In a case-control study at 4 US centers, the effectiveness of influenza vaccine against medically attended polymerase chain reaction–confirmed influenza was 60% (95% confidence interval, 53%–66%). Significant effectiveness was demonstrated in young children, adolescents, and adults, with a lower estimate for those aged ≥65.
Abstract
Background. Influenza vaccines may be reformulated annually because of antigenic drift in influenza viruses. However, the relationship between antigenic characteristics of circulating viruses and vaccine effectiveness (VE) is not well understood. We conducted an assessment of the effectiveness of US influenza vaccines during the 2010–2011 season.
Methods. We performed a case–control study comparing vaccination histories between subjects with acute respiratory illness with positive real-time reverse transcription polymerase chain reaction for influenza and influenza test-negative controls. Subjects with acute respiratory illness of ≤7 days duration were enrolled in hospitals, emergency departments, or outpatient clinics in communities in 4 states. History of immunization with the 2010–2011 vaccine was ascertained from vaccine registries or medical records. Vaccine effectiveness was estimated in logistic regression models adjusted for study community, age, race, insurance status, enrollment site, and presence of a high-risk medical condition.
Results. A total of 1040 influenza-positive cases and 3717 influenza-negative controls were included from the influenza season, including 373 cases of influenza A(H1N1), 334 cases of influenza A(H3N2), and 333 cases of influenza B. Overall adjusted VE was 60% (95% confidence interval [CI], 53%–66%). Age-specific VE estimates ranged from 69% (95% CI, 56%–77%) in children aged 6 months–8 years to 38% (95% CI, −16% to 67%) in adults aged ≥65 years.
Conclusions. The US 2010–2011 influenza vaccines were moderately effective in preventing medically attended influenza during a season when all 3 vaccine strains were antigenically similar to circulating viruses. Continued monitoring of influenza vaccines in all age groups is important, particularly as new vaccines are introduced.
A major consequence of the continuous antigenic evolution of influenza viruses is that influenza vaccines must be evaluated before each Northern and Southern Hemisphere influenza season and possibly reformulated to contain strains that are antigenically similar to the strains predicted to circulate in the upcoming season. Because of the short time available for their manufacture and the unpredictability of influenza epidemiology, these new formulations of influenza vaccine are released for use in the United States based on antigenic composition rather than on clinical trials demonstrating immunogenicity of that specific formulation.
The study reported here is part of an ongoing effort to provide annual estimates of the effectiveness of influenza vaccines licensed for use in the United States. We sought to assess how much effectiveness varies from season to season, including the effects of antigenic match, and to obtain information regarding the effectiveness of vaccines in specific groups at greater risk of complications following influenza infection, including children and adults aged ≥65. These studies utilize an observational study design in which individuals with acute respiratory illness (ARI) are tested by a sensitive and specific real-time reverse-transcription polymerase chain reaction (rRT-PCR) diagnostic test, and the proportion vaccinated among those who test positive for influenza is compared with the proportion vaccinated among those who test negative. This design is sometimes referred to as a test-negative case–control approach [1].
Previous studies conducted by the same sites have evaluated the effectiveness of seasonal influenza vaccine against 2009–2010 seasonal influenza in a year with poor antigenic matching, the effectiveness of the 2009–2010 seasonal vaccine against 2009 pandemic H1N1 in the first wave of the pandemic from May to July 2009 (unpublished), and the effectiveness of unadjuvanted pandemic vaccine against 2009 pandemic H1N1 during the second wave from September 2009 to April 2010 [2]. The current study reports the effectiveness of influenza vaccine against seasonal influenza at 4 geographically diverse sites during the 2010–2011 season, in which a close match existed between the vaccine and the circulating virus for all 3 vaccine components.
METHODS
Enrollment
We enrolled persons seeking care for ARI at medical facilities affiliated with the Marshfield Clinic and St. Joseph's Hospital, Marshfield, Wisconsin; the University of Michigan Health System, Ann Arbor, and Henry Ford Health System, Detroit, Michigan; the University of Rochester (Strong Memorial and Rochester General Hospitals), Rochester, New York; and Vanderbilt University, Summit, St. Thomas, and Baptist Hospitals, Nashville, Tennessee. Details regarding enrollment procedures in each of the sites have been previously published [2].
Surveillance for ARI was conducted in 3 settings: pediatric and adult outpatient clinics and practices, emergency departments, and inpatient pediatric and adult hospital units. The source populations for the study included community-dwelling children and adults who resided in the counties or zip codes surrounding the participating study centers. Subjects were eligible to participate if they were aged ≥6 months and had an ARI with a duration of ≤7 days with documented fever or history of feverishness or cough. Potentially eligible subjects were initially identified by review of admission lists, triage boards, or other lists of current patients. Subjects or their parents or guardians were then approached by trained study staff to assess eligibility and obtain informed consent. Each consented participant (or parent/guardian) completed an interview to ascertain symptoms and date of symptom onset. Age, sex, self-reported race, insurance status, and history of chronic medical conditions were obtained from interview or medical record review. Subjects were defined as high risk if they had documented medical conditions that increase the risk of influenza complications, as defined by the Advisory Committee on Immunization Practices (ACIP) [3].
