The intestinal microbiota was characterized in patients undergoing allogeneic hematopoietic stem cell transplantation. During early transplant, antibiotic administration was associated with intestinal domination by bacterial taxa such as enterococci, streptococci, and Proteobacteria, resulting in an increased risk of bacteremia.
Abstract
Background. Bacteremia is a frequent complication of allogeneic hematopoietic stem cell transplantation (allo-HSCT). It is unclear whether changes in the intestinal microbiota during allo-HSCT contribute to the development of bacteremia. We examined the microbiota of patients undergoing allo-HSCT, and correlated microbial shifts with the risk of bacteremia.
Methods. Fecal specimens were collected longitudinally from 94 patients undergoing allo-HSCT, from before transplant until 35 days after transplant. The intestinal microbiota was characterized by 454 pyrosequencing of the V1-V3 region of bacterial 16S ribosomal RNA genes. Microbial diversity was estimated by grouping sequences into operational taxonomic units and calculating the Shannon diversity index. Phylogenetic classification was obtained using the Ribosomal Database Project classifier. Associations of the microbiota with clinical predictors and outcomes were evaluated.
Results. During allo-HSCT, patients developed reduced diversity, with marked shifts in bacterial populations inhabiting the gut. Intestinal domination, defined as occupation of at least 30% of the microbiota by a single predominating bacterial taxon, occurred frequently. Commonly encountered dominating organisms included Enterococcus, Streptococcus, and various Proteobacteria. Enterococcal domination was increased 3-fold by metronidazole administration, whereas domination by Proteobacteria was reduced 10-fold by fluoroquinolone administration. As a predictor of outcomes, enterococcal domination increased the risk of Vancomycin-resistant Enterococcus bacteremia 9-fold, and proteobacterial domination increased the risk of gram-negative rod bacteremia 5-fold.
Conclusions. During allo-HSCT, the diversity and stability of the intestinal flora are disrupted, resulting in domination by bacteria associated with subsequent bacteremia. Assessment of fecal microbiota identifies patients at highest risk for bloodstream infection during allo-HCST.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment for a range of malignant and nonmalignant disorders. Pre-transplant conditioning transiently ablates circulating granulocytes and monocytes and markedly increases susceptibility to disseminated infections [1, 2]. Mucosal barrier injury is also a complication of allo-HSCT and enables commensal microbes to invade underlying tissues and the bloodstream [3]. As a result, systemic bacterial infections are frequent during the early transplant period [4–6]. Vancomycin-resistant Enterococcus (VRE), viridians-group Streptococcus, and aerobic gram-negative bacteria are the most common causes of bloodstream infection following allo-HSCT [5–8]. Why some patients develop bacteremia while others do not is unclear.
The complex microbial populations colonizing the human intestine provide resistance to infection. The intestinal microbiota also serves as a sanctuary for highly antibiotic-resistant bacteria [9]. Prior studies using in vitro culture methods have characterized microbial populations inhabiting the intestine [10]; more recently, massively parallel pyrosequencing of bacterial 16S ribosomal RNA (rRNA) encoding genes has provided new insights into the composition and complexity of the intestinal microbiota by identifying bacterial taxons that are not readily cultivated in vitro [11–14]. These approaches have demonstrated the compositional changes and resilience of the intestinal microbiota of healthy individuals after antibiotic treatment [12]. Studies with mice demonstrated dramatic changes in the microbiota of the ileum and cecum following antibiotic treatment, and dramatic expansion of antibiotic-resistant microbes such as vancomycin-resistant Enterococcus (VRE) [15]. Intestinal expansion of VRE following antibiotic treatment is also seen in humans, with episodes of VRE domination in patients undergoing allo-HSCT preceding the development of VRE bacteremia in 2 patients [15].
Because the effect of allo-HSCT conditioning, prolonged neutropenia, and antibiotic administration on the gastrointestinal tract microbiota is unknown, we characterized the fecal microbiota of patients undergoing transplant at multiple time points using 454 pyrosequencing of 16S rRNA genes. (Data deposition: 454 pyrosequencing reads have been deposited in the National Center for Biotechnology Information Sequence Read Archive.)
METHODS
Study Patients and Specimen Collection
Subjects consisted of adult patients undergoing allo-HSCT at Memorial Sloan-Kettering Cancer Center (MSKCC), from 4 September 2009 to 4 August 2011. Fecal specimens were collected longitudinally from each patient during their transplant hospitalization. For each patient, serial specimen collection began at the start of pre-transplant conditioning (up to 15 days before stem cell infusion) and continued until 35 days after transplant or at hospital discharge, whichever occurred sooner. Because of the expected variability among patients in the timing and frequency of bowel movement patterns, subjects were excluded from the study if they did not provide at least 1 pre-transplant specimen and at least 2 specimens between transplant and 35 days after transplant. The study protocol was approved by the MSKCC institutional review board; informed consent was obtained from all subjects prior to specimen collection.
Specimen Analysis
For each fecal specimen, DNA was extracted and purified, and the V1-V3 region of the 16S rRNA gene was amplified by means of polymerase chain reaction (PCR) using modified universal bacterial primers. Purified PCR products were sequenced on a 454 GS FLX Titanium platform. Sequence data were compiled and processed using Mothur, version 1.20 [16]. Sequence data were screened and filtered for quality [17], then aligned to the full-length 16S rRNA gene, using as template the SILVA reference alignment [18]. Sequences were grouped into operational taxonomic units of 97% similarity.
