Abstract
Current tuberculosis notification rates in South Africa are among the highest ever recorded. Although the human immunodeficiency virus epidemic has been a critical factor, the density of respiratory contacts in high-risk environments may be an important and underappreciated driver. Using a modified Wells-Riley model for airborne disease transmission, we estimated the risk of tuberculosis transmission on 3 modes of public transit (minibus taxis, buses, and trains) in Cape Town, South Africa, using exhaled carbon dioxide as a natural tracer gas to evaluate air exchange. Carbon dioxide measurements were performed between October and December of 2011. Environmental risk, reflected in the rebreathed fraction of air, was highest in minibus taxis and lowest in trains; however, the average number of passengers sharing an indoor space was highest in trains and lowest in minibus taxis. Among daily commuters, the annual risk of tuberculosis infection was projected to be 3.5%–5.0% and was highest among minibus taxi commuters. Assuming a duration of infectiousness of 1 year, the basic reproductive number attributable to transportation was more than 1 in all 3 modes of transportation. Given its poor ventilation and high respiratory contact rates, public transportation may play a critical role in sustaining tuberculosis transmission in South African cities.
Keywords: HIV, indoor air quality, mathematical model, transmission, tuberculosis
Tuberculosis control is failing in many sub-Saharan African cities despite successful implementation of the World Health Organization's directly observed treatment short-course strategy (1). In Cape Town, South Africa, the notification rate of tuberculosis was 909 cases per 100,000 persons in 2009 (2). Although human immunodeficiency virus (HIV) has been an important driver of the epidemic, the prevalence of tuberculosis among patients not infected with HIV is among the highest ever reported in the world, and it is likely that other factors are contributing to high rates of tuberculosis transmission.
Household contact investigations are key components of tuberculosis control programs in Western countries, as the majority of transmission is thought to occur among close household contacts. However, studies in which the molecular epidemiology of tuberculosis in these settings has been characterized have suggested that the majority of transmission takes place outside of the household (3–5). In addition to the prolonged duration of infectiousness prior to diagnosis (6), the environmental and social conditions present in South Africa may be favorable to tuberculosis transmission in the community.
Few South Africans living in urban townships own automobiles; the majority relies upon formal or informal public transportation methods for transit to school and work (7). These densely crowded and often poorly ventilated environments could pose a significant risk of the transmission of airborne infections, including tuberculosis, as has been documented in various modes of transportation elsewhere (8–12). Studies among transportation sector workers in Lima, Peru, found tuberculosis infection and incidence rates that were several-fold higher than the population averages (13, 14). Little is known about the population-level contributions of public transit to tuberculosis transmission in developing countries.
We estimated ventilation rates in various modes of transportation using continuous carbon dioxide monitoring and applied adjustments to the classic Wells-Riley equation for infection risk in indoor settings to estimate the risk of tuberculosis infection and disease attributable to public transit in a South African city.
MATERIALS AND METHODS
Model development
In the classic Wells-Riley equation, the rate of tuberculosis transmission is proportionate to the number of infectious individuals in a room (I), the per-person breathing rate (p), the rate of generating infectious quanta (q), and the duration of exposure (t) and inversely proportionate to the germ-free ventilation rate (Q) (15). The probability of infection is Poisson-distributed, and the probability (P) of any infection is given by the following equation:
Because this equation requires a steady state of quantum concentration and ventilation rates are difficult to measure directly, Rudnick and Milton (16) revised the equation to represent a non–steady-state relationship in which the ambient carbon dioxide concentration (produced predominantly by human respiration) could be used to estimate the fraction of carbon dioxide that was rebreathed by other occupants of a room. The rebreathed fraction enables estimation of the probability of infection in the space by the following relationship:
Here, 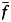 is the average rebreathed fraction, which is derived by estimating the difference between the indoor carbon dioxide concentration and the outdoor air carbon dioxide concentration and dividing that estimation by the carbon dioxide concentration in exhaled air (see the Web Appendix, available at http://aje.oxfordjournals.org/). The annual risk of infection is a commonly used measure of tuberculosis control, as it can be estimated from tuberculin skin test surveys. We used data on use of public transit (see the Data sources section) to estimate the annual risk of infection as a function of time (t) spent in various forms of transit.
is the average rebreathed fraction, which is derived by estimating the difference between the indoor carbon dioxide concentration and the outdoor air carbon dioxide concentration and dividing that estimation by the carbon dioxide concentration in exhaled air (see the Web Appendix, available at http://aje.oxfordjournals.org/). The annual risk of infection is a commonly used measure of tuberculosis control, as it can be estimated from tuberculin skin test surveys. We used data on use of public transit (see the Data sources section) to estimate the annual risk of infection as a function of time (t) spent in various forms of transit.
