Abstract
Lung cancer is the leading cause of cancer death among women in the United States and other Western nations. The predominant cause of lung cancer in women is active cigarette smoking. Secondhand exposure to tobacco smoke is another important cause. The hypothesis that women are more susceptible than men to smoking-induced lung cancer has not been supported by the preponderance of current data, as noted by De Matteis et al. (Am J Epidemiol. 2013;177(7):601–612) in the accompanying article. However, aspects of lung cancer in men and women continue to indicate potential male-female differences in the etiology of lung cancer, based on several observations: 1) among never smokers, women have higher lung cancer incidence rates than men; 2) there is evidence that estrogen may contribute to lung cancer risk and progression; and 3) there are different clinical characteristics of lung cancer in women compared with men, such as the higher percentage of adenocarcinomas in never smokers, the greater prevalence of epidermal growth factor receptor gene (EGFR) mutations in adenocarcinomas among never smokers, and better prognosis. Considered in total, observations such as these offer enticing clues that, even amid cigarette smoking and other commonalities in the etiology of lung cancer in men and women, distinct differences may remain to be delineated that could potentially be of scientific and clinical relevance.
Keywords: cigarettes, estrogen, lung cancer, men, secondhand smoke exposure, sex, smoking, women
Before becoming the most common cause of cancer death worldwide, lung cancer was a rare disease in the early 1900s. The fact that lung cancer was once so rare in both sexes attests to the fact that almost the entire present lung cancer burden is caused by environmental exposures. Tremendous progress has been made in characterizing the environmental causes of lung cancer, with a single etiological agent—cigarette smoking—being the predominant cause, accounting for approximately 80%–90% of lung cancer cases in countries where cigarette smoking is common (1). In both sexes, extensive bodies of evidence have consistently demonstrated extremely strong associations between smoking and lung cancer that follow clear dose-response gradients according to the number of years smoked, the number of cigarettes smoked per day, and ages at starting and stopping smoking (2). Compared with persistent smoking, the risk of lung cancer is reduced following smoking cessation (2).
Cigarette smoking is such a potent cause of lung cancer in both sexes that it can be tracked on a population-wide scale, since spatial and temporal trends in lung cancer occurrence closely mirror trends in smoking prevalence, with rates of occurrence lagging behind smoking rates by about 20 years (3). In the United States, because of historical trends in cigarette smoking prevalence, which peaked approximately 2 decades earlier in men than in women, the epidemic of lung cancer started later in women than in men. Whereas lung cancer incidence rates in men have been declining for the past 2 decades, a significant downturn in lung cancer incidence rates among women was only recently observed in 2003–2007 data (4). Far more men than women still die from lung cancer each year, but the gender gap in lung cancer mortality is steadily narrowing. Thus, from a public health perspective, the message is clear: Effective tobacco control interventions are the central strategy for preventing lung cancer in both men and women.
As the major associations between smoking and lung cancer were characterized, more refined questions were addressed. As De Matteis et al. (5) illustrate in this issue of the Journal, an example is the keen interest in the hypothesis that women may be more susceptible to smoking-induced lung cancer than men.