Microbial diversity was estimated by calculating the Shannon diversity index [19], along with other diversity measures for comparison. Phylogenetic classification to genus level was performed for each 16S sequence using the Ribosomal Database Program naive Bayesian classification scheme [20]. To assess the presence of VRE, specimens with ≥2% enterococcal sequences were screened for the presence of the vancomycin resistance gene vanA (see Supplementary Methods for details).
Patient-Level Analyses
We evaluated trends in microbial diversity over time using moving average filtering. Curvewise 95% confidence intervals and P values were calculated using bootstrap resampling within each subject.
Phylogenetic classification was used to describe the intestinal composition of each subject over the course of transplant. Hierarchical clustering of the specimens was performed [21], based on genus-level phylogeny. Microbial state transitions between consecutive specimens were characterized using Circos plots [22]. The diversity trends and hierarchical clustering analyses were performed using Matlab, version 7.12.
Next we determined whether clinical variables were predictive of changes in microbial composition, and in turn, whether the microbial composition was predictive of clinical outcomes. In our prior findings [15], we observed that some allo-HSCT patients experienced shifts in intestinal composition from a diverse microbiota to one that was largely occupied by a single taxon. We referred to this phenomenon as intestinal domination and defined it as having occurred if a single bacterial genus comprised >30% of sequences and was the most abundant taxon.
First, we examined intestinal domination as a microbial endpoint of interest, using Cox proportional hazards regression. The following clinical variables were assessed as univariate predictors of domination: age, sex, underlying diagnosis (leukemia vs other), receipt of prior antibiotics (within 14 days prior to start of the observation period), conditioning regimen intensity (myeloablative or reduced intensity vs non-myeloablative [23]), whether stem cells were ex vivo T-cell depleted [24], stem cell source (peripheral blood donor vs umbilical cord blood), first onset of fever during the observation period, and antibiotic administration (vancomycin, metronidazole, fluoroquinolones, and beta-lactams). Fever and antibiotic administration were analyzed as time-varying variables, in order to avoid time-dependent bias. Next, we again examined intestinal domination, this time as predictor of clinical outcomes. Domination was analyzed as a time-varying predictor of bloodstream infection with either VRE or aerobic gram-negative bacilli. In order to avoid problems with monotone likelihood for parameter estimate calculations, we employed a penalized maximum likelihood for all Cox hazard calculations [25]. These analyses were performed using R, version 2.15.
RESULTS
Patient Characteristics
During the study period, fecal specimens were obtained from 113 patients during their transplant hospitalization. Nineteen patients provided insufficient specimens, leaving 94 subjects for analysis. No exclusion was due to early death. Time of discharge following allo-HSCT ranged from 13 to 95 days after transplant (median, 21 days). Clinical characteristics of the 94 patients are listed in Table 1. Most patients engrafted within 14 days after transplant. All patients received antibiotics during the study period; in particular, vancomycin and beta-lactam antibiotics were administered to most patients. Bloodstream infection was detected in 22 (23.4%) patients. Of these, 9 were due to VRE and 10 were due to gram-negative bacteria (Table 1).
Table 1.
Characteristics of Patients and Transplant Course
| Parameter | No. (%) of Patients |
|---|---|
| Age, years | |
| <30 | 7 (7.4) |
| 30–39 | 13 (13.8) |
| 40–49 | 19 (20.2) |
| 50–59 | 28 (29.8) |
| ≥60 | 27 (28.7) |
| Sex | |
| Male | 53 (56.4) |
| Female | 41 (43.6) |
| Underlying diagnosis | |
| Leukemia | 44 (46.8) |
| Lymphoma | 26 (27.7) |
| Multiple myeloma | 8 (8.5) |
| Myelodysplastic syndrome | 12 (12.8) |
| Other | 4 (4.3) |
| Prior antibiotics (within 14 days before study period) | |
| No | 52 (55.3) |
| Yes | 42 (44.7) |
| Conditioning regimen | |
| Non-myeloablative | 16 (17) |
| Reduced intensity | 21 (22.3) |
| Myeloablative | 57 (60.6) |
| T-cell depletion | |
| No | 52 (55.3) |
| Yes | 42 (44.7) |
| Stem cell source | |
| Peripheral blood | 71 (75.5) |
| Umbilical cord | 23 (24.5) |
| Time to engraftment, daysa,b | |
| <14 | 70 (74.5) |
| ≥14 | 24 (25.5) |
| Feverb | |
| No | 16 (17) |
| Yes | 78 (83) |
| Antibiotics administeredb,c | |
| Vancomycin | 85 (90.4) |
| Fluoroquinolone | 33 (35.1) |
| Metronidazole | 30 (31.9) |
| Beta-lactamd | 82 (87.2) |
| Bloodstream infectionb,e | |
| Vancomycin-resistant Enterococcus | 9 (9.6) |
| Gram-negative bacilli, aerobic | 10 (10.6) |
| Other organism | 3 (3.2) |
| None | 72 (76.6) |
| Vital statusb | |
| Alive | 92 (97.9) |
| Dead | 2 (2.1) |
| Total | 94 (100) |
a Engraftment was defined as an absolute neutrophil count of >500 cells/µL for 3 consecutive days.
b Assessed during inpatient allogeneic hematopoietic stem cell transplantation hospitalization, from 15 days before transplant to 35 days after transplant.
c Antibiotic variables are not mutually exclusive and do not sum to 100%.
d Beta-lactams include cephalosporins, beta-lactam–beta-lactamase combinations, and carbapenems.
e Positive bacteremia values not meeting standard Centers for Disease Control and Prevention definitions of a laboratory-confirmed bloodstream infection (eg, a single positive blood culture for coagulase-negative Staphylococcus) [44] were excluded.
