This retrospective cohort study of 39 US hospitals found that Centers for Medicare & Medicaid Services nonpayment for hospital-acquired catheter-associated urinary tract infections (UTIs) has not led to overtesting for UTI on admission or to increased antimicrobial prescribing.
Abstract
Background. On 1 October 2008, in an effort to stimulate efforts to prevent catheter-associated urinary tract infection (CAUTI), the Centers for Medicare & Medicaid Services (CMS) implemented a policy of not reimbursing hospitals for hospital-acquired CAUTI. Since any urinary tract infection present on admission would not fall under this initiative, concerns have been raised that the policy may encourage more testing for and treatment of asymptomatic bacteriuria.
Methods. We conducted a retrospective multicenter cohort study with time series analysis of all adults admitted to the hospital 16 months before and 16 months after policy implementation among participating Society for Healthcare Epidemiology of America Research Network hospitals. Our outcomes were frequency of urine culture on admission and antimicrobial use.
Results. A total of 39 hospitals from 22 states submitted data on 2 362 742 admissions. In 35 hospitals affected by the CMS policy, the median frequency of urine culture performance did not change after CMS policy implementation (19.2% during the prepolicy period vs 19.3% during the postpolicy period). The rate of change in urine culture performance increased minimally during the prepolicy period (0.5% per month) and decreased slightly during the postpolicy period (–0.25% per month; P < .001). In the subset of 10 hospitals providing antimicrobial use data, the median frequency of fluoroquinolone antimicrobial use did not change substantially (14.6% during the prepolicy period vs 14.0% during the postpolicy period). The rate of change in fluoroquinolone use increased during the prepolicy period (1.26% per month) and decreased during the postpolicy period (–0.60% per month; P < .001).
Conclusions. We found no evidence that CMS nonpayment policy resulted in overtesting to screen for and document a diagnosis of urinary tract infection as present on admission.
Catheter-associated urinary tract infection (CAUTI) is the most common healthcare-associated infection in the United States [1]. On 1 October 2008, the Centers for Medicare & Medicaid Services (CMS) stopped reimbursing hospitals for treatment of hospital-acquired CAUTI and other hospital-acquired conditions they deemed were “reasonably preventable” [1]. Since then, hospitals only receive reimbursement if a urinary tract infection was demonstrated to have been present at the time the patient was admitted to the hospital (rather than acquired during hospitalization) [2]. There is a debate over whether this policy will decrease the frequency of CAUTI or whether it may create perverse incentives for hospitals to document asymptomatic bacteriuria at the time of admission and inappropriately treat them as urinary tract infections, to avoid loss of payment if a CAUTI is diagnosed after admission. Although the policy explicitly applies to reimbursement for CAUTI, documentation of any urinary tract infection at admission would increase reimbursement. As described in a recent editorial, “if, as a result of the rule change, clinicians were pressured to test the urine of all patients on admission to the hospital, the risk of unnecessary treatment of asymptomatic bacteriuria or inflammation would be substantial” [3]. Past CMS policy designed to improve management of pneumonia has had the unintended consequence of antibiotic overuse [4].
Several groups of patients commonly have bacteria in their urine without any symptoms of infection (ie, asymptomatic bacteriuria) and do not benefit from antimicrobials [5]. For these patients, the possibility of overperformance of urine culture at admission as a screening tool to detect and document urinary tract infection as being present on admission, and thereby to avoid possible nonpayment, is real and concerning [6]. Urinary tract infection is a diagnosis based largely on clinical symptoms, with the support of nonspecific laboratory tests such as urinalysis and urine culture [6, 7]. Patients with urinary catheters are particularly prone to bacteriuria and pyuria, which would fulfill the criteria for CAUTI in the appropriate clinical setting of fever, pain, tenderness, or other nonspecific criteria [7]. Given that fever and abdominal pain are often caused by other conditions, culturing urine more frequently would be expected to yield an increased frequency of urinary tract infection diagnoses, even if symptoms in patients receiving the diagnosis were best explained by another clinical syndrome. This increase in false-positive diagnoses of urinary tract infection at admission would be expected to increase inappropriate use of antimicrobials for patients with a diagnosis of urinary tract infection, resulting in potential increases in bacterial resistance, Clostridium difficile infections, and adverse drug reactions over time. Our aim was to thus evaluate whether implementation of the CMS policy of nonreimbursement for hospital-acquired CAUTI was temporally associated with increased frequency of urine culture on hospital admission. As a secondary outcome, we examined whether implementation of the CMS policy was associated with increased use of antimicrobials commonly used for the treatment of urinary tract infection.