The basic reproductive number (R0) is the average number of infectious individuals created by a single infectious person in a completely susceptible population. R0 can be separated into component modes of transmission; we estimated the transport-associated R0 by estimating the number of secondary infections generated and the probability of each infected individual developing active infectious tuberculosis (Web Appendix). We adjusted natural history risk for primary and reactivation tuberculosis and mortality according to HIV co-infection. We performed 1-way and 2-way sensitivity analyses for key model parameters (Web Figure 1).
Data sources
Using a portable continuous carbon dioxide sampling device (Extech Instruments, Waltham, Massachusetts) sampling at 5- second intervals, research staff collected carbon dioxide data and recorded numbers of passengers commuting via taxis, trains, and buses. Taxis are multipassenger minibuses that travel defined routes, with individuals boarding and disembarking at various points along the route, similar to standard municipal buses. Research staff collected data from 9 to 10 trips for each mode of transit. The monitor was calibrated against an LCA2 infrared gas analyzer (The Analytical Development Co. Ltd., Hoddesdon, United Kingdom).
In the models used to estimate the annual risk of infection and R0, we used tuberculosis and HIV epidemiology parameters from studies conducted in townships around Cape Town and natural history parameters from the literature (Web Table 1). Data on usage of public transit were derived from a national survey (7).
Among the most difficult parameters to estimate was the infectious quanta produced per hour (q) by an infectious individual. In their classic experiments, Wells and Riley built rooms above tuberculosis patient wards to house guinea pig cages, with the ventilation flowing upward (15, 17). Using the number of infectious individuals on the wards, the duration of exposure, and ventilation characteristics, they estimated q to be 1.25 quanta/hour for smear-positive individuals, with considerable variability. Estimates were markedly higher for laryngeal tuberculosis (60 quanta/hour), the most infectious form of tuberculosis. Half a century later, Escombe et al. (18) repeated these experiments among HIV-infected individuals in a Peruvian tuberculosis ward and estimated q at 8.2 quanta/hour. We used 1.25 quanta/hour for our base case estimates.
RESULTS
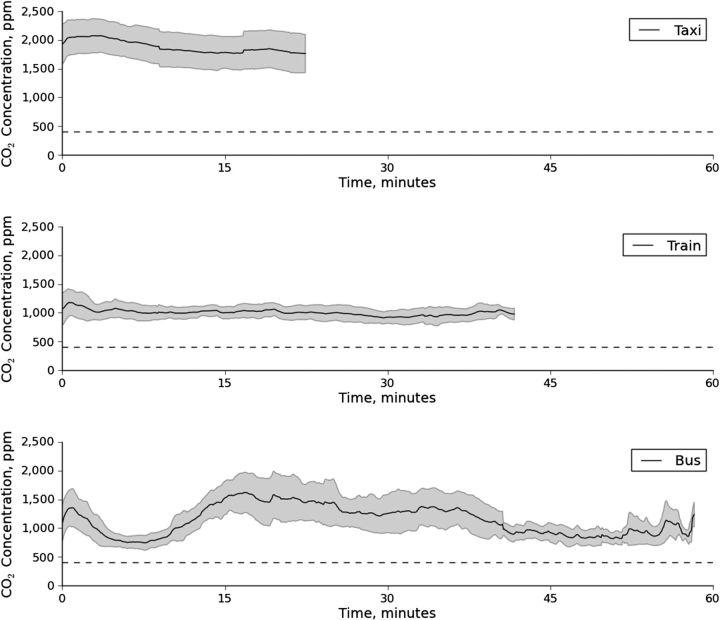
Average carbon dioxide concentrations over time during single trips in the 3 modes of transportation are shown in Figure 1. The lowest carbon dioxide levels were observed in trains (mean = 1,000 ppm); intermediate levels were recorded in buses (mean = 1,150 ppm); and the highest levels were documented in taxis (mean = 1,800 ppm). For reference, the average outdoor ambient carbon dioxide concentration is 410 ppm; guidelines recommend that indoor carbon dioxide concentrations be maintained at less than 1,000 ppm to avoid discomfort and odor (19, 20). Trains exhibited less intertrip temporal variability in carbon dioxide levels concentrations than did taxis and buses, and buses demonstrated the greatest intratrip fluctuations in carbon dioxide concentrations.