Samples of respiratory secretions for diagnostic testing were obtained from all subjects by nasal and throat swabs obtained sequentially or nasal swab alone for children aged <2 years or if throat swab could not be obtained. Samples were transported in transport media on wet ice in a single cryovial to the diagnostic laboratory at each center. In 3 communities, receipt of seasonal 2010–2011 influenza vaccine was ascertained by patient or parental report and confirmed by medical record review and/or state vaccine registries. In Wisconsin, vaccine receipt was confirmed by a real-time Internet-based vaccine registry (http://www.recin.org) that captures 95% of all influenza vaccinations in that population [4]. Strains contained in 2010–2011 trivalent Northern Hemisphere influenza vaccines included an A/California/7/2009 (H1N1)–like virus, an A/Perth/16/2009 (H3N2)–like virus, and a B/Brisbane/60/2008 (B Victoria lineage)–like virus.
Study procedures, informed consent documents, and data collection forms were reviewed and approved by institutional review Bbards representing each of the sites enrolling patients.
Laboratory Methods
Respiratory specimens collected from each enrolled patient were tested for the presence of influenza at each study site using a consensus rRT-PCR protocol (S. Lindstrom, personal communication) using dual-labeled probe (Taqman) chemistry. The Centers for Disease Control and Prevention (CDC) provided primers, probes, control materials, and a proficiency testing panel, which was completed by each laboratory site prior to study initiation. Specific methods are available from the CDC upon request. The rRT-PCR–positive specimens were cultured using Madin-Darby Canine Kidney or rhesus monkey kidney cells, and a subset of viral isolates was antigenically characterized at the CDC by hemagglutination inhibition assay using postinfection ferret antisera [5].
Vaccine Effectiveness Estimates
Vaccine effectiveness (VE) was estimated by using a test-positive case–test-negative control design [1]. Cases were defined as individuals meeting the medically attended ARI definition with rRT-PCR–confirmed influenza, and controls were individuals with similar illnesses in whom rRT-PCR was negative for influenza. The primary exposure was receipt of at least 1 dose of seasonal influenza vaccine at least 14 days before illness onset. The VE was then estimated as 100% × (1 – adjusted odds ratio) using logistic regression models; both crude, unadjusted, and adjusted estimates of effectiveness were provided. Age in years, study community, and enrollment site (outpatient, inpatient, or emergency department) were included in all multivariable models a priori, and other variables were included if they affected the effectiveness point estimate. In the final model, these covariates included race (white or nonwhite), presence of a high-risk medical condition, and insurance status (public or other). Stratified analyses were performed by age category. We separately examined effectiveness of inactivated and live attenuated vaccines, effectiveness against influenza types A and B, and effectiveness against influenza A H1N1 and influenza A H3N2. We also evaluated VE for children aged <9 years by immunized status (fully and partially vaccinated). Finally, we examined effectiveness by enrollment site.
Analyses were conducted using SAS 9.1 software (SAS Institute). A 95% confidence interval (CI) was calculated for each estimate; if this interval excluded 0%, the estimate was considered statistically significant. Comparisons between cases and controls and between vaccinated and unvaccinated patients were conducted by using the χ2 test.
RESULTS
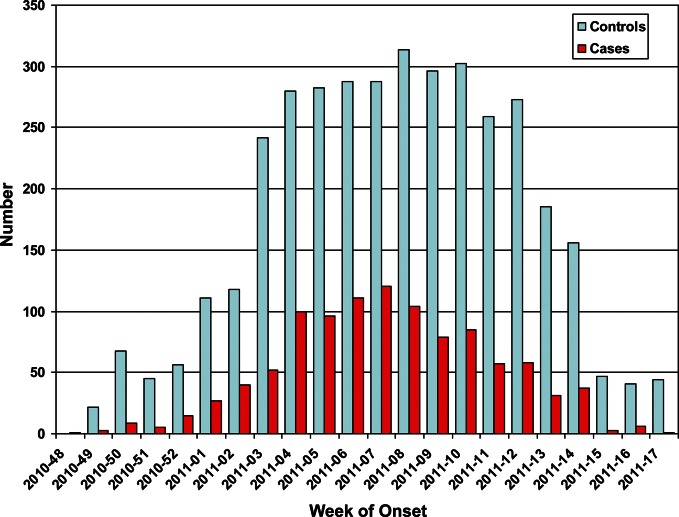
Enrollment at each study site began when the percentage of clinical laboratory specimens testing positive for influenza began to increase in the community or on the week of 17 January 2011, whichever came first, and ended after either 12 weeks of surveillance or 2 weeks without cases. Accordingly, the period of enrollment was 17 January 2011 to 14 April 2011 at the sites in Marshfield, Wisconsin; 1 December 2010 to 27 April 2011 at the sites in Ann Arbor and Detroit, Michigan; 24 December 2010 to 1 May 2011 at the sites in Rochester, New York; and 4 December 2010 to 26 March 2011 at the sites in Nashville, Tennessee. During this period, 5137 subjects were enrolled in the study of which 1113 (22%) were influenza positive. The epidemic curve of enrollment of virus-positive cases and virus-negative controls is shown in Figure 1.
