Abstract
Kv1.3 is a voltage-gated potassium (K) channel expressed in a number of tissues, including fat and skeletal muscle. Channel inhibition improves experimental autoimmune encephalitis, in part by reducing IL-2 and tumor necrosis factor production by peripheral T lymphocytes. Gene inactivation causes mice (Kv1.3-/-) exposed to a high-fat diet to gain less weight and be less obese than littermate control. Interestingly, although Kv1.3-/- mice on the high-calorie diet gain weight, they remain euglycemic, with low blood insulin levels. This observation prompted us to examine the effect of Kv1.3 gene inactivation and inhibition on peripheral glucose homeostasis and insulin sensitivity. Here we show that Kv1.3 gene deletion and channel inhibition increase peripheral insulin sensitivity in vivo. Baseline and insulin-stimulated glucose uptake are increased in adipose tissue and skeletal muscle of Kv1.3-/- mice. Inhibition of Kv1.3 activity facilitates the translocation of the glucose transporter, GLUT4, to the plasma membrane. It also suppresses c-JUN terminal kinase activity in fat and skeletal muscle and decreases IL-6 and tumor necrosis factor secretion by adipose tissue. We conclude that Kv1.3 inhibition improves insulin sensitivity by increasing the amount of GLUT4 at the plasma membrane. These results pinpoint a pathway through which K channels regulate peripheral glucose homeostasis, and identify Kv1.3 as a pharmacologic target for the treatment of diabetes.
Keywords: glucose, body weight, obesity, diabetes
Kv1.3 is a Shaker-related, voltage-gated potassium (Kv) channel expressed in lymphocytes (1-6), CNS (7-9), kidney (10), liver, skeletal muscle, testis, spermatozoa (11), and osteoclasts (12, 13). It is postulated to participate in cellular processes such as apoptosis, cell volume regulation, and T cell stimulation (2, 3, 14, 15). Kv1.3 inhibition is reported to improve experimental autoimmune encephalitis, in part by reducing IL-2 and tumor necrosis factor α (TNFα) production (16). Channel activity is up-regulated by serum-glucocorticoid-activated kinase, one of the main mediators of aldosterone's action at the renal distal tubule (17). Protein kinase C increases (18) and tyrosine kinase inhibits Kv1.3 channel activity (19). Kv1.3 is highly expressed in the olfactory bulb (20), and it appears to mediate a large fraction of the outward current detected in these neurons, which also express the insulin receptor (IR). The available data indicate that insulin down-regulates Kv1.3 activity in the olfactory bulb (19, 21) through the phosphorylation of multiple tyrosine residues in Kv1.3 by IR kinase.
Examination of Kv1.3-deficient mice (Kv1.3-/-), generated by gene targeting, revealed a previously unrecognized role for Kv1.3 in body weight regulation (22). Kv1.3-/- mice weigh less than control littermates and are protected from diet-induced obesity. Indeed, when maintained on a high-fat diet for up to 7 months, they gain less weight than control littermates. Although food intake did not differ significantly between Kv1.3-/- and controls, basal metabolic rate, measured at rest by indirect calorimetry, was significantly higher in knockout animals, suggesting that Kv1.3 channels may participate in the pathways that regulate body weight and that channel inhibition may increase basal metabolic rate.
Interestingly, although Kv1.3-/- mice exposed to a high-fat diet become obese (≈40 g at 7 months), they are euglycemic and have normal blood insulin levels (22). This observation, along with the fact that Kv1.3 activity is regulated by insulin in the olfactory bulb, prompted us to examine the effect of Kv1.3 gene inactivation and inhibition on peripheral glucose homeostasis and insulin sensitivity.
Methods
Insulin Tolerance Test. Male 12- to 24-week-old Kv1.3+/+, Kv1.3-/-, C57BL/6J, db/db, or ob/ob mice (C57BL/6J background, The Jackson Laboratory) were maintained according to National Institutes of Health Guidelines, on standard rodent chow. The experimental group received either Margatoxin (MgTX) (0.1 μg/g body weight) or Kaliotoxin (0.01 μg/g body weight) by i.p. injection 2 h before insulin challenge. Age-matched controls were given PBS i.p.. Both groups then received human insulin (7.5 × 10-4 units/g body weight) by i.p. injection, and blood glucose levels were measured by using a Glucometer (Bayer, West Haven, CT) at the indicated time intervals.
Glucose Uptake in Skeletal Muscle and Adipose Tissue. Baseline and insulin-stimulated glucose uptake was measured in soleus muscle and epididymal fat, isolated from Kv1.3-/- mice and control littermates, by using the methods described by Slentz et al. (23). The insulin concentration was 10 nM.
Immunocytochemistry. Primary cultures of epididymal fat, isolated from Kv1.3-/- mice and control littermates under sterile conditions, were established as described by Cabrero et al. (24). Cells were treated with either PBS, 100 nM insulin, or 1 nM MgTX for 30 min at 37°C, and fixed with 3% paraformaldehyde in PBS for 3 min at room temperature. GLUT4 expression was detected in adipocytes by immunofluorescence (anti-GLUT4 antibody, Cell Signaling Technology, Beverly, MA). The Texas red-conjugated secondary antibody was obtained from Vector Laboratories. Samples were examined by fluorescence microscopy immediately after staining.