METHODS
This study was completed using the Society for Healthcare Epidemiology of America (SHEA) Research Network, a consortium of >200 hospitals that has successfully conducted multicenter research projects in healthcare epidemiology [8, 9]. An invitation to participate in the current study was sent to all SHEA Research Network members. This study received institutional review board (IRB) approval with a waiver of informed consent and a HIPAA waiver from the coordinating center, the University of Maryland School of Medicine, as well as from IRBs at all individual sites.
Data
The CMS nonpayment policy took effect on 1 October 2008. Data were obtained for all adult hospital admissions during a prepolicy period, from 1 June 2007 to 30 September 2008, and a postpolicy period, from 1 October 2008 to 28 February 2010. All hospitals provided daily retrospective data for the study period. To assess the primary outcome (ie, frequency of urine culture on admission after implementation of the CMS policy), variables collected included the daily number of admissions and the number of urine cultures performed in the first 48 hours after the date of admission (including 10 hours prior to admission, to capture urine cultures performed for patients in the emergency department). Urine cultures were assessed within the first 48 hours for 2 reasons: (1) samples requested from admission orders for urine culture may not be collected until up to 48 hours later, and (2) the Centers for Disease Control and Prevention (CDC) considers conditions diagnosed >48 hours after admission to be hospital-acquired conditions [10]. Data on the number of wound cultures performed in the first 48 hours after admission were also requested and reported. Wound cultures were chosen as a nonequivalent, dependent variable to identify changes in culturing practice not related to the CMS policy [11].
To evaluate the secondary outcome of antimicrobial use, we obtained retrospective data from a convenience subsample of hospitals with access to automated pharmacy data to obtain the number of patients who were prescribed antimicrobials that are often (although not exclusively) used for the treatment of urinary tract infection. Antimicrobials included fluoroquinolones, trimethroprim-sulfamethoxazole, cephalexin, amoxicillin-clavulanate, and nitrofurantoin. Fluoroquinolone antimicrobials included ciprofloxacin, levofloxacin, moxifloxacin, and gatifloxacin. Other broad-spectrum antibiotics were not included, as these are more commonly used for other infections. We collected data on antimicrobial use in the first 72 hours after admission and, to better measure how often antimicrobials were being used for suspected urinary tract infection, determined the number of patients who had urine cultures performed in the first 48 hours after admission and were prescribed an antimicrobial in the first 72 hours after admission.
Validation
To validate computer-obtained data on submission of urine specimens for microbiologic culture, we asked participating sites to perform manual validation. Sites were instructed to validate the first 4 patient records for each month by looking up each patient's medical record, checking to see whether they had a urine culture within 48 hours of the date of admission. This was done to evaluate the accuracy of documentation of urine culture submission and the presence or absence of corresponding microbiology results in the clinical record. A total of 13 of 35 sites completed full manual validation. Validation data showed 14 incorrect entries out of 1486 validation admissions reviewed (error rate, 0.9%).
Statistics
Thirty-five hospitals with an average of 30 000 admissions in both the prepolicy and postpolicy periods and an average urine culture frequency of 20% (200 cultures per 1000 admissions) provided >99% power to detect an absolute difference in urine culture frequency of 5% (50 cultures per 1000 admissions), using a 2-sided test with a type I error of 5% and assuming a within-hospital correlation of 5%. Under similar assumptions, 10 hospitals with an average of 7000 admissions with urine cultures in both periods and an average fluoroquinolone prescription frequency of 27% (270 prescriptions per 1000 admissions) provided >85% power to detect an absolute difference in frequencies of 5% in fluoroquinolone use.