Figure 1.
Numbers of influenza-positive acute respiratory illness cases (red bars) and influenza reverse transcription polymerase chain reaction–negative acute respiratory illness controls (blue bars) by week of enrollment.
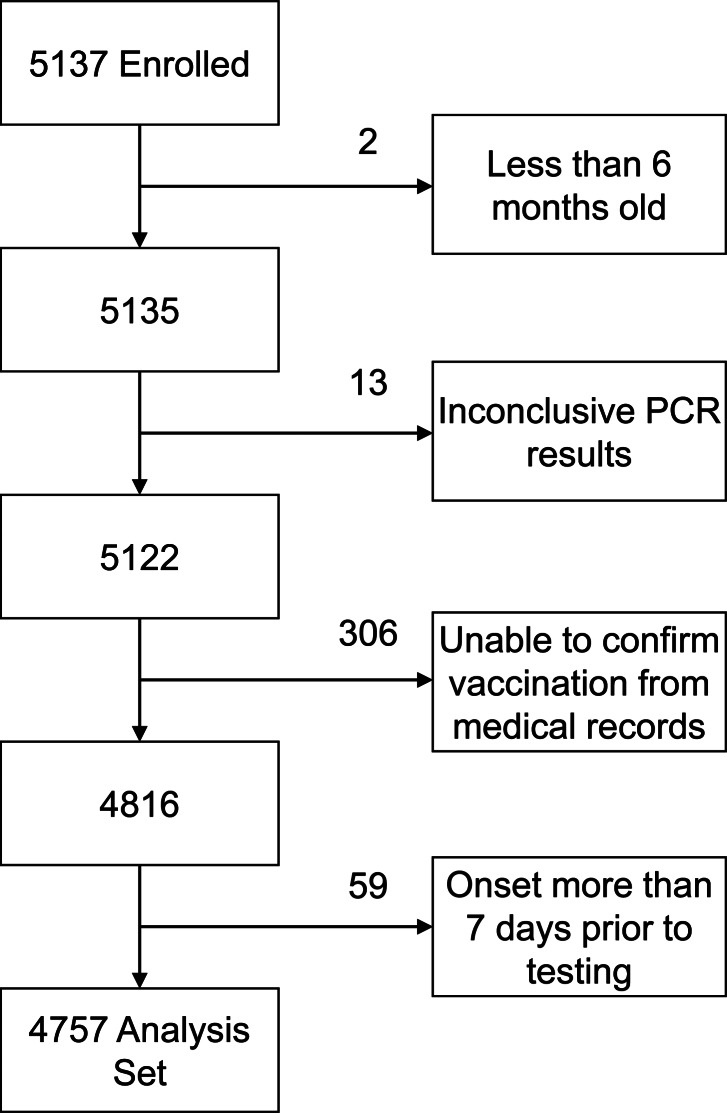
In total, 380 subjects were excluded from the final analysis of effectiveness, including 74 virus-positive and 306 virus-negative subjects (Figure 2). The main reason for exclusion from the analysis was inability to verify vaccination history from medical records, which resulted in exclusion of 306 of 5122 (6%) potential subjects. Other reasons for exclusion included determination that the onset of symptoms was >7 days prior to enrollment (59 subjects), inconclusive rRT-PCR results (13 subjects), and inadvertent enrollment of infants too young to receive influenza vaccine (2 subjects). In addition, 45 subjects who received vaccine within 14 days of illness onset were also excluded from the analysis of VE.
Figure 2.
Numbers of subjects enrolled and excluded from the final analysis of vaccine effectiveness. PCR, polymerase chain reaction.
The characteristics of the subjects analyzed in the study are shown in Table 1. Influenza cases were more likely than rRT-PCR–negative ARI controls to be enrolled in the emergency department or outpatient settings and less likely to be enrolled in the hospital setting. Cases were more likely than controls to be aged 9–49 years, non-white, and uninsured. Cases were less likely than controls to have recognized influenza high-risk conditions, and cases tended to have a shorter duration of symptoms prior to sampling than did the controls.
Table 1.
