). This interaction leads to bi-directional activation of VEGF165 and renilla transgenes driven by the minimal immediate early promoter of cytomegalovirus (PminCMV) (Figure 1a). In the absence of Dox, the rTetR-VP16 fusion protein does not bind to TRE and there is no activation of VEGF165 or renilla. To optimize the dosage for gene induction, stably transfected ES cells were treated with 0, 50, 200, 500, 2000 and 5000 ng/ml of Dox. Quantitative measurements by ELISA and enzyme assays showed peak production of VEGF165 (35.2±2.2 pg/ml) and renilla (123.7±14.3 RLU/mg) at 500 ng/ml of Dox (P<0.01 vs. basal levels). The robust correlation between VEGF165 and renilla (R2=0.92) confirms that Dox can tightly regulate the expression of two different genes via the Bi-Tet system (Figure 1b).
Figure 1. Genetic modification of mouse ES cells with inducible VEGF165 expression.
(a) Schematic diagram showing inducible gene expression system composed of 2 constructs: pTet- On and pBi-Tet. (b) The expression of VEGF165 (left) and renilla (right) can be tightly regulated by doxycycline with 500 ng/ml determined as the optimal dosage. There is a robust correlation between the expression of the two genes (middle).
Induction of VEGF165 in ES cells confers pro-survival advantages
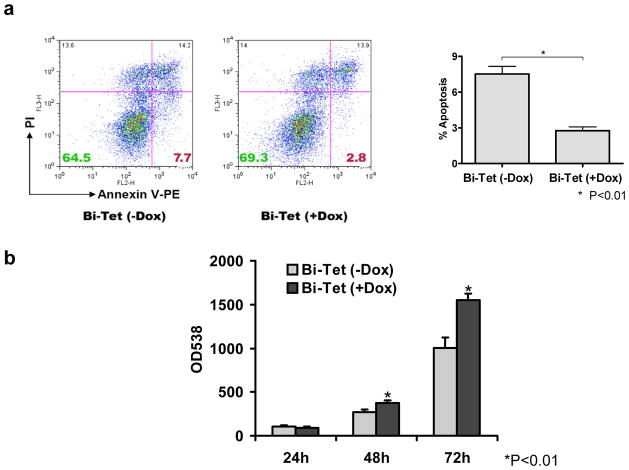
VEGF165 is known to increase angiogenesis and reduce apoptosis in transplanted bone marrow cells (Yau et al., 2005) and skeletal myoblasts (Suzuki et al., 2001). To understand the mechanisms of this improvement, we exposed our Bi-Tet ES cells to 24-hour of 1% O2 hypoxia versus 21% O2 normoxia and analyze the extent of necrosis (propidium iodide staining) and apoptosis (annexin-V staining) by flow cytometry. Figure 2a shows in the presence of hypoxia, inducible-VEGF165 has protective effects by reducing cell apoptosis (2.2±0.5% vs. 7.7±1.1%; P<0.01) in Bi-Tet (+Dox) compared to Bi-Tet (−Dox) cells, respectively. These results are also consistent with increased cell proliferation activity of Bi-Tet (+Dox) cells compared to Bi-Tet (−Dox) cells at 48- and 72-hour time points (Figure 2b).
Figure 2. Expression of VEGF165 promotes cell proliferation and inhibits cell apoptosis in vitro.
(a) Expression of VEGF165 inhibits cell death in vitro. Hypoxia (1% O2 for 24 hours) leads to significant apoptosis in Bi-Tet ES cells without doxycyline induction of VEGF165 (7.7±1.1%) compared to Bi-Tet cells with doxycyline induction of VEGF165 (2.2±0.5%) as determined by flow cytometry of Annexin-V staining (*P<0.01). (b) Expression of VEGF165 promotes cell proliferation. CyQuant assay shows significantly increased proliferation in Bi-Tet (+Dox) cells compared to Bi-Tet (−Dox) cells at 48 and 72 hours (*P<0.01). Data are expressed as mean ± SD.