To quantify the amount of GLUT4 translocated to the plasma membrane, isolated adipocytes were transfected with a reporter gene (HA-GLUT4, gift from Samuel W. Cushman, National Institutes of Health, Bethesda) coding for GLUT4 with a hemagglutinin epitope tag (HA) in the first exofacial loop. Transfection was carried out by using the FuGENE 6 reagent (Roche Diagnostics). Parallel experiments with a plasmid containing a GFP cDNA indicated a transfection efficiency of ≈55%. Four hours after transfection, the cells were plated in culture dishes with glass coverslips in MEM Alpha medium with 10% FCS. Twenty four hours later, the cells were washed in Krebs-Ringer bicarbonate Hepes buffer, pH 7.4, 200 nM adenosine (KRBH buffer) containing 5% BSA, and treated with either PBS, 67 nM insulin, or 5 nM rMargatoxin for 10 min at 37°C. GLUT4 redistribution was stopped by the addition of 2 mM KCN for 5 min. A monoclonal anti-HA-FITC antibody (Santa Cruz Biotechnology) was added at a dilution of 1:50 to nonpermeabilized cells and incubated for 1 h at room temperature. Excess antibody was removed by washing three times with PBS, and cells were mounted onto glass microscope slides and examined by using a Zeiss LSM 510 confocal imaging system.
For colocalization of GLUT4 and Kv1.3 in skeletal muscle, the tissue was embedded in paraffin and 0.5- to 2.0-mm-thick sections were cut and mounted on glass coverslips, deparaffinized by using xylene, and rehydrated in graded ethanol and PBS. Sections were immunolabeled with a rabbit anti-human Kv1.3 polyclonal antibody (Santa Cruz Biotechnology) at a dilution of 1:1,000. A FITC-labeled goat, anti-rabbit secondary antibody (Zymed) was then used at a dilution of 1:1,000. Finally, the slides were washed in PBS, preserved in crystal/mount (Biomeda, Foster City, CA) and examined within 4 h by using a Zeiss LSM 510 confocal imaging system.
Measurement of c-Jun Terminal Kinase (JNK) Activity. Kv1.3-/- mice and control littermates were given either MgTX (0.1 μg/g body weight) or PBS by i.p. injection 2 h before tissue collection. The animals were killed, epididymal fat and soleus muscle were isolated, and JNK activity was measured by using reagents purchased from Cell Signaling Technology, according to the manufacturer's instructions. The amount of JNK protein loaded in each lane was assessed by using an antibody that detects total, phosphorylated and unphosphorylated, JNK1 and JNK2 proteins (Sigma). Signal intensity was quantified by using nih image 1.62 software. For each sample, signal intensity of phosphorylated c-Jun was expressed relative to the amount of JNK protein present.
IL-6 and TNFα Secretion. The periuterinal fat pads of ob/ob mice were dissected under sterile conditions, minced, and incubated in medium (1.5 ml of media per gram of tissue) containing DMEM and Ham's F-12 in a 1:1 mixture, supplemented with 0.5% (wt/vol) endotoxin-free BSA. The incubation solution also contained 1 nM 3,3′,5-triiodo-l-thyronine, 5 mM glutamine, penicillin (6.35 mg/liter), and streptomycin (5 mg/liter). The tissues were incubated at 37°C in six-well plates, which were gently swirled every 15 min. MgTX was added at a concentration of 1 nM as indicated at time 0. Culture medium (250 μl) was collected at 1 h, quickly frozen, and stored at -80°C. TNFα and IL-6 levels were measured by ELISA using the mTNFα Biotrak kit (Amersham Pharmacia) and optEIA mouse IL-6 kit (BD Biosciences, San Diego), respectively. IL-6 and TNFα values were expressed as picograms per milligram of DNA.
Statistical Analysis. The two-tailed Student's t test for unpaired samples was used to analyze the data shown, and P values of <0.05 are indicated as appropriate.
Results and Discussion
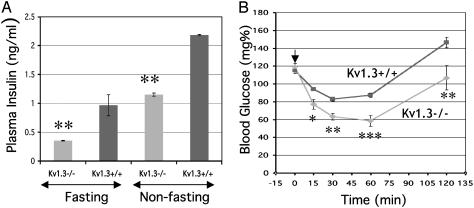
As shown in Fig. 1, although Kv1.3-/- and WT mice had similar glucose levels, fasting and nonfasting plasma insulin concentrations were significantly lower in Kv1.3-/- animals. Glucagon levels were not significantly different (Kv1.3+/+, 84.30 ± 8.54 pg/ml, n = 6 vs. Kv1.3-/-, 76.50 ± 18.5 pg/ml, n = 6). An insulin tolerance test confirmed that Kv1.3-/- mice were more sensitive to the actions of insulin, which caused a significantly greater fall in blood glucose of Kv1.3-/- mice, starting 15 min after injection and lasting at least 2 h (Fig. 1B). These data indicate that disruption of the Kv1.3 gene is associated with an apparent increase in insulin sensitivity.
Fig. 1.