Descriptive Characteristics of Enrolled Patients With Medically Attended Acute Respiratory Infections by Case/Control Status
| Characteristics | Influenza Cases |
Influenza-Negative Controls |
P Value | ||
|---|---|---|---|---|---|
| N = 1040 | % | N = 3717 | % | ||
| Study community | |||||
| Marshfield, WI | 219 | 21 | 1535 | 41 | <.001 |
| Rochester, NY | 145 | 14 | 350 | 9 | |
| Southeast, MI | 377 | 36 | 864 | 23 | |
| Nashville, TN | 299 | 29 | 968 | 26 | |
| Enrollment site | |||||
| Outpatient | 760 | 73 | 2638 | 71 | <.001 |
| Inpatient | 112 | 11 | 634 | 17 | |
| Emergency Dept | 168 | 16 | 445 | 12 | |
| Sex | |||||
| Male | 473 | 45 | 1678 | 45 | .84 |
| Female | 567 | 55 | 2039 | 55 | |
| Age groups | |||||
| 6 months–2 years | 96 | 9 | 688 | 19 | <.001 |
| 3–8 years | 271 | 26 | 767 | 20 | |
| 9–18 years | 141 | 14 | 472 | 13 | |
| 19–49 years | 341 | 32 | 957 | 26 | |
| 50–64 years | 126 | 12 | 473 | 13 | |
| 65–74 years | 38 | 4 | 177 | 5 | |
| ≥75 years | 27 | 3 | 183 | 5 | |
| Race | |||||
| White | 583 | 56 | 2586 | 70 | <.001 |
| Black | 258 | 25 | 651 | 18 | |
| Other | 199 | 19 | 480 | 13 | |
| Insurance status | |||||
| Not insured | 87 | 8 | 200 | 5 | .002 |
| Private insurance | 660 | 63 | 2466 | 66 | |
| Public insurance | 293 | 28 | 1051 | 28 | |
| High-risk condition | |||||
| No | 712 | 68 | 2320 | 62 | <.001 |
| Yesa | 328 | 32 | 1397 | 38 | |
| Onset to test | |||||
| <3 days | 477 | 46 | 1596 | 43 | <.001 |
| 3–4 days | 397 | 38 | 1313 | 35 | |
| 5–7 days | 166 | 16 | 808 | 22 | |
a Presence of at least 1 condition among high-risk–respiratory; high-risk–cardiovascular; high-risk–diabetes; and high-risk–other diseases.
Regardless of whether they were cases or controls, unvaccinated subjects were more likely to be seen in the emergency department, to be aged 9–49 years, to be non-white, and to lack health insurance (Table 2). In addition, presence of an influenza high-risk condition was associated with increased likelihood of vaccination. The great majority of vaccinated subjects in each age group studied received inactivated influenza vaccines, and overall only 8% of the subjects received live attenuated influenza vaccine (LAIV). The most commonly administered vaccine was Fluzone (Sanofi Pasteur; trivalent inactivated vaccine containing 15 μg hemagglutinin of each component), which accounted for 69% of all of the vaccines received by subjects in the analysis. Only 11 subjects received the high-dose Fluzone (Sanofi Pasteur; trivalent inactivated vaccine containing 60 μg HA of each component).
Table 2.
Descriptive Characteristics of Enrolled Patients With Medically Attended Acute Respiratory Infections by Vaccination Status
| Characteristics | Vaccinateda |
Unvaccinated |
P Value | ||
|---|---|---|---|---|---|
| N = 2275 | % | N = 2482 | % | ||
| Study community | |||||
| Marshfield, WI | 870 | 38 | 884 | 36 | <.001 |
| Rochester, NY | 237 | 10 | 258 | 10 | |
| Southeast, MI | 505 | 22 | 736 | 30 | |
| Nashville, TN | 663 | 29 | 604 | 24 | |
| Enrollment site | |||||
| Outpatient | 1670 | 73 | 1728 | 70 | <.001 |
| Inpatient | 373 | 16 | 373 | 15 | |
| Emergency Dept | 232 | 10 | 381 | 15 | |
| Sex | |||||
| Male | 1000 | 44 | 1151 | 46 | .05 |
| Female | 1275 | 56 | 1331 | 54 | |
| Age groups | |||||
| 6 months–8 yearsb | 1029 | 45 | 793 | 32 | <.001 |
| 9–49 years | 638 | 28 | 1273 | 51 | |
| ≥50 years | 608 | 27 | 416 | 17 | |
| Race | |||||
| White | 1586 | 70 | 1583 | 64 | <.001 |
| Black | 350 | 15 | 559 | 23 | |
| Other | 339 | 15 | 340 | 14 | |
| Insurance status | |||||
| Not insured | 50 | 2 | 237 | 10 | <.001 |
| Private insurance | 1493 | 66 | 1633 | 66 | |
| Public insurance | 732 | 32 | 612 | 25 | |
| High-risk condition | |||||
| No | 1297 | 57 | 1735 | 70 | <.001 |
| Yes | 978 | 43 | 747 | 30 | |
| Onset to test | |||||
| <3 days | 985 | 43 | 1088 | 44 | .42 |
| 3–4 days | 825 | 36 | 885 | 36 | |
| 5–7 days | 465 | 20 | 509 | 21 | |
| Influenza test | |||||
| Negative | 1958 | 86 | 1759 | 71 | <.001 |
| Positive | 317 | 14 | 723 | 29 | |
| Influenza A | 212c | 9 | 498d | 20 | <.001 |
| Influenza B | 105 | 5 | 228d | 9 | <.001 |
| Influenza H1N1 | 94 | 4 | 279 | 11 | <.001 |
| Influenza H3N2 | 115 | 5 | 219 | 9 | <.001 |
a Vaccinated subjects are defined by last dose of the vaccine received ≥14 days before symptom onset.
b Partially or fully immunized.
c Three influenza A were unsubtyped.
d Three unvaccinated subjects were coinfected with both influenza A and B.