Data were analyzed as a before/after quasi-experimental study. Culture frequencies during the prepolicy and postpolicy periods were compared using Poisson mixed-effects models to account for within-hospital correlation. Models were created for overall urine culture frequencies (as the primary outcome, dependent variable) in the prepolicy and postpolicy periods, as well as for use of antimicrobials (as the secondary outcome) commonly prescribed for the treatment of urinary tract infections (ie, fluoroquinolones, nitrofurantoin, trimethoprim-sulfamethoxazole, cephalexin, and amoxicillin-clavulanate). Overall antimicrobial prescription frequencies and trends were calculated for all admitted patients and for the subgroup of patients who provided a urine specimen for culture. A mixed-effects segmented regression model compared differences in trends over time (ie, slopes) between the prepolicy and postpolicy periods [11]. The segmented regression model included an indicator for postpolicy period, time (in days), and an indicator-by-time interaction term. The P value from segmented regression for change in slopes tests the indicator-by-time interaction term.
RESULTS
A total of 39 SHEA Research Network hospitals (cited in the Acknowledgments) participated. These hospitals included 15 community hospitals and 22 tertiary care hospitals, with sizes varying from 120 to 1000 beds. Sites represented 22 different states in all geographic areas of the continental United States, accounting for a total of 2 362 742 admissions. Of the 39 hospitals, 4 were not subject to CMS rules (3 are in Maryland, and 1 is a cancer hospital). No hospitals that were part of the Department of Veterans Affairs (VA) participated. All 35 hospitals subject to CMS rules provided urine culture data, and 28 provided control wound culture data. For the secondary outcome, 10 hospitals provided requested antimicrobial prescribing data.
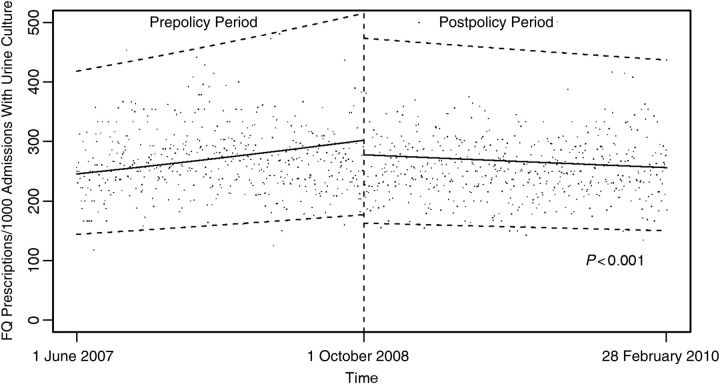
The median percentage of admissions during which urine culture was performed ≤48 hours after admission was 19.2% during the prepolicy period and 19.3% during the postpolicy period. The rate of change in urine culture performance in the 35 hospitals subject to the CMS rule during the prepolicy and postpolicy periods was 0.5% per month during the prepolicy period and –0.25% per month during the postpolicy period (P < .001; Figure 1 and Table 1). The rate of change in the control variable, wound culture performance, also decreased during the postpolicy period (–0.83% per month), compared with the prepolicy period (0.60% per month; P < .001).
Figure 1.
Frequency of urine culture performance among all admissions before and after the Centers for Medicare & Medicaid Services stopped reimbursing hospitals for treatment of hospital-acquired catheter-associated urinary tract infection. Data points represent daily median frequencies, and dashed lines represent 95% confidence intervals. Abbreviation: FQ, fluoroquinolone.
Table 1.