Increased insulin sensitivity in Kv1.3-/- mice. (A) Plasma insulin levels measured in age-matched (5-month-old male, n = 11) mice under fasting and nonfasting conditions. Kv1.3-/- mice have significantly lower plasma insulin levels under both conditions (**, P < 0.01). (B) Insulin tolerance performed in age-matched (5-month-old male, n = 5) conscious mice fasted for 4 h. The arrow indicates when insulin was injected. Blood glucose values of Kv1.3-/- mice are significantly (*, P < 0.05; **, P < 0.01; ***, P < 0.003) lower than those of Kv1.3+/+ mice from 15 to 120 min after insulin injection.
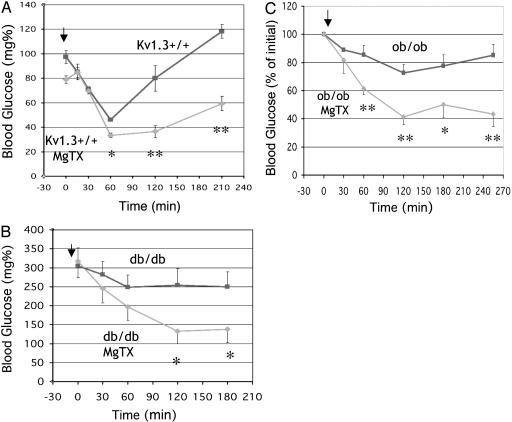
Although deletion of Kv1.3 currents potentiates the hypoglycemic action of insulin, the role of compensatory mechanisms activated by the chronic and complete absence of the Kv1.3 protein is unclear. For instance, Kv1.3-/- mice weigh 10-15% less than littermate controls, and it could be argued that the reduction in body weight could partly account for the apparent increase in insulin sensitivity. To address this issue, we studied the effect of the acute inhibition of Kv1.3 activity on insulin sensitivity in age- and weight-matched WT mice. As shown in Fig. 2A, C57BL/6 mice that received a single injection of MgTX (inhibitor Kv1.2 and Kv1.3; ref. 7) 2 h before study had lower fasting blood glucose compared to mice that were given PBS. Furthermore, MgTX-treated mice exhibited significantly lower blood glucose levels in response to insulin. To examine whether the effect of MgTX was specific for Kv1.3, insulin tolerance tests were carried out in Kv1.3-/- mice pretreated with either PBS or MgTX. No significant differences in blood glucose were observed (PBS, 57.3 ± 4.3 mg/dl at 60 min, n = 5; MgTX, 62.4 ± 5.2 mg/dl at 60 min, n = 5), suggesting that MgTX mediates its action by inhibiting Kv1.3 channel activity. These results show that Kv1.3 inhibition increases peripheral insulin sensitivity independent of changes in body weight, and suggest a direct role of Kv1.3 in glucose metabolism.
Fig. 2.
Acute inhibition of Kv1.3 increases insulin sensitivity in WT, ob/ob, and db/db mice. (A) Insulin tolerance was performed in fasting (4 h) C57BL/6J mice (4-month-old, n = 5). The arrow indicates when insulin was injected. MgTX-treated mice had lower blood glucose than PBS-treated controls (*, P < 0.05; **, P < 0.01; ***, P < 0.003). (B) Insulin tolerance in ob/ob mice (3-month-old male, n = 5). The arrow indicates when insulin was injected. Ob/ob mice given MgTX had lower blood glucose than PBS-treated controls (*, P < 0.05; **, P < 0.01). (C) Insulin tolerance was performed in db/db mice (3-month-old male, n = 5). The arrow indicates when insulin was injected. Blood glucose was significantly lower in db/db mice that received MgTX (*, P < 0.05; **, P < 0.01).
We then examined whether Kv1.3 inhibition could ameliorate the insulin insensitivity observed in mice with type II diabetes mellitus and obesity. db/db mice carry a mutation in the leptin receptor leading to obesity, insulin resistance, and hyperglycemia. At about 8 weeks of age, db/db mice typically have blood glucose levels ranging from 300 to 350 mg/dl (25). Inhibition of Kv1.3 by MgTX resulted in a significantly greater fall in blood glucose in db/db mice receiving insulin compared to those given PBS and insulin (Fig. 2B). The difference in glucose levels was evident within 30 min and persisted for up to 4.5 h. The ob/ob mouse (C57BL/6J background, The Jackson Laboratory) carries a mutation in the leptin gene, and exhibits severe obesity, insulin resistance, hyperinsulinemia, and hyperglycemia. ob/ob mice treated with a single injection of MgTX were significantly more sensitive to insulin than mice receiving PBS, with a fall in blood glucose in response to insulin administration 50% greater than control mice (Fig. 2C). The difference in glucose level was evident within 30 min after the administration of insulin and persisted for at least 3 h. Although MgTX is a specific and potent inhibitor of Kv1.3, it also blocks Kv1.2. To differentiate between these two possibilities, the effect of Kaliotoxin, which inhibits both Kv1.3 (IC50 = 0.6 nM) and Kv1.1 (IC50 = 40 nM) but not Kv1.2, on insulin sensitivity was examined. Animals that received Kaliotoxin and insulin had significantly lower blood glucose than those only receiving insulin (at 270 min, control = 77.3 ± 3.5% vs. Kaliotoxin = 44.2 ± 2.9%, n = 5, P < 0.001). These data indicate that inhibition of Kv1.3 currents increases insulin sensitivity in obese and diabetic mice, and further strengthen the notion that Kv1.3 channels play a key role in peripheral glucose metabolism.