Reporter gene and Bi-Tet systems do not affect ES cell differentiation
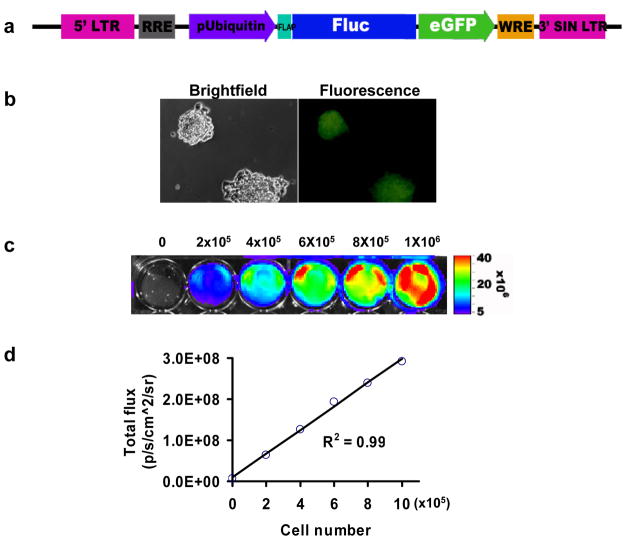
In order to track transplanted ES cells in vivo, a double fusion reporter gene construct (Fluc-eGFP) was introduced into Bi-Tet ES cells (Figure 3a). Stable clones were selected with FACS and confirmed by fluorescence microscopy (Figure 3b). Bioluminescence imaging of Bi-Tet ES cells plated on 24-well plates showed linear increases of Fluc photon signals with cell numbers (R2=0.99) (Figure 3c–d). Both Fluc and eGFP reporter gene expression remained stable for >3 months (~40 passages, data not shown). Signal activity was also maintained during differentiation of ES cells into beating clusters using the hanging drop method (Figure 4a and Supplementary Video 1). Immunostaining of beating cluster cells were positive for cardiac specific markers such as troponin-T and connexin-43 (Figure 4b). Similarly, subcutaneous injection of undifferentiated ES cells retained the ability to differentiate into all three germ layers (i.e., ectoderm, mesoderm, endoderm), including patches of striated cardiomyocytes (Figure 4c). Finally, compared to non-transduced ES cells, Bi-Tet ES cells exhibited no significant differences in expression patterns of known genes underlying germ layer (AFP, Flk-1, Ncam) and cardiac lineage (MLC2v, Nkx2.5, β-MHC) specific differentiation (Figure 4d). Taken together, these studies suggest that self-renewal and pluripotency of mouse ES cells are not affected by introduction of our genetic constructs.
Figure 3. Expression of double fusion reporter gene in ES cells.
(a) Schema of lentiviral construct showing ubiquitin promoter driving Fluc and eGFP linked by a 14-amino acid linker (LENSHASAGYQAST). (b) Brightfield and fluorescence microscopy show robust eGFP expression in undifferentiated ES cells that remain stable for >3 months. (c) Bioluminescence imaging of ES cells plated on 24-well dishes shows photon signals generated by interaction of Fluc reporter protein and D-Luciferin reporter probe. (d) The strong correlation (R2=0.99) between Fluc activity and cell number serves as an in vitro validation that imaging can be used to follow cell survival and proliferation in vivo.
Figure 4. Reporter gene and Bi-Tet systems do not affect ES cell pluripotency.