The overall age-adjusted VE for receipt of inactivated or live attenuated vaccine was 60% (95% CI, 53%–66%; Table 3). Vaccine effectiveness was similar by age group but declined among those aged ≥65 years. In this age group, VE was 38% and was not statistically significant. However, because relatively few elderly subjects were evaluated, the estimate of VE in this age group was imprecise. There was substantial VE in children aged 6 months–2 years (58%; 95% CI, 31%–74%), although the estimate was slightly lower than that in children aged 3–8 years (69%; 95% CI, 56%–77%). The overall VE in children aged 6 months–8 years was 63% (95% CI, 52%–72%). Among vaccinated children aged 6 months–8 years, 704 of 1029 (68%) had been fully vaccinated, and 325 of 1029 (32%) had been partially vaccinated, according to ACIP recommendations [6]. Adjusted VE for fully vaccinated children was 68% (95% CI, 56%–77%) and was 55% (95% CI, 36%–68%) for partially vaccinated children.
Table 3.
Percent Vaccinated by Case/Control Status and Crude and Adjusted Vaccine Effectivenessa by Age Group and Vaccine
| Age Group | Influenza Positive (Cases) |
Influenza Negative (Controls) |
Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|---|
| No. Vaccinated/Total | % Vaccinated | No. Vaccinated/Total | % Vaccinated | VE % | 95% CI | VE %b | 95% CI | |
| Any seasonal vaccine | ||||||||
| All ages | 317/1028 | 31 | 1958/3684 | 53 | 61 | 55–66 | 60 | 54–66 |
| 6 months–2 years | 47/93 | 51 | 465/675 | 69 | 54 | 29–70 | 58 | 31–74 |
| 3–8 years | 79/269 | 29 | 438/756 | 58 | 70 | 59–78 | 69 | 56–77 |
| 9–49 years | 104/479 | 22 | 534/1425 | 37 | 54 | 41–64 | 51 | 36–62 |
| 50–64 years | 47/124 | 38 | 263/470 | 56 | 52 | 29–68 | 51 | 25–68 |
| ≥65 years | 40/63 | 63 | 258/358 | 72 | 33 | −18 to 62 | 36 | −22 to 66 |
| Inactivated seasonal vaccine | ||||||||
| All ages | 269/980c | 27 | 1730/3456d | 50 | 62 | 56–68 | 62 | 55–68 |
| 2–8 years | 66/283 | 23 | 443/833 | 53 | 73 | 64–80 | 71 | 58–78 |
| 9–49 years | 91/466 | 20 | 477/1368 | 35 | 55 | 42–65 | 52 | 37–64 |
| ≥50 years | 79/179 | 44 | 491/798 | 62 | 51 | 32–64 | 47 | 24–63 |
| Live attenuated seasonal vaccine | ||||||||
| 2–49 years | 31/623e | 5 | 162/1443f | 11 | 59 | 39–72 | 65 | 46–77 |
| 2–8 years | 22/239 | 9 | 128/518 | 25 | 69 | 50–81 | 71 | 50–83 |
| 9–49 years | 9/384 | 2 | 34/925 | 4 | 37 | −33 to 70 | 42 | −28 to 74 |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
a Vaccinated subjects are defined by last dose of the vaccine received ≥14 days before symptom onset.
b For all ages models: adjusted for study site, age in years, age group, race, insurance, enrollment site, and high-risk condition. For age-specific models: adjusted for study site, age in years, race, insurance, enrollment site, and high-risk condition.
c Excluded subjects with live attenuated vaccine (n = 31) and unknown vaccine type (n = 17) prior to illness onset date.
d Excluded subjects with live attenuated vaccine (n = 162) and unknown vaccine type (n = 66) prior to illness onset date.
e Excluded subjects aged <2 years or >49 years (n = 240) and those with inactivated vaccine (n = 157) and unknown vaccine type (n = 8) prior to illness onset date.
f Excluded subjects aged <2 years or >49 years (n = 1295) and those with inactivated vaccine (n = 920) and unknown vaccine type (n = 26) prior to illness onset date.
Because LAIVs are currently licensed for use in individuals aged 2–49 years, we also made specific estimates of VE for LAIVs in this age range (Table 3). The effectiveness of at least 1 dose of LAIV in children aged 2–8 years was similar to that of Trivalent Inactivated Vaccine in this age group (adjusted VE, 70%). Effectiveness of LAIVs in those aged 9–49 year olds was lower (adjusted VE, 41%), as was VE for inactivated vaccine (adjusted VE, 52%). However, because LAIVs were rarely used in this age group, the estimate was not precise. Our results suggested that VE for LAIVs was higher among enrolled subjects aged 2–8 years than among those aged 9–49 years.