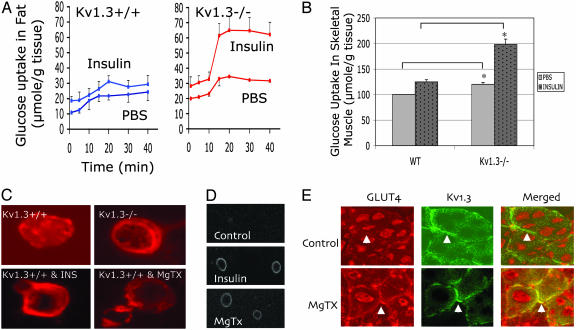
Several mechanisms could account for these observations, including decreased glucose production by the liver or increased glucose uptake by adipose tissue and skeletal muscle. Although skeletal muscle accounts for 80% of peripheral glucose disposal, adipose tissue plays a crucial role in the modulation of peripheral insulin sensitivity. Interestingly, Kv1.3 is expressed in both white and brown adipose tissue, in significantly higher levels than in skeletal muscle and liver. To investigate the mechanisms of increased insulin sensitivity in Kv1.3-/- mice, we examined the role of Kv1.3 on glucose uptake in white adipose tissue and skeletal muscle. Baseline and insulin-stimulated glucose uptake by adipose tissue and skeletal muscle was significantly higher in Kv1.3-/- than in WT mice (Fig. 3 A and B). These data indicate that the observed increase in peripheral insulin sensitivity can largely be explained by the stimulation of glucose uptake in fat and skeletal muscle.
Fig. 3.
Inhibition of Kv1.3 stimulates glucose uptake and GLUT4 translocation in adipose tissue and skeletal muscle. (A) Glucose uptake in adipose tissue. (Left) Baseline and insulin-stimulated glucose uptake in adipose tissue of Kv1.3+/+ mice. (Right) Baseline and insulin-stimulated glucose uptake in adipose tissue of Kv1.3-/- mice. Baseline and insulin-stimulated uptake at 20 min are significantly higher in Kv1.3-/- than in control littermate mice (n = 5, P < 0.001 and P < 0.002). (B) Glucose uptake in skeletal muscle. Values were normalized to baseline glucose uptake, which was set at 100%. Baseline and insulin-stimulated glucose uptake in skeletal muscle of at 20 min are significantly higher in Kv1.3-/- than in control littermate (WT) mice (n = 5, P < 0.05 and 0.01). (C) GLUT4 localization in adipocytes. (Upper Left) GLUT4 is detected by immunofluorescence throughout the cytoplasm and at the plasma membrane. (Upper Right) Kv1.3 deletion increases GLUT4 at the plasma membrane. (Lower Left) Insulin (10 nM) shifts GLUT4 to the plasma membrane in WT mice. (Lower Right) MgTX (1 nM) shifts GLUT4 to the plasma membrane in WT mice. A representative experiment is shown (n = 5). (D) GLUT4 distribution in transfected adipocytes. (Top) In Kv1.3+/+ (WT) adipocytes transfected with HA-GLUT4 incubated with PBS, the GLUT4 signal is undetectable. (Middle) Insulin treatment of transfected adipocytes shifts GLUT4 to the plasma membrane. (Bottom) MgTX treatment stimulates GLUT4 traffic to the plasma membrane. A representative experiment is shown (n = 4). (E) GLUT-4 distribution in skeletal muscle. (Upper Left) Kv1.3+/+ (WT) mice given PBS, GLUT4 (red) is located intracellularly, and the plasma membrane is faintly labeled (arrow). (Upper Center) Kv1.3 (green) is localized to the plasma membrane. (Upper Right) Merged picture. (Lower Left) MgTX treatment of Kv1.3+/+ mice shifts GLUT-4 to the plasma membrane (arrow). (Lower Center) Kv1.3 (green) is localized to the plasma membrane. (Lower Right) Merged picture. A representative experiment is shown (n = 3).
GLUT4 is the major transporter that mediates glucose translocation in insulin sensitive tissues. An increase in glucose transport could occur through the activation of GLUT4 molecules that already reside at the plasma membrane. Alternatively, additional GLUT4 transporters could translocate to the plasma membrane from intracellular stores. To distinguish between these two possibilities, we examined the effect of Kv1.3 deletion or pharmacologic inhibition on GLUT4 translocation. Fig. 3C suggests that, similar to insulin treatment, either Kv1.3 deletion or channel inhibition with MgTX preferentially shifts GLUT4 to the plasma membrane. To refine our analysis and quantify the amount of GLUT4 protein that translocates to the plasma membrane, we transfected freshly isolated adipocytes with GLUT4 containing a HA tag at the first exofacial loop, and examined nonpermeabilized cells for the appearance of GLUT4 protein at the plasma membrane (26). In unstimulated cells, the amount of GLUT4 protein at the plasma membrane was below the limit of detection (Fig. 3D Top), and intracellular labeling was absent because the cells were not permeabilized. As expected, treatment with insulin resulted in a large increase in GLUT4 at the plasma membrane (Fig. 3D Middle). Most importantly, Kv1.3 inhibition with MgTX also resulted in a shift of GLUT4 to plasma membrane (Fig. 3D Bottom).