Specimen Collection and Bacterial Sequences Obtained
A total of 439 fecal specimens were collected, with 3–8 specimens per patient. From these specimens, a total of 1 838 205 high-quality 16S rRNA-encoding sequences were identified. The mean number of sequences obtained per specimen was 4187 (range, 852–9862).
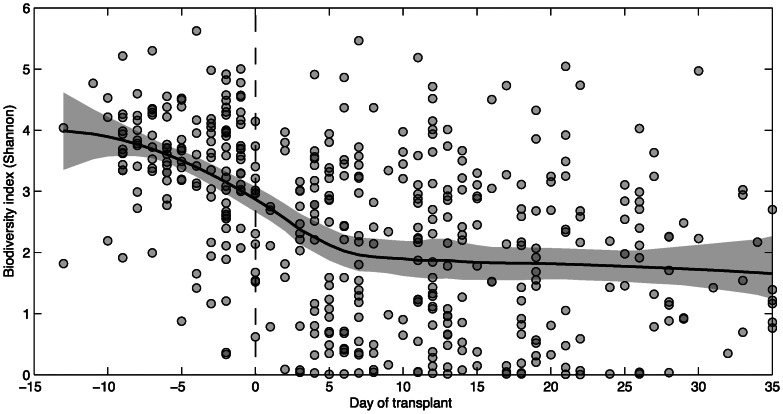
Loss of Microbial Diversity Following Allo-HSCT
There was considerable variation among Shannon diversity index values (Figure 1). During the pre-transplant period, the average Shannon diversity index ranged approximately 3–4, similar to values observed for healthy volunteers in both our prior observations (not shown) and previous reports [12]. However, over the course of allo-HSCT, the mean diversity index decreased to approximately 2 and remained low until the end of our observation period. Similar decreases were observed with other measures of microbial diversity (Supplementary Figure 1). By subgroup, patients with leukemia and those receiving either metronidazole or beta-lactam antibiotics experienced comparatively greater decreases in Shannon diversity index (Supplementary Figure 2).
Figure 1.
Changes in microbial diversity within the intestinal tract during allogeneic hematopoietic stem cell transplantation. Microbial diversity, quantified by the Shannon diversity index, was calculated for each fecal specimen of each patient and plotted over day of transplant (circles). A diversity trend (solid black line; 95% confidence intervals shown in gray) was constructed using moving average filtering.
Development of Intestinal Domination
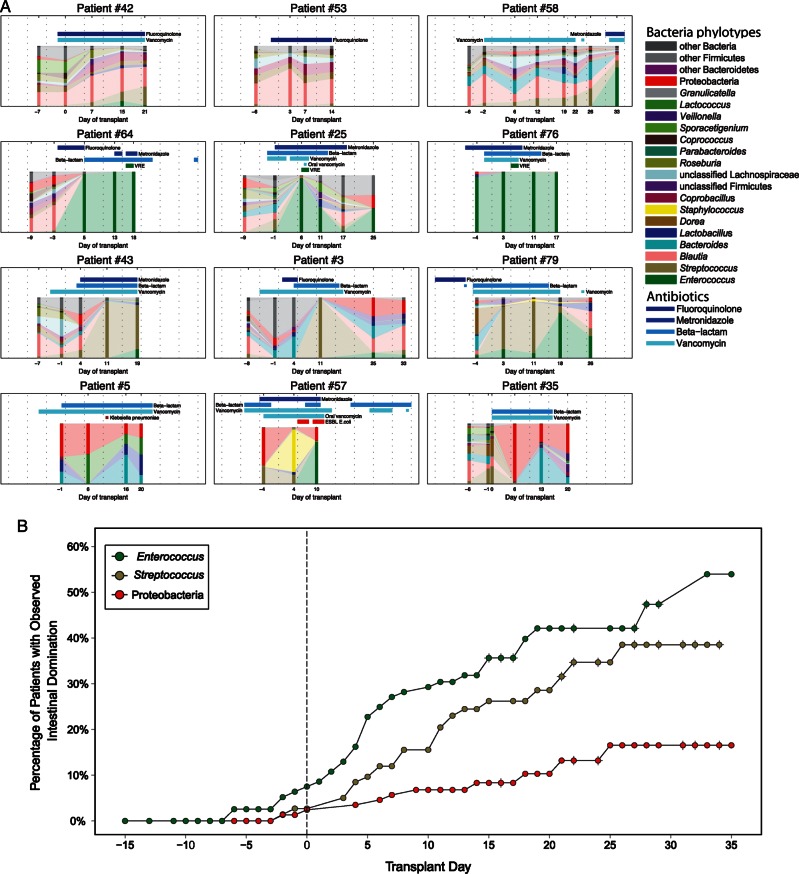
The microbial composition for 12 selected patients over time, with concurrent episodes of bacteremia and administration of antibiotics, is shown in Figure 2A. In some patients, the microbiota remained relatively diverse, experiencing only mild fluctuations in composition. In these patients, the genus Blautia was frequently an abundant inhabitant and appeared to be associated with diminished antibiotic administration.