ENVIRONMENTAL RISK FACTORS OTHER THAN CIGARETTE SMOKING
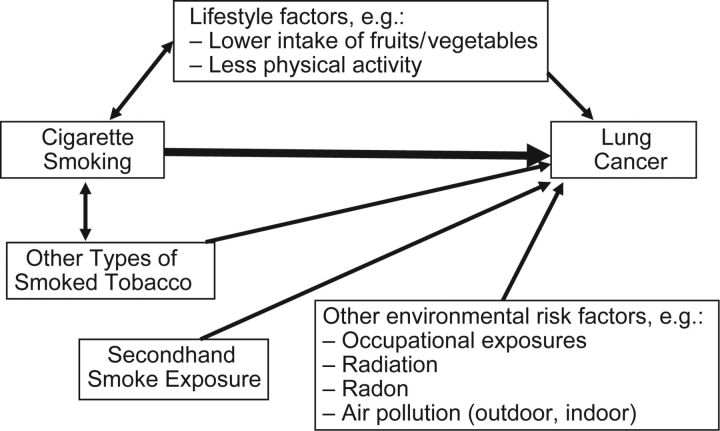
Despite the fact that active cigarette smoking is the most important risk factor for lung cancer, it is but one of a well-characterized set of established risk factors that also includes: smoking types of tobacco other than cigarettes (e.g., cigars, pipes) (6); secondhand exposure to cigarette smoke (7); occupational exposure to lung carcinogens such as asbestos, nickel, chromium, and arsenic (8); exposure to radiation, including radon gas in homes and mines (9); and exposure to indoor (10) and outdoor (11) air pollution. Some of these factors interact synergistically with cigarette smoking to exacerbate the lung cancer risk; well-known examples include the synergistic effect of cigarette smoking on the lung cancer risk associated with exposure to asbestos and radon (12). The accumulating evidence for lifestyle factors, such as diet and exercise, has pointed toward a potential role in influencing lung cancer risk. For example, the evidence was judged to be either “convincing” or “probable” that lung cancer risk was inversely associated with intake of nonstarchy vegetables, intake of fruits, and physical activity (13). An etiological framework of lung carcinogenesis that integrates active cigarette smoking along with these other risk factors is useful for conceptualizing potential male-female differences in lung cancer risk (Figure 1).
Figure 1.
Selected environmental risk factors that contribute to the etiology of lung cancer.
The associations between lung cancer and risk factors other than active smoking would not be expected to differ meaningfully between men and women, but the likelihood of exposure to most of these factors is greater in men. For example, men have been more likely to smoke cigars/pipes (14, 15) and to be exposed to occupational carcinogens (16). To illustrate, in the De Matteis et al. study, compared with women, men were 48 times more likely to have smoked forms of tobacco other than cigarettes and 4.9 times more likely have worked in a high-risk occupation (5). The situation for secondhand smoke exposure is more complicated, with men being more likely than women to be exposed in the United States (17) but prevalence being higher among women than among men in Europe and in Asian countries such as China (18). Of growing concern, particularly in Asia, is indoor air pollution from indoor burning of unprocessed solid fuels, notably soft coal (a fossil fuel) and biomass fuels (10), for cooking and space heating, with exposure patterns that may differentially affect women. With respect to lifestyle characteristics, women generally have higher levels of fruit and vegetable consumption but are less physically active than men (19). This implies that—holding all else equal, including intrinsic susceptibility factors—to the extent that these environmental factors considered as a whole interact synergistically with cigarette smoking, one would anticipate greater risk associated with cigarette smoking in men compared with women because of the greater burden of exposure to effect modifiers in men.
This line of reasoning was supported by the results of De Matteis et al., who observed stronger, though not statistically significantly different, associations between cigarette smoking and lung cancer in men than in women (5). However, as De Matteis et al. note (5), the observation of an interaction in this direction has been the exception rather than the norm in previous studies of this topic.
ARE WOMEN MORE SUSCEPTIBLE TO SMOKING-INDUCED LUNG CANCER?
Rather, in contrast, some initial studies in the 1990s observed the opposite, leading to the hypothesis that women may be more susceptible to smoking-induced lung cancer than men. The study by De Matteis et al. adds to the previous 7 case-control studies and 13 cohort studies that have evaluated this topic. As De Matteis et al. describe, the results of this substantial body of evidence have been at best equivocal and, more often than not, have not supported the hypothesis (5). When viewed in light of the new evidence reported by De Matteis et al., it is becoming increasingly clear that the evidence does not support the hypothesis that women are more susceptible to smoking-induced lung cancer. This inference is firmly reinforced by the substantial bodies of consistent evidence indicating that women are not more susceptible than men to secondhand smoke exposure as measured by spousal smoking in never smokers. At exposure doses considerably lower than those for active smoking, associations in men and women are of similar magnitudes; in a meta-analysis, the association was actually slightly stronger for men whose wives smoked (relative risk (RR) = 1.37, 95% confidence interval (CI): 1.05, 1.79) than for women whose husbands smoked (RR = 1.20, 95% CI: 1.11, 1.29) (7).