We then asked whether Kv1.3 inhibition also affected GLUT4 translocation in skeletal muscle. WT mice were treated with MgTX and killed 2 h later, and skeletal muscle sections were examined for the distribution of Kv1.3 (plasma membrane protein) and GLUT4. In mice given PBS, GLUT4 protein is located intracellularly (Fig. 3E Upper Left), with faint labeling of the plasma membrane (arrow). As expected, the anti-Kv1.3 antibody preferentially labels the plasma membrane (Fig. 3E Upper Center), and the merged picture (Fig. 3E Upper Right) reveals little overlap of GLUT4 and Kv1.3 at the plasma membrane. In contrast, Kv1.3 inhibition with MgTX stimulates GLUT4 movement to the plasma membrane (Fig. 3E Lower Left) and colocalization with Kv1.3 is clearly evident in the merged picture (Fig. 3E Lower Right).
These data strongly indicate that the apparent increase in insulin sensitivity observed in whole-animal studies, can be attributed, in part, to a stimulation of glucose uptake in insulin-sensitive tissues, which is mediated by increased GLUT4 translocation to the plasma membrane. It should be noted that, although the effect on GLUT4 traffic to the plasma membrane is robust enough to account for the observed increase in glucose transport, our data do not completely exclude a role for GLUT4 activation.
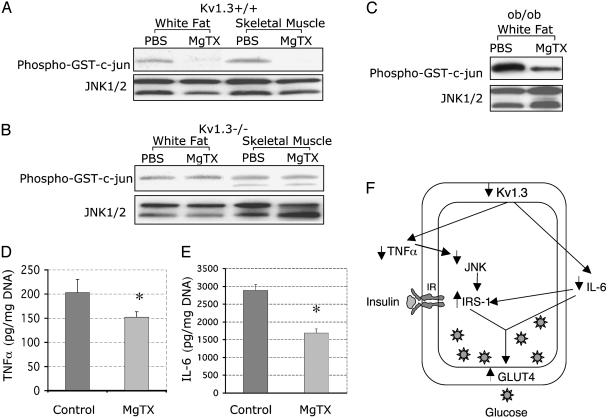
Recent experimental data indicate that adipocytes secrete a number of molecules, including leptin, TNFα, IL-6, and resistin, that modulate peripheral insulin sensitivity (27, 28). In peripheral T lymphocytes, Kv1.3 inhibition decreases the production of inflammatory cytokines such as IL-2 and TNFα (15, 16, 29, 30). Although the mechanisms underlying the action of TNFα on insulin sensitivity are not completely understood, the stimulation of lipolysis in adipose tissue appears to be an important event (31). Recent data indicate that mitogen-activated protein kinases, including JNK, are critical mediators of TNFα's action on lipolysis (32, 33). Furthermore, JNK activity is increased in obesity, and JNK1-deficient mice exposed to a high-fat diet are less obese than controls and exhibit improved insulin sensitivity (28), suggesting that JNK plays a central role in obesity and insulin sensitivity. JNK activity was measured, by using c-jun as JNK substrate, in skeletal muscle and white adipose tissue 2 h after injection of either PBS or MgTX in WT and Kv1.3-/- mice. As shown in Fig. 4A, compared to PBS, MgTX decreased JNK activity in WT mice (adipose tissue, 86.3 ± 6.7%, n = 3, P < 0.001; skeletal muscle, 92.8%±11.3, n = 3, P < 0.001), but had no effect on JNK activity in Kv1.3 null mice (Fig. 4B). It is noteworthy that, although baseline JNK activity was detected in Kv1.3 null mice (perhaps secondary to up-regulation of JNK2), it was insensitive to inhibition by MgTX, strongly suggesting that the decrease in JNK activity detected in WT mice given MgTX was specifically mediated by Kv1.3 channel inhibition. Because JNK is an important mediator of peripheral insulin sensitivity, we conclude that it may play a key role in the molecular mechanism that underlie the action of Kv1.3 on insulin sensitivity.
Fig. 4.
Kv1.3 inhibition down-regulates JNK activity in skeletal muscle and adipose tissue. (A) MgTX decreases JNK activity in Kv1.3+/+ mice. Phopho-GST-c-jun indicates JNK kinase activity. JNK1/2 reflects total JNK protein in each lane. Representative experiment shown. MgTX decreased JNK activity in adipose tissue and skeletal muscle of WT mice by 86.3 ± 6.7%, n = 3, P < 0.001, and 92.8 ± 11.3%, n = 3, P < 0.001, respectively. (B) MgTX has no effect on JNK activity in Kv1.3-/- mice. Phopho-GST-c-jun indicates JNK kinase activity. JNK1/2 reflects total JNK protein in each lane. A representative experiment is shown (n = 3). (C) MgTX decreases JNK activity in ob/ob mice. Phopho-GST-c-jun indicates JNK kinase activity. JNK1/2 reflects total JNK protein in each lane. A representative experiment is shown (n = 3). (D) MgTX decreases TNFα secretion by adipose tissue. Control, incubation medium. MgTX, incubation medium with 1 nM MgTX. TNFα was measured at 1 h after incubation with MgTX (n = 4, P < 0.03). (E) MgTX decreases IL-6 secretion by adipose tissue. Control, incubation medium; MgTX, incubation medium with 1 nM MgTX. IL-6 was measured at 1 h after incubation with MgTX (n = 4, P < 0.02). (F) Cellular mechanisms of increased glucose uptake with Kv1.3 deletion. IRS-1, IR substrate 1; JNK, c-Jun terminal kinase; GLUT4, glucose transporter 4; IL-6, interleukin-6; up and down arrows, increased and decreased activity, respectively.