Figure 2.
Characterization of the intestinal microbiota during allogeneic hematopoietic stem cell transplantation. A, Genus-level taxonomy of 12 selected patients, plotted over day of transplant. Each stacked bar represents the microbial composition of a single fecal specimen, based on taxonomic classification. Shading between specimens are provided for interpolation and visualization of changes in relative abundances. Concurrent antibiotic administration (vancomycin, fluoroquinolones, metronidazole, and beta-lactams) and detected bacteremias are shown for each patient. Patients in the first row generally maintained diversity with few changes in composition; patients in the second row developed intestinal domination by Enterococcus; patients in the third row developed intestinal domination by Streptococcus; and patients in the fourth row developed domination with Proteobacteria. Note that patient 58 in the top row maintained diversity but developed Enterococcus domination shortly after metronidazole administration. (Plots of all 94 patients can be found in Supplementary Figure 3.) B, Kaplan-Meier plot of intestinal domination as a survival event. Crosshairs represent censored observations. Intestinal domination by Enterococcus, Streptococcus, and Proteobacteria was common and occurred at various times throughout the observation period.
However, other patients demonstrated marked changes in composition, with transitions from a relatively diverse microbiota to a simpler one with fewer members. In many instances, the microbial composition became dominated by a single bacterial taxon. Enterococcus was the most frequent dominating genus, occurring in 38 (40.4%) patients. Of these patients, most (92%) had detectable expression of the vanA gene, suggesting the presence of VRE. Streptococcus was the next most frequent dominating genus, observed in 35 (37.2%) patients. Domination by phylum Proteobacteria, which includes Enterobacteriaceae and other aerobic gram-negative bacteria, was observed in 12 (12.8%) patients. Domination by these bacteria occurred at various times relative to transplant (Figure 2B). The composition of all 94 patients is provided (Supplementary Figure 3).
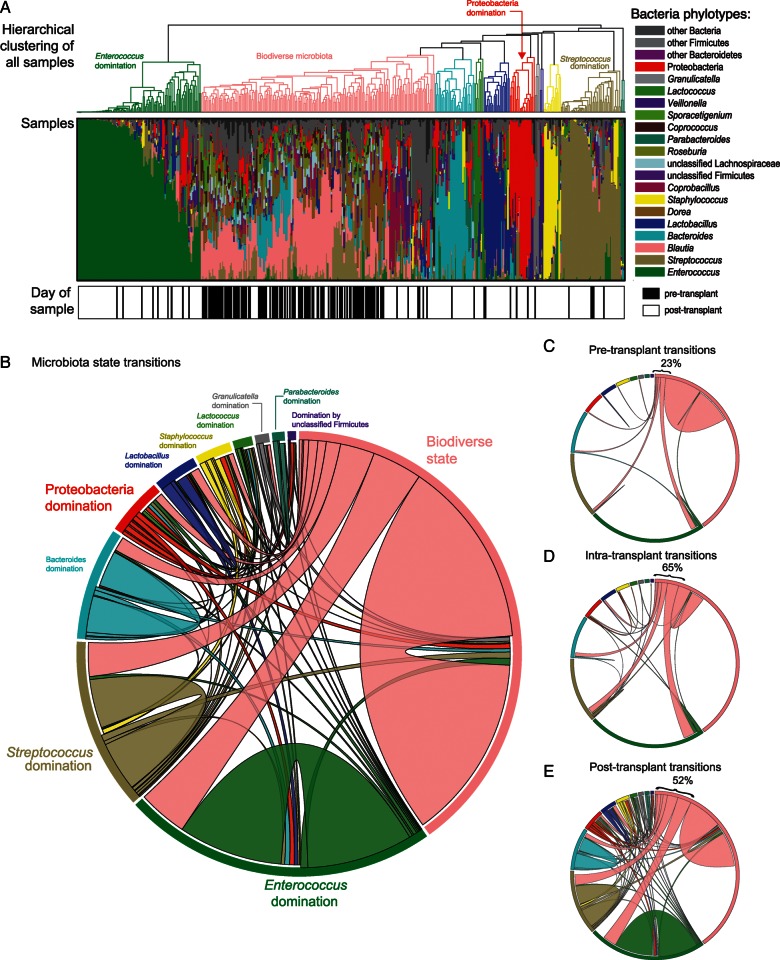
Hierarchical clustering of the phylogenetic composition of all 439 specimens demonstrated that specimens can be categorized into specific states of either biodiversity or domination by specific taxa (Figure 3A), with biodiverse specimens being more common during the pre-transplant period. Circos plots demonstrated a large number of state-to-state transitions (Figure 3B–E). Although most pre-transplant specimens remained biodiverse, transitions to states of domination were most prevalent between the pre-transplant specimen and the first post-transplant specimen and intermediate between sequential post-transplant specimens.