SEX DIFFERENCES IN LUNG CANCER NOT CAUSED BY ACTIVE SMOKING
Even as the evidence coalesces around the absence of a smoking-by-sex interaction, greater interest in the broader question of a potential sex difference in the etiology of lung cancer is emerging. Among never smokers of cigarettes, men would be expected to have higher lung cancer rates than women based on the fact that typically the prevalence of exposure to lung carcinogens other than active cigarette smoking is higher in men than in women, largely because of occupational exposures. It is thus surprising that lung cancer incidence rates among never smokers appear to be greater in women than in men. In 3 cohort studies that included both sexes, lung cancer incidence rates among never smokers were 51%–200% greater in women than in men (20). In data from 35 cohort and cancer registry studies conducted worldwide, among never smokers lung cancer incidence rates were 15% greater in females than in males (21). If this is true, among never smokers, the greater observed lung cancer incidence rates in women than in men despite women's ostensibly lower risk profile raises the hypothesis that women may be more susceptible than men to lung malignancy that is not caused by active smoking.
A potential role of hormones in the etiology of lung cancer has emerged from varied lines of evidence. Estrogen has been observed to promote the growth of lung cancer cells (22). Estrogen receptor β is expressed in the majority of non-small-cell lung carcinoma cell lines (23). In women, a significantly increased risk of lung cancer (RR = 1.4, 95% CI: 1.03, 1.8) was observed in a meta-analysis of results from 2 large-scale randomized controlled trials of hormonal therapy that included an estrogen-plus-progestin formulation (24). The lung cancer risk associated with hormonal therapy may be specific to estrogen-plus-progestin formulations, since the results from 1 randomized trial were null for estrogen-only formulations (25). In a prospective cohort study, increased lung cancer risk was observed for estrogen-plus-progestin formulations, with null results for estrogen-only formulations (26), but when viewed as a whole, the heterogeneous results of observational studies do not lend themselves to such a clear-cut interpretation. Among lung cancer patients, use of hormone replacement therapy has been consistently associated with disease progression (27).
SEX DIFFERENCES IN LUNG CANCER PRESENTATION AND TREATMENT
In addition to the evidence that there may be hormone-mediated pathways to lung carcinogenesis, several unique clinical features of lung cancer in women have been characterized. Among lung cancer patients, women are more likely than men to be diagnosed with adenocarcinoma. Among lung cancer patients diagnosed with adenocarcinoma, tumors in women are more likely than those in men to have epidermal growth factor receptor gene (EGFR) mutations, which are more responsive to treatment with EGFR tyrosine kinase inhibitors. Factors such as the relative female predominance of EGFR mutation-positive adenocarcinomas contribute to the better lung cancer survival in women than in men. These observations may relate back to the potential estrogenic role, as the estrogen receptor and the EGFR engage in bidirectional signaling in both normal and malignant cells (28). This can potentially be exploited to therapeutic advantage, as evidenced by the fact that in non-small-cell lung cancer cells, combined targeting of both estrogen receptor and EGFR results in enhanced antiproliferative effects (29, 30).
It is a challenging proposition to tease apart the complex interplay of factors that contribute to lung cancer risk in never smokers, let alone attempt to differentiate the risk factors according to histological type and activating mutation status. Further discerning the extent to which these unique clinical features of lung cancer in women represent true male-female differences in etiology introduces an added layer of complexity. For example, EGFR mutation-positive tumors are significantly more likely to occur in never smokers (31); and, as was apparent in the data of De Matteis et al., where 78% of the never-smoking cases were women (5), a preponderance of never-smoking lung cancer patients are women. Piecing this puzzle together will require systematically addressing key questions in a focused way that holistically accounts for the important risk factor and clinical variables. For example, in a study of lung cancer patients who had never smoked, both female sex and secondhand smoke exposure were significantly associated with the presence of EGFR mutations after adjustment for age and other factors (32).