The JNK signal transduction cascade integrates a large number of extracellular signals, including TNFα, which modulates the activity of mitogen-activated protein kinases such as extracellular signal-related kinase and JNK in adipocytes. Changes in TNFα levels lead to reciprocal variations in JNK activity. Previous studies indicate that Kaliotoxin, a Kv channel blocker, inhibits TNFα secretion by peripheral T lymphocytes (16). Based on the forgoing, we hypothesized that Kv1.3 could inhibit JNK activity in adipocytes by decreasing TNFα secretion, and examined the effect of MgTX on JNK activity and TNFα secretion in adipocytes of ob/ob mice. Fig. 4C shows that 1 nM MgTX significantly inhibits JNK activity in ob/ob mice (63.2 ± 3.5%, n = 4). Furthermore, MgTX decreased TNFα secretion by ob/ob adipocytes (Fig. 4D). These data confirm our hypothesis and indicate that the increased in insulin sensitivity observed with Kv1.3 inhibition is, in part, mediated by a decrease in TNFα secretion by adipocytes.
Adipocytes produce large quantities of IL-6, and contribute ≈30% of the IL-6 detected in the systemic circulation (34). They express active IL-6 receptors and become insulin resistant when exposed to IL-6, which inhibits of IR substrate 1 and GLUT4 gene transcription (27). We tested whether Kv1.3 inhibition altered IL-6 production by adipocytes. Fig. 4E shows that 1 nM MgTX significantly inhibits IL-6 secretion by ob/ob adipocytes (42.4 ± 5.1%, P < 0.02, n = 4). Taken together, these data indicate that Kv1.3 inhibition increases insulin sensitivity by down-regulating the production of TNFα and IL-6 by adipocytes (Fig. 4F).
The decrease in IL-6 and TNFα secretion observed with Kv1.3 inhibition can account for the down-regulation of JNK activity. On the other hand, the mechanisms that underlie Kv1.3's action on cytokine production are not entirely clear. Kv1.3 is known to regulate the resting membrane potential of nonexcitable cells, and channel inhibition has been shown to depolarize the plasma membrane and to alter intracellular calcium levels (15). For each particular cell, the net effect on intracellular calcium concentration would depend on the nature and net balance of the calcium entry pathways present at the plasma membrane. For instance, membrane depolarization will decrease calcium influx through calcium release-activated calcium channel (inactivated by depolarization), but increase calcium entry via voltage-gated calcium channels, which are stimulated by membrane depolarization. We speculate that Kv1.3 might regulate cytokine synthesis and secretion through its action on intracellular calcium levels.
K channels play an important role in insulin secretion and glucose metabolism. The ATP-sensitive potassium (KATP) and other Kv channels (Kv1.x and Kv2.x) regulate glucose homeostasis by controlling the membrane potential and insulin secretion by pancreatic β cells (35). Inhibition of KATP by sulfonylurea compounds depolarizes the cell and activates voltage-sensitive Ca2+ channels, leading to an increase in intracellular Ca2+ concentration and a stimulation of insulin secretion. In addition to their effect on insulin secretion, these compounds can also increase insulin sensitivity in insulin-responsive tissues. KATP-deficient mice develop hypoglycemia and have a defect in glucagon secretion. In contrast to what is observed with KATP inhibition or deletion (36), Kv1.3 disruption does not cause an increase in insulin secretion and is not associated with abnormalities in glucagon secretion. Furthermore, because we could not detect Kv1.3 expression in the pancreas, we hypothesize that its role in glucose homeostasis may be quite distinct from that of other K channels. Increased insulin sensitivity is observed as a side effect of clinically useful compounds. For instance, fluxetine (37-39) and verapamil (40) are known to increase insulin sensitivity. The precise mechanisms mediating their effect on insulin sensitivity remains unknown. Interestingly, therapeutic levels of both drugs (41-44) inhibit Kv1.3 activity, suggesting that they may modulate insulin sensitivity through inhibition of Kv1.3 currents.
In conclusion, this work provides support for the notion that Kv1.3 is an important component of the pathways that regulate glucose homeostasis. Inhibition of channel activity, either by gene deletion or by channel blockers, significantly increases peripheral insulin sensitivity by recruiting GLUT4 to the plasma membrane by down-regulating IL-6 and TNFα secretion and JNK activity. The channel and its signaling pathway represent potential targets for the development of drugs useful in the management of diabetes.