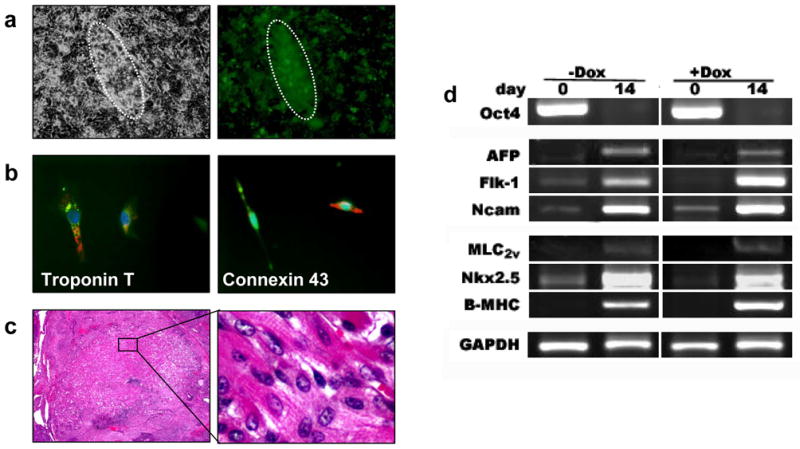
(a) Brightfield and fluorescence microscopy of a representative beating embryoid body at day 14 of differentiation. (b) Immunostaining of isolated single cells confirms the expression of cardiac specific markers such as troponin-T and connexin-43. Green, GFP; Red, TRITC-antibody stain; Blue, DAPI-stained nuclei. (c) Histology of ES cells injected subcutaneously showing in vivo differentiation into striated cardiomyocytes. (d) RT-PCR comparison of Bi-Tet (−Dox) and Bi-Tet (+Dox) cells at day 0 and day 14 of differentiation. There is no significant difference in expression of ES cell (Oct4), endoderm (AFP), mesoderm (Flk-1), ectoderm (Ncam), and cardiac specific markers (MLC2v, Nkx2.5, β-MHC) between the 2 groups, suggesting that reporter gene and Bi-Tet systems do not affect ES cell pluripotency.
Assessment of transplanted cell survival, proliferation, and function after transplantation
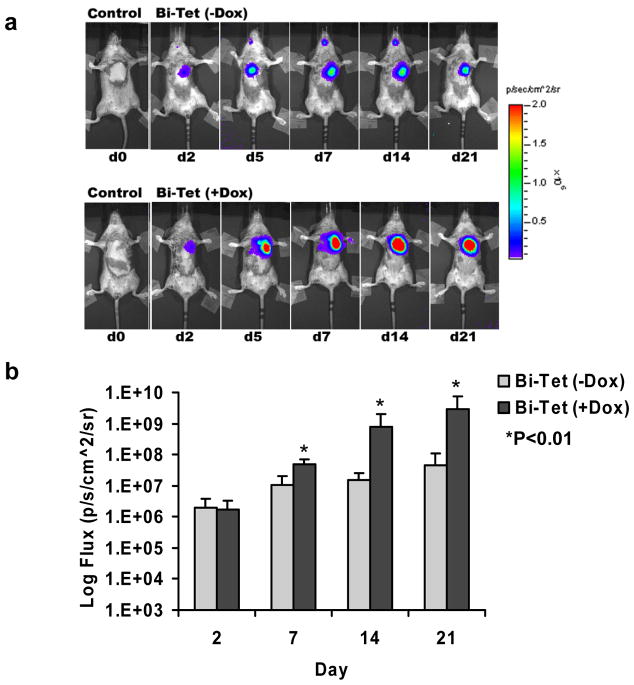
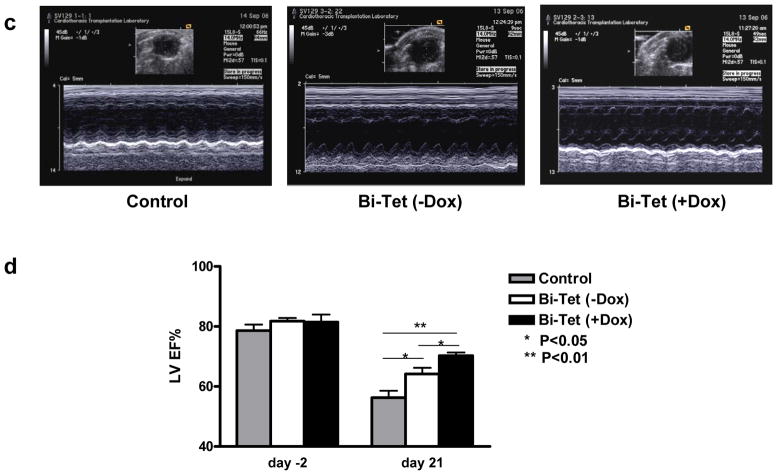
For in vivo assessment, animals first underwent ligation of the LAD artery to induce myocardial infarction followed by transplantation with 5×105 cells harvested from beating embryoid bodies. To determine the spatiotemporal kinetics of transplanted cell survival, all animals underwent longitudinal bioluminescence imaging (Figure 5a). As expected, control animals injected with saline showed background signals only (3.14±0.09 × 103 photons/sec/cm2/sr). Both the Bi-Tet (−Dox induction) and Bi-Tet (+Dox induction) groups showed progressive increase in Fluc signals from day 2 to day 21, indicating both cell survival and proliferation in the heart. Detailed quantitative analysis confirmed that cell proliferation was significantly more robust under +Dox induction starting at day 7 (Figure 5b), which was consistent with the in vitro assay results showing that VEGF165 induction improved