Vaccine effectiveness did not vary by enrollment site. For subjects enrolled as outpatients, adjusted VE was 59% (95% CI, 52%–67%); for those enrolled in the emergency department, it was 61% (95% CI, 40%–75%); and for those enrolled as inpatients, VE was 61% (95% CI, 38%–76%). Adjusted VE was slightly higher in individuals without a known influenza high-risk condition (62%; 95% CI, 53%–69%) than in those with high-risk conditions (54%; 95% CI, 40%–64%).
During the study period, we detected 334 cases of influenza A H3N2, 373 cases of influenza A H1N1, and 333 cases of influenza B. All viral isolates assessed at the CDC for antigenic relatedness to the vaccine strains by hemagglutination inhibition tests using postinfection ferret antisera were antigenically similar to the vaccine strains. Adjusted VE estimates by influenza type and subtype are shown in Table 4. Vaccine effectiveness was similar for each of the 3 components of the vaccine among children, and although lower overall, VE was also similar between types and subtypes in adults aged ≥50 years. However, the estimates of VE for influenza A H3N2 and influenza B in adults aged 9–49 years were substantially lower than for influenza A H1N1 and were also lower than the estimates in other age groups.
Table 4.
Adjusted Vaccine Effectiveness for Influenza Type and Subtype
| Influenza Positive (Cases) |
Influenza Negative (Controls) |
Adjusteda |
||||
|---|---|---|---|---|---|---|
| No. Vaccinated/Total | % Vaccinated | No. Vaccinated/Total | % Vaccinated | VE % | 95% CI | |
| Influenza A | ||||||
| All ages | 212/700 | 30 | 1958/3684 | 53 | 60 | 53–67 |
| 6 months–8 years | 71/193 | 37 | 903/1431 | 63 | 64 | 50–74 |
| 9–49 years | 71/357 | 20 | 534/1425 | 37 | 55 | 39–66 |
| ≥50 years | 70/150 | 47 | 521/828 | 63 | 46 | 21–63 |
| Influenza B | ||||||
| All ages | 105/325 | 32 | 1958/3684 | 53 | 60 | 48–69 |
| 6 months–8 years | 55/167 | 33 | 903/1431 | 63 | 62 | 45–74 |
| 9–49 years | 33/121 | 27 | 534/1425 | 37 | 38 | 2–61 |
| ≥50 years | 17/37 | 46 | 521/828 | 63 | 47 | −6 to 74 |
| Influenza A H1N1 | ||||||
| All ages | 94/369 | 25 | 1958/3684 | 53 | 66 | 56–74 |
| 6 months–8 years | 28/73 | 38 | 903/1431 | 63 | 60 | 33–77 |
| 9–49 years | 34/220 | 15 | 534/1425 | 37 | 66 | 47–76 |
| ≥50 years | 32/76 | 42 | 521/828 | 63 | 45 | 9–67 |
| Influenza A H3N2 | ||||||
| All ages | 115/328 | 35 | 1958/3684 | 53 | 54 | 42–64 |
| 6 months–8 years | 43/120 | 36 | 903/1431 | 63 | 66 | 48–78 |
| 9–49 years | 35/135 | 26 | 534/1425 | 37 | 39 | 7–60 |
| ≥50 years | 37/73 | 51 | 521/828 | 63 | 52 | 18–72 |
Abbreviations: CI, confidence interval; VE, vaccine effectiveness.
a Adjusted for study site, age in years, race, insurance, enrollment site, and high-risk condition.
DISCUSSION
In this large study conducted during the 2010–2011 US influenza season, during which all 3 components of the vaccine were antigenically similar to circulating influenza viruses and >1000 cases were enrolled, we found an overall VE of 60% for prevention of laboratory-confirmed, medically attended influenza illness due to any influenza virus. Because of the large number of cases, we were able to estimate VE with considerable precision (95% CI, 53%–66%). These results extend those of earlier studies conducted by the same centers in which we estimated the effectiveness of monovalent pandemic H1N1 vaccine at 56% [2] and found VE of seasonal trivalent vaccine against seasonal H1N1 in the 2008–2009 season at 47% and against a lineage mismatched influenza B virus at 28% (unpublished).
Other studies of influenza VE using similar study designs have shown variable levels of effectiveness. A recent study performed over 3 years did not show VE in the 2004–2005 season during which a mismatched influenza A/H3N2 virus was the main virus detected, demonstrated 28% VE in the 2005–2006 season during which a lineage mismatch influenza B virus predominated, and demonstrated 52% VE in the 2006–2007 season during which antigenically matched influenza A H3N2 and H1N1 were isolated [7]. Other studies have shown also shown variable VE and consistently low levels of effectiveness for influenza B during seasons with a lineage mismatch [8, 9]. In the current study, we have been able to determine with good precision VE against all 3 components of the vaccine in a year when estimates are not complicated by issues of antigenic mismatching.