Frequency of Urine Cultures and Fluoroquinolone Use Before and After the Centers for Medicare & Medicaid Services Stopped Reimbursing Hospitals for Treatment of Hospital-Acquired Catheter-Associated Urinary Tract Infection
| Outcome (No. of Hospitals) | Prepolicy Period (1 June 2007–30 September 2008) |
Postpolicy Period (1 October 2008–28 February 2010) |
|||
|---|---|---|---|---|---|
| Median Frequencya (95% CI) | Relative Change,b % (95% CI) | Median Frequencya (95% CI) | Relative Change,b % (95% CI) | Pc | |
| Urine cultures (n=35) | 180 (150–216) | 0.50 (0.40–0.59) | 185 (154–222) | −0.25 (−0.34 to −0.17) | <.001 |
| Wound cultures (control variable; n = 28) | 27.0 (20.9–34.9) | 0.60 (0.32–0.88) | 26.2 (20.3–33.9) | −0.83 (−1.11 to −0.58) | <.001 |
| FQ use in all admissions (n = 10) | 128 (92–177) | 0.30 (0.10–0.50) | 123 (89–171) | −1.24 (−3.08 to −0.59) | <.001 |
| FQ use in patients with a urine culture at time of admission (n = 10) | 272 (158–468) | 1.28 (0.98–1.58) | 267 (155–458) | −0.47 (−0.74 to −0.19) | <.001 |
Abbreviations: CI, confidence interval; FQ, fluoroquinolone.
a Defined as the No. of cultures or No. of FQ prescriptions per 1000 admissions, from Poisson mixed-effects regression.
b Defined as the slope, expressed as a relative change in frequency of cultures or FQ prescriptions since the previous month, from Poisson mixed-effects segmented regression.
c For comparison of the relative change in frequency (ie, slopes) during the prepolicy and postpolicy periods.
In the 4 control hospitals not subject to the CMS rule, the median percentage of admissions during which urine culture was performed ≤48 hours after admission was 23.4% during the prepolicy period and 24.8% during the postpolicy period. The rate of change in urine culture frequency in these 4 hospitals decreased during the postpolicy period (–0.60% per month), compared with the prepolicy period (1.26% per month; P < .001).
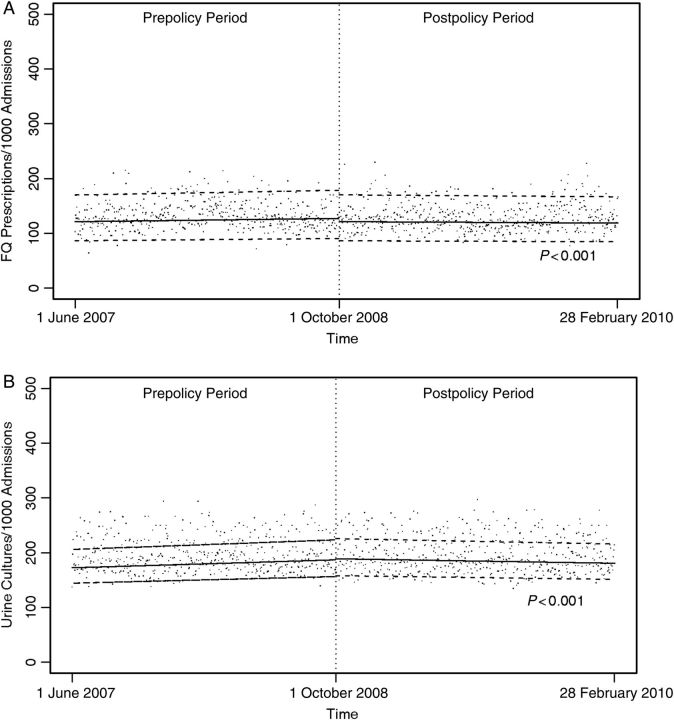
In the 10 hospitals that submitted data on antibiotic use, a median of 14.6% of patients were treated with a fluoroquinolone antimicrobial during the prepolicy period; this decreased to a median of 14.0% during the postpolicy period. Among patients with urine cultures performed ≤2 days after admission, a median of 29.2% were prescribed a fluoroquinolone antimicrobial during the prepolicy period, and a median of 28.2% were prescribed a fluoroquinolone antimicrobial during the postpolicy period. The rate of change in fluoroquinolone antimicrobial use decreased during the postpolicy period (–1.24% per month), compared with the prepolicy period (0.30% per month; P < 0.001). The rate of change in fluoroquinolone antimicrobial use for patients who had urine culture performed decreased during the postpolicy period (–0.74% per month), compared with the prepolicy period (1.28% per month; P < .001). Overall prescription frequencies and trends are presented in Table 1 and Figure 2. The only antimicrobials used in >5% of admissions were fluoroquinolones. Other antimicrobials were used much less frequently than fluoroquinolones (data not shown; these included trimethroprim-sulfamethoxazole, cephalexin, amoxicillin-clavulanate, and nitrofurantoin).