cell proliferation and decreased cell apoptosis (Figure 2). To assess for functional changes, echocardiography was performed pre-operatively (day −2) and post-operatively (day +21) (Figure 5c). The LVEF was comparable in all groups pre-operatively: 78.6±4.9% for control, 81.7±2.6% for Bi-Tet (−Dox), and 81.4±6.1% for Bi-Tet (+Dox) (P=N.S.). Postoperatively, the LVEF was significantly higher in the Bi-Tet (+Dox) group compared to the control group (70.2±2.6 vs. 56.2±5.6%; P<0.01). In addition, the degree of improvement in LVEF was also significantly higher in the Bi-Tet (+Dox) group compared to Bi-Tet (−Dox) group (70.2±2.6 vs. 64.1±5.0%; P<0.05) (Figure 5d). Taken together, these results suggest that inducible VEGF165 expression by transplanted cells confers survival advantage (assessed by bioluminescence imaging) which translates into functional benefit following myocardial infarction (assessed by echocardiography).
Figure 5. Cell-mediated gene therapy improves cell survival and cardiac contractility following ischemia-induced myocardial injury.
(a) Bioluminescence imaging of transplanted cells expressing the Fluc reporter gene over 3 weeks. Animals were randomized to receive saline (control), Bi-Tet cells without doxycycline induction (top row), and Bi-Tet cells with doxycyline induction (bottom row). (b) Quantitative analysis shows significantly higher photon signals (reflecting enhanced Bi-Tet cell survival) in animals with +Dox induction of VEGF (*P<0.01). (c) Representative M-mode echocardiogram images of animals injected with saline as control, Bi-Tet cells without doxycycline induction, and Bi-Tet cells with doxycyline induction. (d) Quantitative analysis shows significant improvement in LVEF in the Bi-Tet cells with doxycyline induction group compared to the Bi-Tet cells without doxcycyline (*P<0.05) as well as control group three weeks after cell transplantation (**P<0.01).