Figure 3.
Microbiota states and their transitions. A, Hierarchical clustering of all fecal specimens according to microbiota state based on phylogenetic classification. There was wide variation in phylogenetic composition; some specimens showed a relatively biodiverse membership, whereas others were largely dominated by a single bacterial taxon. B, Circos plots representing microbiota state transitions between consecutive specimens within the observation period. Subplots of transitions during specific transplant periods are shown in C–E. C, Pre-transplant transitions revealing that patients in the “biodiverse microbiota” state tend to remain in that state; resilience of the “biodiverse” state, measured as the percentage of consecutive specimens that remain in that state, is 77%. D, Resilience of “biodiverse” state decreasing to 35% during the intra-transplant period (consecutive specimens straddling the day of stem cell infusion). E, Post-transplant transitions with the “biodiverse” state resilience staying low at 48%.
Clinical Factors Associated With Development of Intestinal Domination
Clinical predictors of intestinal domination with Enterococcus, Streptococcus, and Proteobacteria are shown in Table 2. Age, sex, conditioning regimen intensity, and stem cell source were not associated with intestinal domination by the 3 organisms evaluated. An underlying diagnosis of leukemia was associated with a higher risk of domination by Enterococcus.
Table 2.
Clinical Predictors of Intestinal Domination
|
Enterococcus Domination |
Streptococcus Domination |
Proteobacteria Domination |
||||
|---|---|---|---|---|---|---|
| Predictor | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P |
| Age, years | 1.00 (.98–1.04) | .790 | 0.99 (.97–1.03) | .681 | 1.00 (.95–1.05) | .978 |
| Female sex | 0.84 (.42–1.64) | .611 | 1.07 (.50–2.27) | .852 | 1.12 (.33–3.78) | .854 |
| Underlying diagnosis (leukemia vs other) | 3.22 (1.60–6.94) | .001 | 0.71 (.32–1.51) | .375 | 0.66 (.18–2.19) | .498 |
| Prior antibiotics (14 days)a | 1.49 (.77–2.94) | .237 | 1.03 (.48–2.17) | .945 | 1.31 (.39–4.44) | .651 |
| Conditioning regimen (myeloablative or reduced intensity vs non-myeloablative) | 1.01 (.44–2.84) | .977 | 0.61 (.25–1.75) | .329 | 0.98 (.22–9.25) | .983 |
| T-cell depleted graft | 0.81 (.40–1.61) | .551 | 0.91 (.39–2.00) | .812 | 1.07 (.29–3.62) | .910 |
| Stem cell source (cord vs other) | 1.22 (.55–2.52) | .607 | 0.54 (.19–1.34) | .196 | 1.36 (.36–4.69) | .633 |
| Feverb | 1.68 (.78–3.74) | .182 | 0.90 (.36–2.39) | .826 | 1.28 (.30–6.34) | .747 |
| Antibioticsb | ||||||
| Vancomycin | 2.12 (.67–10.21) | .222 | 0.95 (.33–3.77) | .938 | 5.17 (.52–707.15) | .192 |
| Metronidazole | 3.38 (1.65–6.73) | .001 | 1.94 (.81–4.30) | .131 | 1.73 (.41–6.03) | .426 |
| Fluoroquinolonesc | 1.09 (.49–2.24) | .832 | 1.19 (.51–2.60) | .677 | 0.09 (.00–.75) | .020 |
| Beta-lactamd | 1.64 (.74–3.99) | .232 | 1.69 (.62–5.64) | .319 | 1.23 (.27–7.50) | .800 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Defined as administration of any antibacterial drug within 14 days prior to observation period.
b Analyzed as a time-varying predictor.
c Fluoroquinolones consist of ciprofloxacin and levofloxacin.
d Beta-lactams include cephalosporins, beta-lactam–beta-lactamase combinations, and carbapenems.
Antibiotics, given at various time points for various indications (eg, prophylaxis or empiric/directed treatment) were associated with subsequent enterococcal domination. Metronidazole was associated with a 3-fold increase in risk of enterococcal domination. Metronidazole was given for various reasons, including treatment for Clostridium difficile infection (14 of 30 metronidazole recipients). Vancomycin, fluoroquinolone, and beta-lactam administration did not increase the risk of domination. Fluoroquinolone administration was associated with a 10-fold decreased risk of intestinal domination by Proteobacteria.
Association of Intestinal Domination With Bacteremia
Analysis of intestinal domination as predictor of bloodstream infection is shown in Table 3. Patients with enterococcal domination had a 9-fold increased risk of VRE bacteremia, and intestinal domination by Proteobacteria increased the risk of bacteremia with aerobic gram-negative bacilli 5-fold. In the time leading up to bloodstream infection, enterococcal domination with detectable vanA was observed in 8 of 9 patients with VRE bacteremia, and proteobacterial domination was observed in 2 of 10 patients with gram-negative bacteremia. In total, 11 of the 22 patients with bacteremia demonstrated preceding intestinal domination by a corresponding organism; the median time between domination and bacteremia in these patients was 7 days.
Table 3.