Figure 2.
Frequency of fluoroquinolone use among all admissions (A) and among patients suspected of having urinary tract infection (B) before and after the Centers for Medicare & Medicaid Services stopped reimbursing hospitals for treatment of hospital-acquired catheter-associated urinary tract infection. Data points represent daily median frequencies, and dashed lines represent 95% confidence intervals. Abbreviation: FQ, fluoroquinolone.
DISCUSSION
In a national sample of 39 hospitals, we found no evidence that the CMS policy resulted in overtesting for urinary tract infections on admission. Furthermore, the secondary outcome of antimicrobial prescribing was also not found to increase following implementation of the CMS policy.
CMS nonpayment for hospital-acquired conditions reflects a fundamental and controversial change in hospital payment structure. The potential for unintended consequences of nonpayment for hospital-acquired CAUTI, including adverse outcomes associated with excessive use of antimicrobials, such as selection pressure promoting the emergence of multidrug-resistant organisms and increased risks for C. difficile infection, were a concern for many [3, 12, 13]. Importantly, our study did not identify adverse consequences associated with the CMS policy on hospital-acquired CAUTI. However, our data reflect only the first 16 months after the CMS policy. The long-term impact of public reporting of CAUTI and the 1% decrease in all diagnosis-related group payments in 2015 for hospitals in the worst quartile for rates of hospital-acquired conditions may have unintended consequences and must be carefully assessed as they are implemented [14].
Previous CMS policies intended to improve hospital treatment of infections have had unintended negative consequences. For example, the CMS policy relating to pneumonia prevention has been associated with inappropriate use of antimicrobials [4]. The CMS process-of-care measure for pneumonia care initially included a measure requiring that patients with pneumonia receive an antimicrobial ≤4 hours after emergency department arrival. Emergency medicine physicians found this time frame to be narrow and, in response to pressure to improve the measure, began prescribing antimicrobials to most patients with pulmonary syndromes [4]. Subsequently, the window for receiving antimicrobials was widened to 6 hours, which helped decrease overprescribing [15].
Instead of an increase in testing for urinary tract infections on admission, we found a slight decrease in testing after implementation of the CMS policy. The clinical relevance of this finding is unclear. With >2 million hospital admissions, our study was powered to detect very small differences. Our finding that wound culture frequencies also decreased during this period suggests that there may be secular trends independent of the CMS policy that have resulted in less microbiologic culturing for a range of specimen types. Although the secondary outcome of antimicrobial prescribing was only measured in 10 hospitals, this outcome still had significant power to detect a small difference. A small decrease in fluoroquinolone antimicrobial prescribing, from 14.6% during the prepolicy period to 14.0% during the postpolicy period, was observed. This finding could be related to improved antimicrobial stewardship for fluoroquinolone antimicrobials or to a shift away from fluoroquinolone antimicrobials to broader-spectrum antimicrobials in response to increasing rates of infection due to multidrug-resistant organisms [9, 10].
Whether the CMS policy has had a beneficial effect on patient care has yet to be fully determined. Krein et al recently reported the results of a large national study of VA hospitals (which are not subject to the CMS rule changes) and non-VA hospitals in which surveys were conducted in 2005 and 2009 to assess the use of practices to prevent central line–associated bloodstream infection (CLABSI), ventilator-associated pneumonia (VAP), and CAUTI after the CMS policy was implemented [15]. These investigators found that the use of key practices to prevent CLABSI, VAP, and CAUTI increased in both VA and nonfederal hospitals, suggesting that, despite its perceived importance, the CMS policy may not be the primary driver of practice change [15]. Additionally, while approximately two-thirds of nonfederal hospitals reported a moderate or large increase in preventing CAUTI as a facility priority, use of practices to prevent CAUTI remains low, compared with use of practices to prevent CLABSI and VAP [15].