Histology confirming engraftment of ES cell-derived beating embryoid bodies
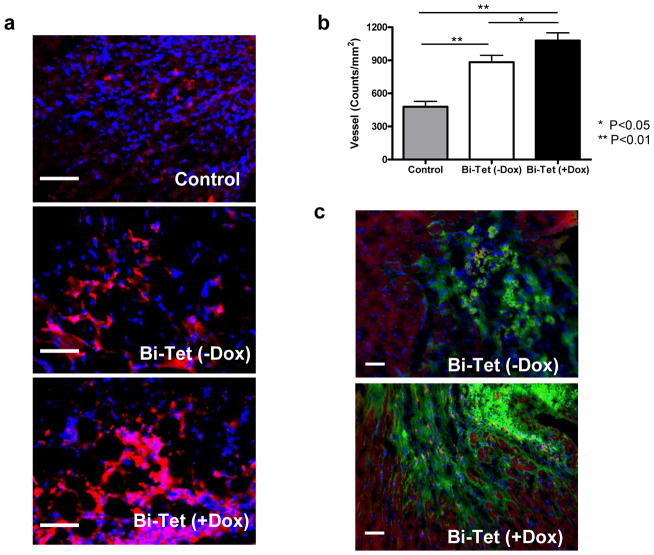
After imaging, all animals were sacrificed and histology of explanted hearts was performed for postmortem confirmation. Three weeks after transplantation, both Bi-Tet (−Dox) and Bi-Tet (+Dox) groups had higher neoangiogenesis compared to the control saline group as assessed by anti-vWF staining (Figure 6a). Near the peri-infarct zone, the myocardial vessel density was significantly enhanced in the Bi-Tet (+Dox) compared to the Bi-Tet (−Dox) group (1079±141 vs. 883±125 counts/mm2; P<0.05). Not surprisingly, both cell groups had higher vessel density compared to the saline control group (479±98; P<0.01) (Figure 6b). Engraftment of transplanted beating embryoid cells was confirmed by immunofluorescence staining showing GFP positive cells (green) near troponin-T positive (red) host myocardium as shown in Figure 6c.
Figure 6. Histology confirming engraftment of transplanted cells.
(a) Representative anti-vWF stainings from animals transplanted with Bi-Tet (+Dox) cells, Bi-Tet (−Dox) cells, and saline control at day 21. (b) Quantitative analysis of capillary density shows improved angiogenesis in the Bi-Tet (+Dox) group compared to the Bi-Tet (−Dox) (*P<0.05) and saline control group (**P<0.01). (c) Immunostaining with troponin-T (red) and GFP (green) demonstrate surviving engrafted cells can incorporate into the host myocardium. Double-positive cells can be found as isolated pockets of cellular islands within the graft as well as in the boundary of transplantation. Nuclei are identified by DAPI staining (blue). Scale bar=50μm.
Long term follow-up reveals teratoma formation
For a subset of the −Dox and +Dox treated animals (n=5 per group), we followed transplanted cell fate for an additional 3–4 weeks by weekly bioluminescence imaging. At these later time points, robust signals were detected in both intra-cardiac and extra-cardiac regions (Figure 7a). Despite having harvested cells from ES cell-derived beating embryoid bodies, histological analysis showed morphology consistent with teratoma formation in both groups (Figure 7b). Our imaging analysis also showed that the Bi-Tet (+Dox) group, with VEGF165 induction, had more dissemination of the teratomas. There were also significantly fewer apoptotic nuclei in the +Dox group compared to the −Dox group, again consistent with our in vitro observation showing protective effects of VEGF165 expression.
Figure 7. Long-term follow-up of transplanted beating embryoid bodies reveals teratoma formation.
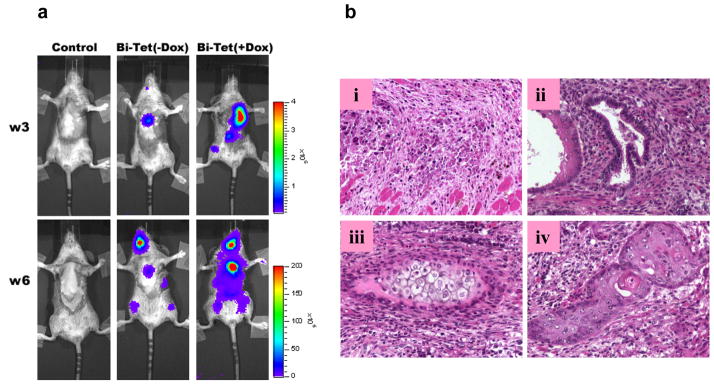
(a) Representative bioluminescence imaging shows teratoma formation in both the Bi-Tet (−Dox) and Bi-Tet (+Dox) animals. (b) Representative postmortem histology of the explanted hearts shows (i) apoptosis of some transplanted cells in the BiTet (−Dox) group as well as differentiation into (ii) glandular epithelia (endoderm), (iii) cartilage (mesoderm), and (iv) squamous epithelia (ectoderm) in the heart.