Association of Intestinal Domination With Bacteremiaa
| VRE Bacteremia |
Gram-negative Bacteremia |
|||
|---|---|---|---|---|
| Dominating Taxonb | HR (95% CI) | P | HR (95% CI) | P |
| Enterococcus | 9.35 (2.43–45.44) | .001 | 1.35 (.25–5.08) | .690 |
| Streptococcus | 0.21 (.00–1.75) | .184 | 0.82 (.09–3.65) | .823 |
| Proteobacteria | 0.75 (.01–6.14) | .837 | 5.46 (1.03–19.91) | .047 |
Abbreviations: CI, confidence interval; HR, hazard ratio; VRE, Vancomycin-resistant Enterococcus.
a Bacteremia for each organism was defined as at least one positive blood culture within the study period.
b Intestinal domination was analyzed as a time-varying predictor.
DISCUSSION
Patients undergoing allo-HSCT are subjected to chemotherapy, radiation, and antibiotics within a short time frame. In this study we observed extreme shifts in the intestinal microbiota during transplant. In many instances, domination by a single bacterial taxon occurred. These perturbations correlated with subsequent development of a corresponding bloodstream infection with either VRE or gram-negative bacteria.
The intestinal microbiota is engaged in a complex relationship with the mucosal epithelium. Intestinal epithelial cells, underlying immune tissues, and the microbiota establish a state of equilibrium that optimizes resistance to infection and facilitates the absorption of nutrients [26]. Allo-HSCT disrupts this equilibrium, resulting in dramatic compositional fluctuations that can result in domination by bacteria that invade the bloodstream. Radiation and chemotherapy-mediated destruction of gut epithelial stem cells, inhibition of microbial populations by antibiotic administration, and suspension of innate immune defenses each affect intestinal environment and host-microbe interactions.
We find that microbial diversity is generally not re-established during allo-HSCT hospitalization, perhaps due in part to continued antibiotic pressure. Other studies of the intestinal microbiota have shown the resilience of bacterial populations following antibiotic therapy [12, 27], and experimental studies in mice demonstrate that decreased diversity increases susceptibility to dense colonization by VRE [15]. Although the benefits of diversity remain unproven, strategies to reintroduce complex microbial populations into the gut following allo-HSCT may be of benefit.
How the complex microbiota prevents domination by rogue bacterial species such as VRE or Enterobacteriaceae remains unclear. Antibiotic administration results in expansion of Enterobacteriaceae and enterococci in the gut and increases susceptibility to infection by these organisms. The suppression of newly introduced bacteria by the intestinal microbiota is referred to as colonization resistance [28–30]. Although commensal microbes may outcompete VRE by depleting nutrients or occupying spatial niches, other mechanisms such as production of inhibitory short-chain fatty acids may play a more important role [31, 32].
In this and our previous studies [15], bloodstream infections during allo-HSCT are frequently preceded by intestinal domination by the same organism. In this study, two-thirds of patients developed either enterococcal or streptococcal domination during the course of transplant. Interestingly, viridians-group streptococci and VRE were historically the most frequently encountered bloodstream infections in allo-HSCT patients during the pre-engraftment period [4–8]. Prior to 2002, viridans streptococci were an important cause of bacteremia at our center, with significant morbidity [4, 6]. Prophylactic vancomycin administration during transplant has virtually eliminated viridans-group Streptococcus bacteremia in our center [7]. Because streptococcal domination remained common in this study, vancomycin prophylaxis may have served to prevent bloodstream invasion but not intestinal domination.
In contrast to Streptococcus domination, enterococcal domination was highly associated with VRE bacteremia. Prior studies of allo-HSCT patients have demonstrated associations between VRE colonization and the development of VRE bacteremia [7, 8, 33], leading many centers to routinely test for VRE colonization by means of rectal swab cultures prior to transplant. Compared with routine surveillance culture findings, enterococcal domination was detected in a greater percentage of subjects (40% vs 21%, respectively), and we identified more patients with VRE bacteremia (8 of 9 instead of 6). It is possible that bacteremia occurred with greater frequency than was documented, because blood cultures were largely obtained in the setting of fever or other evidence of septicemia. Indeed, a study involving surveillance blood cultures of steroid-treated allo-HSCT patients revealed bacteremia in more than one-third of patients [34].
Our finding that metronidazole administration is strongly associated with the development of Enterococcus domination is consistent with prior studies demonstrating that antibiotics with anaerobic activity promote VRE colonization [35, 36] and supports the notion that anaerobic bacteria contribute to colonization resistance [30, 31]. Because vanA was present in most cases of enterococcal domination, and because most cases of enterococcal bloodstream infections were vancomycin resistant, we presume antibiotic resistance contributes to microbial shifts within the microbiota; however, indirect effects of antibiotic treatment resulting from interdependencies of bacterial taxa in the gut are likely important as well [37]. The Shannon diversity index was also lower in recipients of metronidazole, presumably reflecting our observed association with enterococcal domination. However, beta-lactam recipients also lost diversity but were not associated with enterococcal domination. Although this disparity may reflect the ability of beta-lactams to inhibit enterococcal growth in the intestine, an alternative explanation is that beta-lactams were administered later during the course of transplant, as empiric therapy for fever in the setting of neutropenia, and thus were largely unevaluated in the Cox model as a preceding risk factor for domination.