Figure 1.
Average carbon dioxide (CO2) concentrations (solid lines) and 95% confidence intervals (shaded regions) over time in single trips on taxis, trains, and buses, Cape Town, South Africa, 2011. The horizontal dashed line indicates the average outdoor carbon dioxide concentration (410 ppm).
We evaluated the risk of tuberculosis infection for a passenger traveling in a vehicle with 1 infectious individual according to mode of transit, as infectiousness (q) varied. For the mean q estimated using the Wells-Riley equation (1.25 quanta/hour), the probability of infection was less than 0.5% in a single trip (Web Figure 2). At the q estimate derived using the formula of Escombe et al. (8.2 quanta/hour), the risk of infection was less than 1% for trains and buses but was nearly 1.5% in taxis. As q increased to that of laryngeal tuberculosis (60 quanta/hour), the risk of infection from a single taxi journey was projected to be greater than 11%, though it was significantly lower for trains and buses.
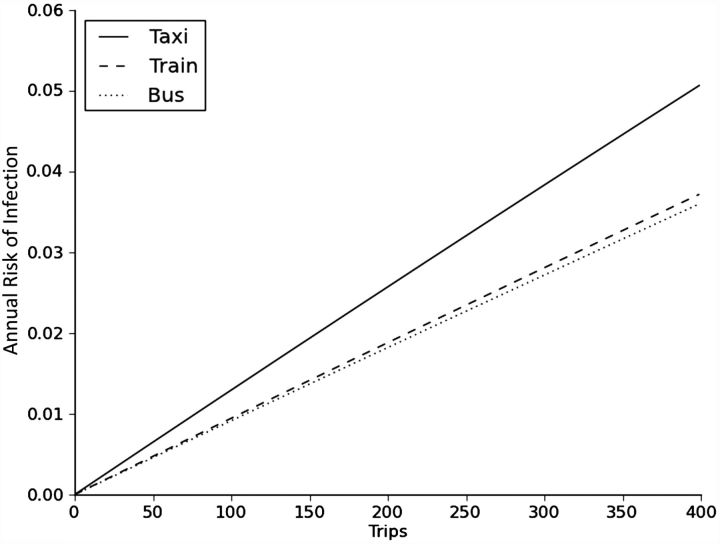
We projected the annual risk of infection attributable to using mass transit according to the number of (1-way) trips taken in a year in various modes of transit (Figure 2). For individuals who took 400 trips per year, as in the case of daily commuters (accounting for holidays), the projected risk of infection associated with transit ranged from 3.5% to 5.0%, with the lowest risk on buses and the highest risk on taxis.
Figure 2.
Annual risk of tuberculosis infection by number of trips taken in a year, stratified by mode of transit (not including combinations of modes), Cape Town, South Africa, 2011.
We estimated the R0 attributable to various modes of public transportation and examined its sensitivity to the frequency of use of transit and the duration of infectiousness (Figure 3). For individuals using taxis, daily use of taxis and moderate estimate of infectiousness (8 months) was associated with a R0 greater than 1. Slightly higher rates of usage or longer duration of infectiousness (11 months) were needed to acheive a R0 greater than 1 on buses, which carried more susceptible passengers but had better ventilation. On all 3 modes of transit, daily commuting (10 trips/week) was associated with a R0 greater than 1 for an infectious period of 1 year.
Figure 3.