DISCUSSION
In this study, we validated the novel approach of imaging genetically modified ES cells designed for inducible gene expression. Our findings are summarized as follows: (i) the Bi-Tet system allows inducible expression of two genes (VEGF165 and renilla) with strong correlation; (ii) VEGF165 expression confers resistance against cellular apoptosis during hypoxia in vitro and ischemia in vivo; (iii) the reporter gene and Bi-Tet systems do not affect two hallmarks of ES cell biology–self renewal and pluripotency; (iv) noninvasive imaging shows that +Dox induction of VEGF165 expression enhances cell survival and proliferation in living animals; (v) despite differentiation of ES cells into beating embryoid bodies prior to transplantation, these cells still have the potential to form teratomas in the long term.
Presently, available methods of monitoring transplanted cells are quite limited. In animal studies, typical reporter markers (e.g., β-galactosidase and GFP) require the animals to be sacrificed for histological analysis, which provide only single “snapshots” of cell engraftment. Thus, monitoring the outcomes of stem cell therapy longitudinally requires the development of noninvasive strategies capable of identifying the location, magnitude, and duration of transplanted cells. The reporter gene imaging system that we have developed provides several unique advantages over other existing techniques. First, in contrast to radionuclide or iron labeling techniques (Kraitchman et al., 2005), only viable cells are detected as expression of the reporter gene product requires the cells be alive. Second, the reporter gene can be integrated into the cellular chromosomes and passed on from mother to daughter cells, thereby permitting the tracking of cell proliferation. Third, multiple reporter genes can be combined for multi-modality visualization, and can also be linked to tissue specific promoters for precise analysis of differentiation pathways (Chang et al., 2006).
Although stem cell transplantation has emerged in recent years as a promising therapy for myocardial disease, its application may be limited by low donor cell survival. The exact reasons for cell death are still unclear, but inflammation, ischemia, apoptosis, and immune response all appear to play a role (Smythe et al., 2000). Using PCR analysis of male-specific Sry gene, Muller-Ehmsen et al. quantified the survival of transplanted fetal cardiomyocytes as 57±9% at 1 hour and only 24±6% at 24 hours (Muller-Ehmsen et al., 2002). A recent study by Yau et al. noted acute cell loss of ~30% immediately following bone marrow cell injection followed by a progressive decrease over time (Yau et al., 2005). Ex vivo modification with pro-angiogenic genes such as VEGF165 appears to be a viable strategy for improving the survival of transplanted bone marrow cells (Yau et al., 2005), skeletal myoblasts (Suzuki et al., 2001), as well as ES cell-derived beating clusters as shown in this study. Expression of VEGF can induce upregulation of VEGF receptors such as Flk-1 and Flt-1 in host myocardium through paracrine signaling pathways, leading to enhanced angiogenesis that promotes cell survival (Yla-Herttuala and Alitalo, 2003). The benefit that VEGF exert to stem cells may include activation of the mitogen-activated protein kinase, protein kinase C, and Akt pathways. These pathways are known to play a pivotal role in cell survival, proliferation, and angiogenesis (Zachary and Gliki, 2001). Expression of VEGF can also enhance mitosis and connexin-43 expression on ischemic heart tissues in animal models (Laguens et al., 2002). Likewise, clinical gene therapy trials using VEGF have shown slight beneficial effects on cardiac viability and functional recovery (Yla-Herttuala and Alitalo, 2003). However, prolonged and unregulated VEGF expression can result in hemangioma formation in the heart (Lee et al., 2000). As shown in this study, VEGF expression might also increase the possibility of teratoma formation by enhancing angiogenesis or by providing a microenvironment favorable for teratoma growth. Thus, to circumvent these problems, further validation of a Bi-Tet system in which VEGF can be selectively activated, such as the one employed in the present study, could have attractive advantages for future stem cell-based gene therapy (Melo et al., 2004).