CAUTI is the most common hospital-associated infection, with significant costs and morbidity [1]. However, hospital coding that is used for reimbursement only detects a small fraction of CAUTIs, and the CMS policy is not expected to have an appreciable effect on reimbursement [13]. A more reliable method of CAUTI detection would improve policies designed to encourage prevention. Methods of detection will need to be objective to decrease variability in how frequencies are reported and to avoid penalizing hospitals that make more rigorous efforts to identify CAUTI.
Strengths of our study include its use of data from a nationally representative group of community and tertiary care hospitals in 22 states. This was achievable through the SHEA Research Network. Second, hospitals submitted validated primary data in a standardized manner. Finally, a proportion of hospitals were able to collect the secondary outcome of antimicrobial use to better measure potential unintended consequences of the policy.
Our findings should be interpreted in the context of the following important limitations. First, this study was based on retrospective, clinically collected data. Second, we did not examine use of urinalysis, which may have been performed to justify a diagnosis of urinary tract infection, Third, fluoroquinolone antibiotics are used for diagnoses other than urinary tract infection. Fourth, the CMS policy was publicized 2 years before the date it started, and hospital practices may have changed before 1 October 2008. Last, this study did not examine whether CMS nonpayment policy resulted in a decrease in CAUTI.
In a large, nationally representative set of hospitals, we did not identify overtesting for urinary tract infection to document the infection as present on admission and avoid nonreimbursement for CAUTI. Robust consortia of hospitals such as the SHEA Research Network are necessary to examine the positive and negative effects of policy changes [16]. The CMS policy did not promote overtesting for or unnecessary treatment of urinary tract infection.
Notes
Acknowledgments. Members of the SHEA Research Network participating in this study are as follows: Dianna Appelgate, MS, MPH, Sacred Heart Medical Center; Alexandra Yamshchikov, MD, Rochester General Hospital; Mohamed Fakih (principal investigator) and Janice Rey (coinvestigator), St John Hospital and Medical Center; David Fisk, MD, Santa Barbara Cottage Hospital; Sarah Haessler, MD, Baystate Medical Center; David A. Pegues, MD, Ronald Reagan UCLA Medical Center; Carol Sulis, MD, Boston Medical Center; Michael Sweet, PharmD, BCPS, West Virginia University Hospitals; Marc-Oliver Wright, MS, Evanston Hospital, Glenbrook Hospital, and Highland Park Hospital; Dan Morgan, MD, and Anthony Harris, MD, MPH, University of Maryland Medical Center; Jan Patterson, MD, and Jason Bowling, MD, The University of Texas Health Science Center at San Antonio; Deverick Anderson, MD, MPH, Duke University Medical Center; Trish Perl, MD, and Aaron Milstone, MD, Johns Hopkins; Sarah Boyd, MD, Nick Bennett, PharmD, and Kristin Repp, PharmD, St Luke's Northland Hospital; Nasia Safdar, MD, PhD, and Christopher Harper, University of Wisconsin Hospital; Brandon Bookstaver, PharmD, BCPS, and Janet B. Craig, DHA, MBA, MSN, South Carolina Health Sciences and University of South Carolina Hospitals; Marci Drees, MD, MS, Christiana Hospital and Wilmington Hospital; Len Mermel, DO, ScM, AM, Rhode Island Hospital; Sharon B. Wright, MD, MPH, Beth Israel Deaconess Medical Center; Ebbing Lautenbach, MD, MPH, Hospital of the University of Pennsylvania; Jesse T. Jacob, MD, Emory University Hospital Midtown; Amy Kressel, MD, MS, Indiana University Hospital; Margaret Turner, RN, MEd, Maricopa Integrated Health System; Timothy Burke and Julia Pattison-Crisostomo, RN, St Mary's Medical Center and St Mary's Medical Center–Superior; Tara N. Palmore, MD, NIH Clinical Center; Julie E. Mangino, MD, Ohio State University Hospital, Ohio State University Hospital East, Ohio State University James Cancer Hospital, and Ross Heart Hospital; Carol Chenoweth, MD, and Latoya Kuhn, MPH, University of Michigan; and Kyle B. Enfield, MD, MS, University of Virginia.