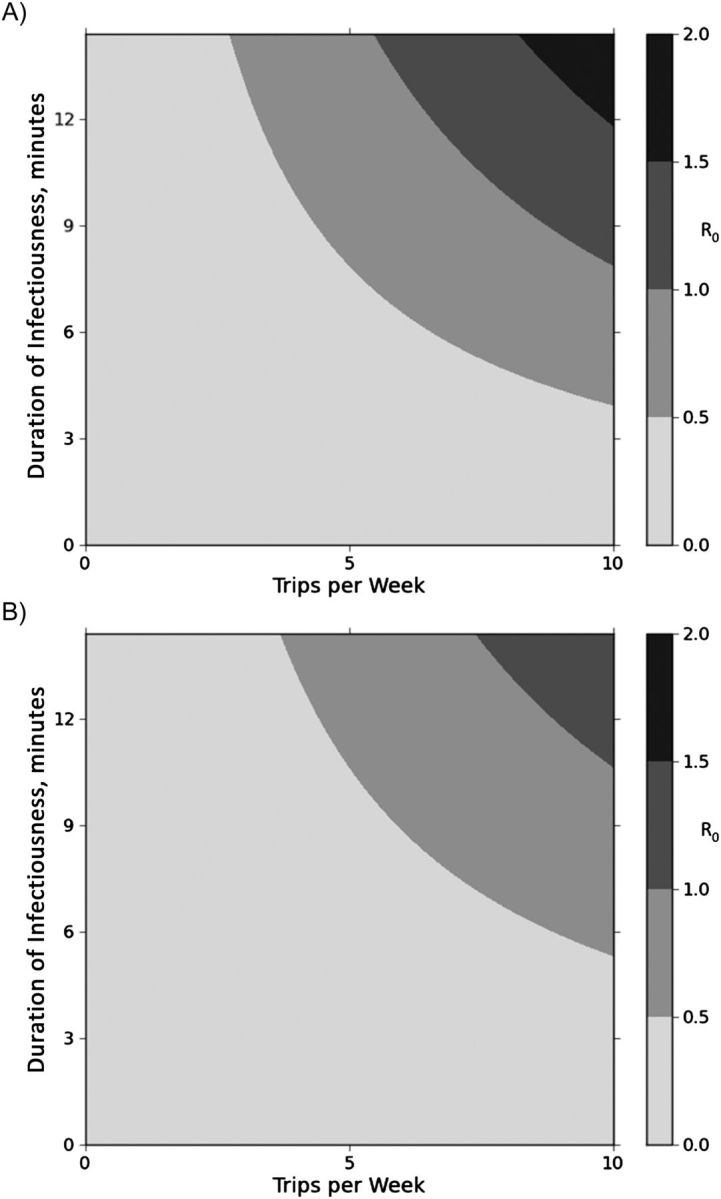
Two-way sensitivity analysis demonstrating how the basic reproductive number (R0) varies as a function of frequency of trips and duration of infectiousness in commuters using taxis (A) and buses (B), Cape Town, South Africa, 2011. R0 is shown with different shades of gray, with darker shades indicating higher values, as shown in bar on the right.
A substantial proportion of contacts in public transit may be recurrent (similar routes or schedules). Because of the saturation of infection among local contacts, recurrent contacts attenuate the R0. We examined the impact of this association in each mode of transit. The impact of contact recurrence was greater for transit in taxis, which carry a smaller number of passengers, than for trains or buses (Web Figure 3). Although trains have much higher ventilation rates than do taxis, as the proportion of contacts that are recurrent increased, the reproductive number of taxis approached that of trains.
DISCUSSION
The frequency and nature of social contacts are key determinants of epidemics of infectious diseases spread by a respiratory route. A previous study in a South African township found that residents had close indoor contact with more than twice as many people per day than did people in studies from Europe (21, 22). These social conditions may be underappreciated drivers of the tuberculosis epidemic in this setting. We demonstrated that the environmental conditions in public transit, where many of these contacts take place, may be important determinants of tuberculosis transmission. More than three-fourths of public transit commutes in urban South Africa take place in minibus taxies, and these taxies were projected to be the highest-risk environment because of crowding and poor ventilation, despite the fact that they carry the smallest number of passengers (7).
The annual risk of tuberculosis infection in townships near Cape Town has been estimated to be 3%–7% (23, 24). We projected the annual risk of tuberculosis infection attributable to public transit among daily commuters to be 3.5%–5.0%. The R0 attributable to transit for a daily commuter was greater than 1, suggesting that tuberculosis could be very difficult to control under the current socioenvironmental conditions in urban South Africa.