Previously, several groups have reported that intra-cardiac transplantation of undifferentiated mouse ES cells or beating embryoid bodies (Behfar et al., 2002; Hodgson et al., 2004; Min et al., 2002; Min et al., 2003) improved ventricular function without concomitant teratoma formation. Although the exact reasons for this discrepancy is unclear (e.g., variation between surgical models, cell dosages, and animal species), it is possible that conventional histology-based sampling employed by these studies may not have identified all the potential sites of cell survival, migration, and subsequent teratoma formation. Whole body optical imaging circumvents this sampling bias issue and, as our results demonstrate, allows the ability to track cells as they migrate and proliferate to sites distant from the original area of transplantation. Thus, the observation of teratoma formation in our cohort again cautions against the indiscriminate use of undifferentiated ES cells for myocardial regeneration purpose as well as call for the needs for noninvasive monitoring.
Our imaging results are also concordant with findings from a recent study by Kolossov et al. that compared cardiac injection of undifferentiated ES cells, ES-cell derived beating embryoid bodies, and ES cell-derived cardiac cells (Kolossov et al., 2006). The investigators showed that injection of undifferentiated ES cells led to teratoma formation in 13/13 animals. Similar to our study, injection of ES-cell derived beating embryoid bodies led to teratoma formation in 5/6 animals with delayed onset. Interestingly, injection of ES cell-derived cardiac cells (genetically modified with cardiac specific α-MHC promoter driving puromycin resistance and eGFP genes) still revealed tumor formation in 5% of the animals. Hence, the two studies indicate that “highly purified” ES cell-derived cardiac cells are required to minimize the risk of tumor formation. However, achieving the goal of 100% purified cardiac cells remains a daunting task and, to date, no study has reported such a feat. We believe imaging will play a critical role in evaluating tumorigenc potential of ES cell-derived cell lineages tailored for the treatment of cardiac disease as well as other debilitating conditions such as diabetes and spinal cord injury. For future clinical application, positron emission tomography (PET) reporter gene such as the herpes simplex virus type 1 thymidine kinase (HSV1-tk) may be used to track human ES cell-based therapies as well as other cell types (Cao et al., 2006; Chang et al., 2006; Wu et al., 2003).
In summary, this is the first study to image ES cells with inducible expression of VEGF165. We demonstrate that this approach can be used to monitor the spatiotemporal kinetics of cell survival, proliferation, and migration. While stem cell therapy for cardiac repair is an exciting area of investigation in both the laboratory and clinic, a fundamental understanding of optimal therapeutic cellular candidates, mechanisms underlying myocardial repair, and the long-term fates of transplanted cells remain elusive. Application of molecular imaging will be an extremely useful and perhaps necessary tool to aid in the continuing development of this field.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NHLBI, AHA, ACCF-GE, Stanford CVI (JCW) as well as the Stanford Dean’s Fellowship (XX).
Footnotes
CONFLICT OF INTERESTS: The authors declare that they have no conflicts to disclose.