We found that fluoroquinolones protected against proteobacterial domination, which in turn protected against gram-negative bloodstream infections. This finding supports prior studies of fluoroquinolone prophylaxis in neutropenic patients [38, 39] and provides insight into the mechanism of protection. The routine practice of fluoroquinolone prophylaxis in neutropenia has been controversial, in part due to concerns about resistance or disturbance of the microbiota. Other studies show fluoroquinolones having effects of varying duration in healthy humans [12, 37]. In those subjects, there were no clinically apparent consequences resulting from these microbial disturbances. Whether fluoroquinolone prophylaxis in patients undergoing allo-HSCT has any deleterious consequences remains unclear.
Patients with leukemia were more likely to develop enterococcal domination than those with other underlying diseases. The reason for this association is unclear but is likely multifactorial, including greater pre-transplant exposure to antibiotics, more intense conditioning prior to transplant, and/or greater exposure to VRE in conjunction with prior treatment for leukemia. However, our analysis did not identify associations between these factors and enterococcal domination.
Our study has several limitations. Specimens were collected with variable frequency of approximately 1 week apart. Thus, our microbiologic data could be viewed as interval-censored since we do know the state of the microbiota between successive specimens. At times we observed rapid transitions, and thus there may have been transient microbial states which were not captured. For example, episodes of domination, perhaps preceding some episodes of bloodstream infection, may have occurred but went undetected. To obviate this, we used a cutoff definition of 30% for intestinal domination, even though in many instances the relative abundance was much higher. We chose this cutoff primarily on the basis of resultant grouping of microbial states in our hierarchical cluster analysis, but this cutoff likely enhanced the identification of domination states, given the frequency of collection. The number of 16S sequences obtained per specimen was lower than that of some other studies [37]. Thus, the depth of coverage may have been insufficient to determine whether certain infrequent bacterial taxons were present. Since we focused on dominating (ie, highly abundant) microbes, we expect that our results would be unaffected by deeper sequencing. Furthermore, Good's coverage was ≥98% in >96% of specimens in this study.
Although our studies are limited to allo-HSCT patients at one medical center, we suspect similar microbiota shifts are also occurring at other centers, and for that matter in other populations of patients who might be at risk for bloodstream infections due to neutropenia, such as those receiving cytotoxic chemotherapy for hematologic malignancies. Based on evidence from prior studies, it is also likely that disturbances of the microbiota are implicated in the pathogenesis of other complications of allo-HSCT, such as such as graft-versus-host disease of the gut and C. difficile associated diarrhea [40–43]. Further study is needed in order to fully grasp the clinical implications of microbial derangements in these patient populations.
Our study demonstrates that intestinal domination in a subset of allo-HSCT patients precedes bloodstream infection by a median of 7 days, suggesting that determination of the fecal microbiota composition can identify patients at highest risk for bacteremia. This longitudinal study provides an example of how analysis of the intestinal microbiota can have relevance in clinical disease. Although current deep sequencing platforms for the analysis of the microbiota require substantial time for specimen preparation, amplification, sequencing, and analysis, it is likely that these platforms will evolve in the near future to provide microbiota analyses within a clinically meaningful period. With this progress, microbiota analyses such as that presented in our study will increasingly guide the treatment of pertinent populations such as allo-HSCT recipients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This study was supported by the National Institutes of Health (grants 1K23 AI095398-01 to Y. T., DP2OD008440 to J. B. X., and 1RO1 AI42135 to E. G. P.), the Lucille Castori Center for Microbes, Inflammation, and Cancer, and the Tow Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Junghanss C, Marr KA, Carter RA, et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem cell transplantation: a matched control study. Biol Blood Marrow Transplant. 2002;8:512–20. doi: 10.1053/bbmt.2002.v8.pm12374456. [DOI] [PubMed] [Google Scholar]
- 2.Blijlevens NM, Donnelly JP, de Pauw BE. mucosal barrier injury depends on conditioning regimen. Bone Marrow Transplant. 2005;35:707–11. doi: 10.1038/sj.bmt.1704863. [DOI] [PubMed] [Google Scholar]
- 3.van Vliet MJ, Harmsen HJ, de Bont ES, Tissing WJ. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 2010;6:e1000879. doi: 10.1371/journal.ppat.1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffe D, Jakubowski A, Sepkowitz K, et al. Prevention of peritransplantation viridans streptococcal bacteremia with early vancomycin administration: a single-center observational cohort study. Clin Infect Dis. 2004;39:1625–32. doi: 10.1086/425612. [DOI] [PubMed] [Google Scholar]
- 5.Castagnola E, Bagnasco F, Faraci M, et al. Incidence of bacteremias and invasive mycoses in children undergoing allogeneic hematopoietic stem cell transplantation: a single center experience. Bone Marrow Transplant. 2008;41:339–47. doi: 10.1038/sj.bmt.1705921. [DOI] [PubMed] [Google Scholar]