As with results from all model-based analyses, our results must be considered in light of the model assumptions and quality of data utilized. In particular, the quantum generation rate parameter was derived from studies in which investigators observed rates of infection in guinea pigs that shared air with hospitalized patients, who may have had greater burden of disease and therefore have been more infectious. The 2 available estimates differed significantly, and we utilized the more conservative estimate. Notably, other estimates have been even higher (25). As anticipated, we found this to be the most important model parameter, which limited any quantitative projections. We did not use differential infectivity for HIV-infected and -uninfected individuals, as data have been conflicting. We did not explore the relevance of interindividual variability in infectiousness, which has implications for the speed and probability of epidemic emergence (15, 18, 26). Additionally, we did not examine individual variability in infectiousness over time, as there is a paucity of data to inform this parameter. More research is needed to improve our understanding of infectious quanta generation and facilitate more accurate quantitative modeling.
We assumed that individuals with active tuberculosis continued to use public transit, though it is possible that once symptomatic enough, individuals would stay home or otherwise decrease their use of public transportation. However, as the best estimates of the duration of infectiousness exceed 1 year (6) and individuals may be infectious long before they are debilitated, this approximation is likely reasonable. If individuals curtailed their use of public transit earlier in the course of disease, the duration of their infectiousness would be reduced, as examined in sensitivity analysis.
The measurement of carbon dioxide to assess ventilation was first described over 135 years ago (27); however, the use of carbon dioxide as a natural tracer gas to assess risk of airborne infectious diseases has been limited. As devices become smaller and decrease in cost, it may be possible to evaluate individuals' environmental risks over time. By defining variation in environmental risk, models of tuberculosis transmission might account for this key element of heterogeneity, thereby improving our understanding of approaches to tuberculosis control.
The public health approach to controlling tuberculosis has focused on 2 components: improving case detection and maintaining high rates of treatment success. Our results suggest that even with substantial reduction in the duration of infectiousness through enhanced case detection, tuberculosis will remain difficult to control in South African cities. Future interventions should target high-risk congregation environments, such as public transit, for interruption transmission, by using active case detection, improving ventilation standards, and potentially implementing “air scrubbing” techniques (filters, ultraviolet light, etc). Control of tuberculosis in South African cities may benefit from new approaches that target these high-transmission environments.
ACKNOWLEDGMENTS
Author affiliations: Division of Infectious Diseases, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts (Jason R. Andrews); and Desmond Tutu HIV Centre, Institute of Infectious Diseases and Molecular Medicine, University of Cape Town, Cape Town, South Africa (Carl Morrow, Robin Wood).
This work was supported by the National Institute of Allergy and Infectious Diseases (grants R01 AI058736 and T32AI007433) and the Harvard Global Health Institute.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, which played no role in the study design, methods, interpretation of results, the content of this manuscript, or the decision to submit it for publication.