Financial support. This work was supported by the Society for Hospital Epidemiology of America.
Potential conflicts of interest. D. J. M. has received an unrestricted research grant from Merck. D. A. has received an unrestricted research grant from Merck and has served on a speakers’ bureau for Merck. A. D. H. and D. A. have received payment for contributions to UpToDate Online. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Klevens RM, Edwards JR, Richards CL, et al. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007;122:160. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Medicare and Medicaid Services (CMS), HHS. Medicare program; changes to the hospital inpatient prospective payment systems and fiscal year 2008 rates. Fed Regist. 2007;72:47129–8175. [PubMed] [Google Scholar]
- 3.Wald HL, Kramer AM. Nonpayment for harms resulting from medical care. JAMA. 2007;298:2782–4. doi: 10.1001/jama.298.23.2782. [DOI] [PubMed] [Google Scholar]
- 4.Wachter RM, Flanders SA, Fee C, Pronovost PJ. Public reporting of antibiotic timing in patients with pneumonia: lessons from a flawed performance measure. Ann Intern Med. 2008;149:29. doi: 10.7326/0003-4819-149-1-200807010-00007. [DOI] [PubMed] [Google Scholar]
- 5.Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis. 2005;40:643–54. doi: 10.1086/427507. doi:10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 6.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International clinical practice guidelines from the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:625–63. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 7.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–32. doi: 10.1016/j.ajic.2008.03.002. doi:10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Lautenbach E, Saint S, Henderson DK, Harris AD. Initial response of health care institutions to emergence of H1N1 influenza: experiences, obstacles, and perceived future needs. Clin Infect Dis. 2010;50:523. doi: 10.1086/650169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Research Committee of the Society of Healthcare Epidemiology of America. Enhancing patient safety by reducing healthcare-associated infections: the role of discovery and dissemination. Infect Control Hosp Epidemiol. 2010;31:118–23. doi: 10.1086/650198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the national healthcare safety network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31:528–31. doi: 10.1086/652152. [DOI] [PubMed] [Google Scholar]
- 11.Shardell M, Harris AD, El-Kamary SS, Furuno JP, Miller RR, Perencevich EN. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis. 2007;45:901–7. doi: 10.1086/521255. doi:10.1086/521255. [DOI] [PubMed] [Google Scholar]
- 12.Saint S, Meddings JA, Calfee D, Kowalski CP, Krein SL. Catheter-associated urinary tract infection and the medicare rule changes. Ann Intern Med. 2009;150:877. doi: 10.7326/0003-4819-150-12-200906160-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meddings J, Saint S, McMahon LF., Jr Hospital-acquired catheter-associated urinary tract infection: documentation and coding issues may reduce financial impact of Medicare's new payment policy. Infect Control Hosp Epidemiol. 2010;31:627–33. doi: 10.1086/652523. [DOI] [PubMed] [Google Scholar]
- 14.Patient Protection and Affordable Care Act. 2010. Pub L 111-148 §3008 124 Stat 119. http://www.gpo.gov/fdsys/pkg/PLAW-111publ148/pdf/PLAW-111publ148.pdf . Available at: Accessed 1 March 2012. [Google Scholar]
- 15.Bratzler DW. Antibiotic timing for pneumonia. Clin Infect Dis. 2008;47:1115–6. doi: 10.1086/591800. author reply 1156–7 doi:10.1086/591800. [DOI] [PubMed] [Google Scholar]
- 16.Shekelle PG, Pronovost PJ, Wachter RM, et al. Advancing the science of patient safety. Ann Intern Med. 2011;154:693. doi: 10.7326/0003-4819-154-10-201105170-00011. [DOI] [PubMed] [Google Scholar]