- 6.Almyroudis NG, Fuller A, Jakubowski A, et al. Pre- and post-engraftment bloodstream infection rates and associated mortality in allogeneic hematopoietic stem cell transplant recipients. Transpl Infect Dis. 2005;7:11–7. doi: 10.1111/j.1399-3062.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 7.Kamboj M, Chung D, Seo SK, et al. The changing epidemiology of vancomycin-resistant Enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol Blood Marrow Transplant. 2010;16:1576–81. doi: 10.1016/j.bbmt.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstock DM, Conlon M, Iovino C, et al. Colonization, bloodstream infection, and mortality caused by vancomycin-resistant enterococcus early after allogeneic hematopoietic stem cell transplant. Biol Blood Marrow Transplant. 2007;13:615–21. doi: 10.1016/j.bbmt.2007.01.078. [DOI] [PubMed] [Google Scholar]
- 9.Donskey CJ. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis. 2004;39:219–26. doi: 10.1086/422002. [DOI] [PubMed] [Google Scholar]
- 10.Gorbach SL. Microbiology of the gastrointestinal tract. In: Baron S, editor. Medical microbiology. 4th ed. Galveston, TX: 1996. [PubMed] [Google Scholar]
- 11.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008;6:e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Lozupone C, Hamady M, Bushman FD, Knight R. Short pyrosequencing reads suffice for accurate microbial community analysis. Nucleic Acids Res. 2007;35:e120. doi: 10.1093/nar/gkm541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Relman DA. Microbial genomics and infectious diseases. N Engl J Med. 2011;365:347–57. doi: 10.1056/NEJMra1003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest. 2010;120:4332–41. doi: 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One. 2011;6:e27310. doi: 10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schloss PD. A high-throughput DNA sequence aligner for microbial ecology studies. PLoS One. 2009;4:e8230. doi: 10.1371/journal.pone.0008230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magurran AE. Measuring biological diversity. Malden, Ma: Blackwell Pub.; 2004. [Google Scholar]
- 20.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krzywinski M, Schein J, Birol I, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–45. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jakubowski AA, Small TN, Kernan NA, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinze G, Schemper M. A solution to the problem of monotone likelihood in Cox regression. Biometrics. 2001;57:114–9. doi: 10.1111/j.0006-341x.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 26.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42:1203–6. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vollaard EJ, Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–14. doi: 10.1128/aac.38.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endt K, Stecher B, Chaffron S, et al. The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog. 2010;6:e1001097.. doi: 10.1371/journal.ppat.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Waaij D, Berghuis-de Vries JM, Lekkerkerk L-V. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971;69:405–11. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pultz NJ, Stiefel U, Subramanyan S, Helfand MS, Donskey CJ. Mechanisms by which anaerobic microbiota inhibit the establishment in mice of intestinal colonization by vancomycin-resistant Enterococcus. J Infect Dis. 2005;191:949–56. doi: 10.1086/428090. [DOI] [PubMed] [Google Scholar]
- 32.Bohnhoff M, Miller CP, Martin WR. Resistance of the mouse's intestinal tract to experimental Salmonella infection. II. Factors responsible for its loss following streptomycin treatment. J Exp Med. 1964;120:817–28. doi: 10.1084/jem.120.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zirakzadeh A, Gastineau DA, Mandrekar JN, Burke JP, Johnston PB, Patel R. Vancomycin-resistant enterococcal colonization appears associated with increased mortality among allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2008;41:385–92. doi: 10.1038/sj.bmt.1705912. [DOI] [PubMed] [Google Scholar]
- 34.Chizuka A, Kami M, Kanda Y, et al. Value of surveillance blood culture for early diagnosis of occult bacteremia in patients on corticosteroid therapy following allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2005;35:577–82. doi: 10.1038/sj.bmt.1704830. [DOI] [PubMed] [Google Scholar]
- 35.Donskey CJ, Hanrahan JA, Hutton RA, Rice LB. Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J Infect Dis. 1999;180:384–90. doi: 10.1086/314874. [DOI] [PubMed] [Google Scholar]
- 36.Donskey CJ, Chowdhry TK, Hecker MT, et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med. 2000;343:1925–32. doi: 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bucaneve G, Micozzi A, Menichetti F, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. N Engl J Med. 2005;353:977–87. doi: 10.1056/NEJMoa044097. [DOI] [PubMed] [Google Scholar]
- 39.Gafter-Gvili A, Fraser A, Paul M, Leibovici L. Meta-analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Ann Intern Med. 2005;142(12 Pt 1):979–95. doi: 10.7326/0003-4819-142-12_part_1-200506210-00008. [DOI] [PubMed] [Google Scholar]
- 40.Vossen JM, Heidt PJ, van den Berg H, Gerritsen EJ, Hermans J, Dooren LJ. Prevention of infection and graft-versus-host disease by suppression of intestinal microflora in children treated with allogeneic bone marrow transplantation. Eur J Clin Microbiol Infect Dis. 1990;9:14–23. doi: 10.1007/BF01969527. [DOI] [PubMed] [Google Scholar]
- 41.Pollard M, Chang CF, Srivastava KK. The role of microflora in development of graft-versus-host disease. Transplant Proc. 1976;8:533–6. [PubMed] [Google Scholar]
- 42.Chang JY, Antonopoulos DA, Kalra A, et al. Decreased diversity of the fecal Microbiome in recurrent Clostridium difficile-associated diarrhea. J Infect Dis. 2008;197:435–8. doi: 10.1086/525047. [DOI] [PubMed] [Google Scholar]
- 43.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–9. [PubMed] [Google Scholar]
- 44.CDC. Central Line-Associated Bloodstream Infection (CLABSI) Event. Available at: http://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf . Accessed 23 October 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.