Acknowledgments
We thank Dr. Samuel Cushman for his generous gift of the HA-Glut4 plasmid; Drs. Gerald I. Shulman and Jason K. Kim for their helpful discussion; and Ms. Emma Matos for expert technical assistance. Grant support for J.X. is from the National Institutes of Health (R21DK064317 and K08DK02917) and for G.V.D. from the Veteran Administration (Merit Review award) and the National Institutes of Health (DK48105B).
Abbreviations: IR, insulin receptor; MgTX, Margatoxin; HA, hemagglutinin; JNK, c-Jun terminal kinase; TNFα, tumor necrosis factor α.
References
- 1.Cahalan, M. D., Chandy, K. G. & Grissmer, S. (1991) Curr. Topics Membr. 39, 357-394. [Google Scholar]
- 2.Attali, B., Romey, G., Honoré, E., Schmid-Alliana, A., Mattéi, M.-G., Lesage, F., Ricard, P., Barhanin, J. & Lazdunski, M. (1992) J. Biol. Chem. 267, 8650-8657. [PubMed] [Google Scholar]
- 3.Attali, B., Honoré, E., Lesage, F., Lazdunski, M. & Barhanin, J. (1992) FEBS Lett. 303, 229-232. [DOI] [PubMed] [Google Scholar]
- 4.Ghanshani, S., Wulff, H., Miller, M. J., Rohm, H., Neben, A., Gutman, G. A., Cahalan, M. D. & Chandy, K. G. (2000) J. Biol. Chem. 275, 37137-37149. [DOI] [PubMed] [Google Scholar]
- 5.Levite, M., Cahalon, L., Peretz, A., Hershkoviz, R., Sobko, A., Ariel, A., Desai, R., Attali, B. & Lider, O. (2000) J. Exp. Med. 191, 1167-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grissmer, S., Dethlefs, B., Wasmuth, J. J., Goldin, A. L., Gutman, G. A., Cahalan, M. D. & Chandy, K. G. (1990) Proc. Natl. Acad. Sci. USA 87, 9411-9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mourre, C., Chernova, M. N., Martin-Eauclaire, M. F., Bessone, R., Jacquet, G., Gola, M., Alper, S. L. & Crest, M. (1999) J. Pharmacol. Exp. Ther. 291, 943-952. [PubMed] [Google Scholar]
- 8.Chandy, K. G., Williams, C. B., Spencer, R. H., Aguilar, B. A., Ghanshani, S., Tempel, B. L. & Gutman, G. A. (1990) Science 247, 973-975. [DOI] [PubMed] [Google Scholar]
- 9.Stuhmer, W., Ruppersberg, J. P., Schroter, K. H., Sakmann, B., Stocker, M., Giese, K. P., Perschke, A., Baumann, A. & Pongs, O. (1989) EMBO J. 8, 3235-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao, X., Chang, A. Y., Boulpaep, E. L., Segal, A. S. & Desir, G. V. (1996) J. Clin. Invest. 97, 2525-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob, A., Hurley, I. R., Goodwin, L. O., Cooper, G. W. & Benoff, S. (2000) Mol. Hum. Reprod. 6, 303-313. [DOI] [PubMed] [Google Scholar]
- 12.Arkett, S. A., Dixon, J., Yang, J. N., Sakai, D. D., Minkin, C. & Sims, S. M. (1994) Recept. Channels 2, 281-293. [PubMed] [Google Scholar]
- 13.Komarova, S. V., Dixon, S. J. & Sims, S. M. (2001) Curr. Pharm. Des. 7, 637-654. [DOI] [PubMed] [Google Scholar]
- 14.Deutsch, C. & Chen, L.-Q. (1993) Proc. Natl. Acad. Sci. USA 90, 10036-10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, Q. H., Fleischmann, B. K., Hondowicz, B., Maier, C. C., Turka, L. A., Yui, K., Kotlikoff, M. I., Wells, A. D. & Freedman, B. D. (2002) J. Exp. Med. 196, 897-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beeton, C., Wulff, H., Barbaria, J., Clot-Faybesse, O., Pennington, M., Bernard, D., Cahalan, M. D., Chandy, K. G. & Beraud, E. (2001) Proc. Natl. Acad. Sci. USA 98, 13942-13947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warntges, S., Friedrich, B., Henke, G., Duranton, C., Lang, A., Waldegger, S., Meyermann, R., Kuhl, D., Speckmann, J., Obermuller, N., et al. (2002) Pflugers Arch. 443, 617-624. [DOI] [PubMed] [Google Scholar]
- 18.Chung, I. & Schlichter, L. C. (1997) J. Membr. Biol. 156, 73-85. [DOI] [PubMed] [Google Scholar]
- 19.Fadool, D. A., Tucker, K., Phillips, J. J. & Simmen, J. A. (2000) J. Neurophysiol. 83, 2332-2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veh, R. W., Lichtinghagen, R., Sewing, S., Wunder, F., Grumbach, I. M. & Pongs, O. (1995) Eur. J. Neurosci. 7, 2189-2205. [DOI] [PubMed] [Google Scholar]