Our VE estimate is consistent with the results of recently conducted randomized controlled trials of inactivated influenza vaccines against laboratory documented influenza in healthy adults. In 1 study in which the predominant influenza isolates were well-matched A/H1N1 viruses, efficacy against culture-confirmed illness was 63% [10]; in another study in which the viruses were predominantly antigenically or genetically matched A/H3N2 viruses, efficacy against culture-confirmed illness was 73% [11]. In an earlier study in the same population, TIV efficacy was 75% even against mostly drifted viruses [12]. In other randomized studies done in years with poor antigenic match, efficacy has generally been lower. In a trial in the 2005–2006 influenza season during which influenza B viruses predominated and the overall attack rate was low, the protective efficacy of TIV was only 22.3% [13], and in a trial conducted over 2 seasons (2005–2007) during which most cases were infected with antigenically variant viruses, TIV efficacy was 49.3% [14]. A recent meta-analysis of these and other randomized trials estimated the pooled efficacy of inactivated vaccine of 59% (95% CI, 51%–67%) [15]. However, a limitation of these trials is that they are generally conducted in selected healthy populations, so the results, although important, are not easily extended to other more high-risk populations. The current study gives an estimate of the potential impact of influenza vaccine under real-world conditions in the populations for which vaccination is particularly important, young children and the elderly.
Although we enrolled substantial numbers of both influenza-positive cases and influenza-negative controls during this season, age-specific vaccine assessments are limited by small numbers in some age groups. Nevertheless, VE among individuals aged ≥65 years appears to have been lower than in the other age groups. This finding is consistent with the well-documented decreased immunogenicity of influenza vaccine in older adults and should continue to be monitored. As pointed out recently [15], there have been no published, randomized, placebo-controlled trials of inactivated influenza vaccine efficacy in older adults that utilized culture- or RT-PCR–confirmed endpoints and only 1 such study of live vaccine, in which the efficacy was 43% [16]. Given the substantial resource allocations to the US influenza vaccine program, annual assessments of vaccine effects are warranted to understand opportunities for improving the population-level impact of the program, particularly among older adults at high risk of serious influenza complications.
At the other end of the age spectrum, the large sample size of our study also allowed us to obtain estimates of VE in young children. These estimates provide reassuring evidence of influenza VE even in the youngest populations for which vaccine is licensed. We also obtained identical estimates of the effectiveness of at least 1 dose of LAIV or of inactivated vaccine (both 71%) in children aged 2–8 years. In randomized, controlled studies, LAIVs have demonstrated high levels of efficacy in young children [17, 18]. In contrast, some previous randomized trials have suggested that inactivated vaccines may have better efficacy in adults than LAIVs [11]. However, only 43 subjects in the current study who were aged 9–49 years received LAIV, limiting our ability to make an accurate, specific estimate of LAIV effectiveness in this age group.
We found little evidence of confounding by the variables we collected in this study, as evidenced by the small differences in the crude and adjusted VE estimates. This finding may not be surprising because controls were enrolled in the same settings as cases and presented with similar clinical findings. In the test-negative case–control design, controls are meant to represent persons who would have sought care if they were ill with influenza. It is possible that an important yet unmeasured confounder may have biased our results, and further investigation of possible confounding by health behaviors is planned. Unmeasured confounding and the possibility of selection bias are important considerations for all observational studies of prevention interventions.
The results of this study demonstrate that influenza vaccination offers a health benefit in individuals aged <50 years and is likely beneficial in older age groups as well. However, the level of benefit could be described as modest, and a substantial proportion of influenza cases (30%) occurred in vaccinated individuals. Recently, a high-dose vaccine formulation that is more immunogenic in elderly recipients [19] has been licensed in the United States. Only 11 subjects received this vaccine in the current study. Annual assessments are needed to monitor the potential impact of this vaccine and other new strategies, such as use of adjuvants [20] to improve the performance of influenza vaccines, that may be implemented in the near future. Ultimately, more effective vaccines, and possibly other policies to limit transmission, such as programs to increase the vaccination rates in school-aged children [21], will be needed to control this important public health problem in all age groups.
Notes
Acknowledgments. Additional members of the US Flu-VE Network include Marshfield Clinic: J. Donahue, S. Irving, B. Kieke, S. Kjos, S. Koptizke, P. Squires, M. Vandermause, and S. Waring; University of Michigan: L. Blythe, R. Cross, E. Johnson, M. Zervos (Henry Ford Health System), and L. Lamerato (Henry Ford Health System); University of Rochester: A. Falsey, B.-K. Yoo, N. Bennett, P. Szylagyi, and K. Schnabel; Vanderbilt University: K. Edwards, Y. Zhu, D. Wyatt, D. Kent, Z. Liu, A. Storrow, L. Laya (Summit Hospital), P. McNabb (Baptist Hospital), and S. VanHook (St. Thomas Hospital); and Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention: S. Lindstrom, X. Xian, A. Klimov, S. Sambhara, J. Katz, and N. Cox.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Financial support. This study was funded by the Centers for Disease Control and Prevention (CDC) through cooperative agreements with the Marshfield Clinic Research Foundation (U01 IP000183), the University of Michigan (U01 IP000170), the University of Rochester (U01 IP000172), and Vanderbilt University (U01 IP000184). Internal CDC funds were used to support CDC investigators and to provide laboratory validation of RT-PCR methods at the enrolling site laboratories. As part of the cooperative agreement, CDC investigators participated in study design, analysis, decision to publish, and preparation of the manuscript. H. K. T. received salary support from the NIAID (1K23AI074863-01).