References
- Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. Faseb J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, et al. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GY, Xie X, Wu JC. Overview of stem cells and imaging modalities for cardiovascular diseases. J Nucl Cardiol. 2006;13:554–569. doi: 10.1016/j.nuclcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Collins KA, Korcarz CE, Lang RM. Use of echocardiography for the phenotypic assessment of genetically altered mice. Physiol Genomics. 2003;13:227–239. doi: 10.1152/physiolgenomics.00005.2003. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson DM, Behfar A, Zingman LV, Kane GC, Perez-Terzic C, Alekseev AE, Puceat M, Terzic A. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H471–479. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguens R, Cabeza Meckert P, Vera Janavel G, Del Valle H, Lascano E, Negroni J, Werba P, Cuniberti L, Martinez V, Melo C, et al. Entrance in mitosis of adult cardiomyocytes in ischemic pig hearts after plasmid-mediated rhVEGF165 gene transfer. Gene Ther. 2002;9:1676–1681. doi: 10.1038/sj.gt.3301844. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium: deleterious effects of unregulated expression. Circulation. 2000;102:898–901. doi: 10.1161/01.cir.102.8.898. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ Res. 1994;75:233–244. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- Melo LG, Pachori AS, Kong D, Gnecchi M, Wang K, Pratt RE, Dzau VJ. Molecular and cell-based therapies for protection, rescue, and repair of ischemic myocardium: reasons for cautious optimism. Circulation. 2004;109:2386–2393. doi: 10.1161/01.CIR.0000128597.37025.00. [DOI] [PubMed] [Google Scholar]
- Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, Xiao YF. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol. 2002;92:288–296. doi: 10.1152/jappl.2002.92.1.288. [DOI] [PubMed] [Google Scholar]
- Min JY, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, Morgan JP, Xiao YF. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- Murry CE, Wiseman RW, Schwartz SM, Hauschka SD. Skeletal myoblast transplantation for repair of myocardial necrosis. J Clin Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Smythe GM, Hodgetts SI, Grounds MD. Immunobiology and the future of myoblast transfer therapy. Mol Ther. 2000;1:304–313. doi: 10.1006/mthe.2000.0049. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Murtuza B, Smolenski RT, Sammut IA, Suzuki N, Kaneda Y, Yacoub MH. Cell transplantation for the treatment of acute myocardial infarction using vascular endothelial growth factor-expressing skeletal myoblasts. Circulation. 2001;104:I207–212. doi: 10.1161/hc37t1.094524. [DOI] [PubMed] [Google Scholar]
- Swijnenburg RJ, Tanaka M, Vogel H, Baker J, Kofidis T, Gunawan F, Lebl DR, Caffarelli AD, de Bruin JL, Fedoseyeva EV, Robbins RC. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation. 2005;112:I166–172. doi: 10.1161/CIRCULATIONAHA.104.525824. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, Fishbein MC, Gambhir SS. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Chen IY, Wang Y, Tseng JR, Chhabra A, Salek M, Min JJ, Fishbein MC, Crystal R, Gambhir SS. Molecular imaging of the kinetics of vascular endothelial growth factor gene expression in ischemic myocardium. Circulation. 2004;110:685–691. doi: 10.1161/01.CIR.000013815302213.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Spin JM, Cao F, Lin S, Xie X, Gheysens O, Chen IY, Sheikh AY, Robbins RC, Tsalenko A, et al. Transcriptional profiling of reporter genes used for molecular imaging of embryonic stem cell transplantation. Physiol Genomics. 2006;25:29–38. doi: 10.1152/physiolgenomics.00254.2005. [DOI] [PubMed] [Google Scholar]
- Yau TM, Kim C, Li G, Zhang Y, Weisel RD, Li RK. Maximizing ventricular function with multimodal cell-based gene therapy. Circulation. 2005;112:I123–128. doi: 10.1161/CIRCULATIONAHA.104.525147. [DOI] [PubMed] [Google Scholar]
- Yla-Herttuala S, Alitalo K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med. 2003;9:694–701. doi: 10.1038/nm0603-694. [DOI] [PubMed] [Google Scholar]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.