- 21.Fadool, D. A. & Levitan, I. B. (1998) J. Neurosci. 18, 6126-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu, J., Koni, P. A., Wang, P., Li, G., Kaczmarek, L., Wu, Y., Li, Y., Flavell, R. A. & Desir, G. (2002) Hum. Mol. Genet. 12, 551-559. [DOI] [PubMed] [Google Scholar]
- 23.Slentz, C. A., Gulve, E. A., Rodnick, K. J., Henriksen, E. J., Youn, J. H. & Holloszy, J. O. (1992) J. Appl. Physiol. 73, 486-492. [DOI] [PubMed] [Google Scholar]
- 24.Cabrero, A., Alegret, M., Sanchez, R. M., Adzet, T., Laguna, J. C. & Vazquez, M. (2001) Diabetes 50, 1883-1890. [DOI] [PubMed] [Google Scholar]
- 25.Manchem, V. P., Goldfine, I. D., Kohanski, R. A., Cristobal, C. P., Lum, R. T., Schow, S. R., Shi, S., Spevak, W. R., Laborde, E., Toavs, D. K., et al. (2001) Diabetes 50, 824-830. [DOI] [PubMed] [Google Scholar]
- 26.Quon, M. J., Butte, A. J., Zarnowski, M. J., Sesti, G., Cushman, S. W. & Taylor, S. I. (1994) J. Biol. Chem. 269, 27920-27924. [PubMed] [Google Scholar]
- 27.Rotter, V., Nagaev, I. & Smith, U. (2003) J. Biol. Chem. 278, 45777-45784. [DOI] [PubMed] [Google Scholar]
- 28.Hirosumi, J., Tuncman, G., Chang, L., Gorgun, C. Z., Uysal, K. T., Maeda, K., Karin, M. & Hotamisligil, G. S. (2002) Nature 420, 333-336. [DOI] [PubMed] [Google Scholar]
- 29.Lin, C. S., Boltz, R. C., Blake, J. T., Nguyen, M., Talento, A., Fischer, P. A., Springer, M. S., Sigal, N. H., Slaughter, R. S., Garcia, M. L., et al. (1993) J. Exp. Med. 177, 637-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedman, B. D., Price, M. A. & Deutsch, C. J. (1992) J. Immunol. 149, 3784-3794. [PubMed] [Google Scholar]
- 31.Boden, G. (1997) Diabetes 46, 3-10. [PubMed] [Google Scholar]
- 32.Aguirre, V., Uchida, T., Yenush, L., Davis, R. & White, M. F. (2000) J. Biol. Chem. 275, 9047-9054. [DOI] [PubMed] [Google Scholar]
- 33.Ryden, M., Dicker, A., van Harmelen, V., Hauner, H., Brunnberg, M., Perbeck, L., Lonnqvist, F. & Arner, P. (2002) J. Biol. Chem. 277, 1085-1091. [DOI] [PubMed] [Google Scholar]
- 34.Mohamed-Ali, V., Goodrick, S., Rawesh, A., Katz, D. R., Miles, J. M., Yudkin, J. S., Klein, S. & Coppack, S. W. (1997) J. Clin. Endocrinol. Metab. 82, 4196-4200. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald, P. E., Ha, X. F., Wang, J., Smukler, S. R., Sun, A. M., Gaisano, H. Y., Salapatek, A. M., Backx, P. H. & Wheeler, M. B. (2001) Mol. Endocrinol. 15, 1423-1435. [DOI] [PubMed] [Google Scholar]
- 36.Miki, T., Liss, B., Minami, K., Shiuchi, T., Saraya, A., Kashima, Y., Horiuchi, M., Ashcroft, F., Minokoshi, Y., Roeper, J. & Seino, S. (2001) Nat. Neurosci. 4, 507-512. [DOI] [PubMed] [Google Scholar]
- 37.Breum, L., Bjerre, U., Bak, J. F., Jacobsen, S. & Astrup, A. (1995) Metabolism 44, 1570-1576. [DOI] [PubMed] [Google Scholar]
- 38.Potter van Loon, B. J., Radder, J. K., Frolich, M., Krans, H. M., Zwinderman, A. H. & Meinders, A. E. (1992) Int. J. Obes. Relat. Metab. Disord. 16, Suppl. 4, S55-S61. [PubMed] [Google Scholar]
- 39.Potter van Loon, B. J., Radder, J. K., Frolich, M., Krans, H. M., Zwinderman, A. H. & Meinders, A. E. (1992) Int. J. Obes. Relat. Metab. Disord. 16, 79-85. [PubMed] [Google Scholar]
- 40.Dal Ponte, D. B., Fogt, D. L., Jacob, S. & Henriksen, E. J. (1998) Metabolism 47, 982-987. [DOI] [PubMed] [Google Scholar]
- 41.Choi, J. S., Hahn, S. J., Rhie, D. J., Yoon, S. H., Jo, Y. H. & Kim, M. S. (1999) J. Pharmacol. Exp. Ther. 291, 1-6. [PubMed] [Google Scholar]
- 42.Yeung, S. Y., Millar, J. A. & Mathie, A. (1999) Br. J. Pharmacol. 128, 1609-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madeja, M., Muller, V., Musshoff, U. & Speckmann, E. J. (2000) Neuropharmacology 39, 202-210. [DOI] [PubMed] [Google Scholar]
- 44.Robe, R. J. & Grissmer, S. (2000) Br. J. Pharmacol. 131, 1275-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]