Potential conflicts of interest. J. T. reports grant or clinical trial support from Sanofi, GlaxoSmithKline (GSK), Protein Sciences, and Vaxinnate, and is on the scientific advisory boards of Novartis and Immune Targeting Systems. H. K. T. has clinical trial support from Sanofi Pasteur. S. E. O. has clinical trial support and royalty income from Sanofi. J. V. W. is on the scientific advisory boards of MedImmune and Quidel. C. B. H. has performed consulting work for Medimmune and GSK. A. S. M. has grant support from Sanofi and is a consultant for GSK and Novartis. E. B. has grant support from Medimmune. All other authors: No potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Orenstein EW, de Serres G, Haber MJ, et al. Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int J Epidemiol. 2007;36:623–31. doi: 10.1093/ije/dym021. [DOI] [PubMed] [Google Scholar]
- 2.Griffin MR, Monto AS, Belongia EA, et al. Effectiveness of non-adjuvanted pandemic influenza A vaccines for preventing pandemic influenza acute respiratory illness visits in 4 U.S. communities. PLoS One. 2011;6:e23085. doi: 10.1371/journal.pone.0023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1–62. [Google Scholar]
- 4.Irving SA, Donahue JG, Shay DK, Ellis-Coyle TL, Belongia EA. Evaluation of self-reported and registry-based influena vaccination status in a Wisconsin cohort. Vaccine. 2009;27:6543–9. doi: 10.1016/j.vaccine.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Kendal A, Pereira M, Skehel J. Hemagglutination-inhibtion: concepts and procedures for laboratory-based influenza surveillance. 1982 US Department of Health and Human Services, Atlanta GA. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1128–32. [PubMed] [Google Scholar]
- 7.Belongia EA, Kieke BA, Donahue JG, et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004–2005 season to the 2006–2007 season. J Infect Dis. 2009;199:159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 8.Belongia E, Kieke B, Coleman L, et al. Interim within-season estimate of the effectiveness of trivalent inactivated influenza vaccine—Marshfield, Wisconsin, 2007–2008 influenza season. MMWR Morb Mortal Wkly Rep. 2008;57:393–8. [PubMed] [Google Scholar]
- 9.Skowronski DM, De Serres G, Dickinson J, et al. Component-specific effectiveness of trivalent influenza vaccine as monitored through a sentinel surveillance network in Canada, 2006–2007. J Infect Dis. 2009;199:160–79. doi: 10.1086/595862. [DOI] [PubMed] [Google Scholar]
- 10.Frey S, Vesikari T, Szymczakiewicz-Multanowska A, et al. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51:997–1004. doi: 10.1086/656578. [DOI] [PubMed] [Google Scholar]
- 11.Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361:1260–7. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 12.Ohmit SE, Victor JC, Rotthoff JR, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med. 2006;355:2513–22. doi: 10.1056/NEJMoa061850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beran J, Wertzova V, Honegr K, et al. Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis. 2009;9:2. doi: 10.1186/1471-2334-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson L, Gaglani M, Keyserling H, et al. Safety, efficacy, and immunogenicity of an inactivated influenza vaccine in healthy adults: a randomized, placebo-controlled trial over two influenza seasons. BMC Infect Dis. 2010;10:71–7. doi: 10.1186/1471-2334-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osterholm MT, Kelley NS, Sommer A, Belongia E. Influenza vaccine efficacy and effectiveness: a new look at the evidence. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 16.de Villiers PJT, Steele AD, Hiemstra LA, et al. Efficacy and safety of a live attenuated influenza vaccine in adults 60 years of age and older. Vaccine. 2009;28:228–34. doi: 10.1016/j.vaccine.2009.09.092. [DOI] [PubMed] [Google Scholar]
- 17.Belshe RB, Mendelman PM, Treanor J, et al. The efficacy of live attenuated cold-adapted trivalent, intranasal influenzavirus vaccine in children. N Engl J Med. 1998;358:1405–12. doi: 10.1056/NEJM199805143382002. [DOI] [PubMed] [Google Scholar]
- 18.Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 19.Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Superior immunogenicity of high dose influenza vaccine compared with standard influenza vaccine in adults >65 years of age: a randomized double-blinded controlled phase 3 trial. J Infect Dis. 2008;200:172–80. doi: 10.1086/599790. [DOI] [PubMed] [Google Scholar]
- 20.Vesikari T, Knuf M, Wutzler P, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–16. doi: 10.1056/NEJMoa1010331. [DOI] [PubMed] [Google Scholar]
- 21.Loeb M, Russell ML, Moss L, et al. Effect of influenza vaccination of children on infection rates in Hutterite communities: a randomized trial. JAMA. 2010;303:943–50. doi: 10.1001/jama.2010.250. [DOI] [PubMed] [Google Scholar]