Conflict of interest: None.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Control 2012. Geneva, Switzerland: World Health Organization; 2012. http://www.who.int/tb/publications/global_report/en/index.html. ). (Accessed January 12, 2013) [Google Scholar]
- 2.Wood R, Lawn SD, Caldwell J, et al. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One. 2011;6(10):e25098.. doi: 10.1371/journal.pone.0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verver S, Warren RM, Munch Z, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363(9404):212–214. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 4.Classen CN, Warren R, Richardson M, et al. Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax. 1999;54(2):136–140. doi: 10.1136/thx.54.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockman S, Sheppard JD, Braden CR, et al. Molecular and conventional epidemiology of Mycobacterium tuberculosis in Botswana: a population-based prospective study of 301 pulmonary tuberculosis patients. J Clin Microbiol. 2001;39(3):1042–1047. doi: 10.1128/JCM.39.3.1042-1047.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175(1):87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Transport. Key Results of the National Household Travel Survey. Pretoria: Republic of South Africa:; 2005. Department of Transport. [Google Scholar]
- 8.Kenyon TA, Valway SE, Ihle WW, et al. Transmission of multidrug-resistant Mycobacterium tuberculosis during a long airplane flight. N Engl J Med. 1996;334(15):933–938. doi: 10.1056/NEJM199604113341501. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf HR, Braden CR, Greenberg AJ, et al. Tuberculosis transmission among five school bus drivers and students in two New York counties. Pediatrics. 1997;100(3):E9. doi: 10.1542/peds.100.3.e9. [DOI] [PubMed] [Google Scholar]
- 10.Houk VN. Spread of tuberculosis via recirculated air in a naval vessel: the Byrd study. Ann N Y Acad Sci. 1980;353:10–24. doi: 10.1111/j.1749-6632.1980.tb18901.x. [DOI] [PubMed] [Google Scholar]
- 11.Miller MA, Valway S, Onorato IM. Tuberculosis risk after exposure on airplanes. Tuber Lung Dis. 1996;77(5):414–419. doi: 10.1016/s0962-8479(96)90113-6. [DOI] [PubMed] [Google Scholar]
- 12.Feske ML, Teeter LD, Musser JM, et al. Giving TB wheels: public transportation as a risk factor for tuberculosis transmission. Tuberculosis (Edinb) 2011;91(suppl 1):S16–S23. doi: 10.1016/j.tube.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Horna-Campos OJ, Bedoya-Lama A, Romero-Sandoval NC, et al. Risk of tuberculosis in public transport sector workers, Lima, Peru. Int J Tuberc Lung Dis. 2010;14(6):714–719. [PubMed] [Google Scholar]
- 14.Horna-Campos OJ, Consiglio E, Sánchez-Pérez HJ, et al. Pulmonary tuberculosis infection among workers in the informal public transport sector in Lima, Peru. Occup Environ Med. 2011;68(2):163–165. doi: 10.1136/oem.2009.051128. [DOI] [PubMed] [Google Scholar]
- 15.Riley RL, Mills CC, O'Grady F, et al. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi: 10.1164/arrd.1962.85.4.511. [DOI] [PubMed] [Google Scholar]
- 16.Rudnick SN, Milton DK. Risk of indoor airborne infection transmission estimated from carbon dioxide concentration. Indoor Air. 2003;13(3):237–245. doi: 10.1034/j.1600-0668.2003.00189.x. [DOI] [PubMed] [Google Scholar]
- 17.Sultan L, Nyka W, Mills C, et al. Tuberculosis disseminators. A study of the variability of aerial infectivity of tuberculous patients. Am Rev Respir Dis. 1960;82:358–369. doi: 10.1164/arrd.1960.82.3.358. [DOI] [PubMed] [Google Scholar]
- 18.Escombe AR, Moore DAJ, Gilman RH, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5(9):e188.. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Persily A. Evaluating building IAQ and ventilation with indoor carbon dioxide. ASHRAE Trans. 1997;103(2):1–12. [Google Scholar]
- 20.Lindgren T, Norbäck D. Cabin air quality: indoor pollutants and climate during intercontinental flights with and without tobacco smoking. Indoor Air. 2002;12(4):263–272. doi: 10.1034/j.1600-0668.2002.01121.x. [DOI] [PubMed] [Google Scholar]
- 21.Mossong J, Hens N, Jit M, et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnstone-Robertson SP, Mark D, Morrow C, et al. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol. 2011;174(11):1246–1255. doi: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middelkoop K, Bekker L-G, Myer L, et al. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis. 2008;47(3):349–355. doi: 10.1086/589750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middelkoop K, Bekker L-G, Liang H, et al. Force of tuberculosis infection among adolescents in a high HIV and TB prevalence community: a cross-sectional observation study. BMC Infect Dis. 2011;11:156. doi: 10.1186/1471-2334-11-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nardell EA, Keegan J, Cheney SA, et al. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144(2):302–306. doi: 10.1164/ajrccm/144.2.302. [DOI] [PubMed] [Google Scholar]
- 26.Fennelly KP, Martyny JW, Fulton KE, et al. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med. 2004;169(5):604–609. doi: 10.1164/rccm.200308-1101OC. [DOI] [PubMed] [Google Scholar]
- 27.Chaumont FD. On the theory of ventilation: an attempt to establish a positive basis for the calculation of the amount of fresh air required for an inhabited air-space. Proc R Soc Lond. 1874;23:187–201. [Google Scholar]