SUMMARY AND CONCLUSIONS
The results of the De Matteis et al. study add to a growing body of evidence that, when considered in total, fails to support the hypothesis that women are more susceptible than men to cigarette smoking-induced lung cancer. As clarity is achieved on this question, increased attention is being directed toward other potential differences in lung cancer etiology between men and women. There is ample justification to pursue a research agenda in this direction based on the following reasons: 1) the higher incidence rates among never smokers in women than in men; 2) the emerging evidence of a potential link between estrogen and lung carcinogenesis; and 3) differences in the clinical characteristics of lung cancer in women compared with men. Observations such as these offer enticing clues that, even amid active and passive cigarette smoking and other commonalities in the etiology of lung cancer in men and women, distinct differences may remain to be delineated that could potentially be of scientific and clinical relevance.
ACKNOWLEDGMENTS
Author affiliations: Hollings Cancer Center, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina (Anthony J. Alberg, Kristin Wallace, Gerard A. Silvestri); Division of Biostatistics and Epidemiology, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina (Anthony J. Alberg, Kristin Wallace); Division of Pulmonary, Critical Care, Allergy, and Sleep Medicine, Department of Medicine, Medical University of South Carolina, Charleston, South Carolina (Gerard A. Silvestri); Kimmel Comprehensive Cancer Center at Johns Hopkins, Johns Hopkins Medical Institutions, Baltimore, Maryland (Malcolm V. Brock); and Department of Surgery, Johns Hopkins Medical Institutions, Baltimore, Maryland (Malcolm V. Brock).
This work was carried out with funding from the National Institutes of Health (grants P30 CA138313, UL1 RR029882, K07CA151864, and NCI 3P50 CA058184).
Conflict of interest: none declared.
REFERENCES
- 1.Peto R, Lopez AD, Boreham J, et al. Mortality From Smoking in Developed Countries 1950–2000: Indirect Estimates From National Vital Statistics. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 2.Office of the Surgeon General, US Public Health Service. The Health Effects of Active Smoking: A Report of the Surgeon General. Washington, DC: US Public Health Service; 2004. [Google Scholar]
- 3.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest. 2003;123(1 suppl):21S–49S. doi: 10.1378/chest.123.1_suppl.21s. [DOI] [PubMed] [Google Scholar]
- 4.Kohler BA, Ward E, McCarthy BJ, et al. Annual report to the nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst. 2011;103(9):714–736. doi: 10.1093/jnci/djr077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Matteis S, Consonni D, Pesatori AC, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol. 2013;177(7):601–612. doi: 10.1093/aje/kws445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Cigars: Health Effects and Trends. Bethesda, MD: National Cancer Institute; 1998. (Smoking and Tobacco Control monograph no. 9) NIH publication no. 98-4302) [Google Scholar]
- 7.Office of the Surgeon General, US Public Health Service. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: US Public Health Service; 2006. [Google Scholar]
- 8.International Agency for Research on Cancer. A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts. Lyon, France: International Agency for Research on Cancer; 2012. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol 100C) [Google Scholar]
- 9.National Research Council, Committee on Health Risks of Exposure to Radon. Health Effects of Exposure to Radon (BEIR VI) Washington, DC: National Academy Press; 1999. [Google Scholar]
- 10.Hosgood HD, 3rd, Boffetta P, Greenland S, et al. In-home coal and wood use and lung cancer risk: a pooled analysis of the International Lung Cancer Consortium. Environ Health Perspect. 2010;118(12):1743–1747. doi: 10.1289/ehp.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner MC, Krewski D, Pope CA, 3rd, et al. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med. 2011;184(12):1374–1381. doi: 10.1164/rccm.201106-1011OC. [DOI] [PubMed] [Google Scholar]
- 12.Saracci R. The interactions of tobacco smoking and other agents in cancer etiology. Epidemiol Rev. 1987;9(1):175–193. doi: 10.1093/oxfordjournals.epirev.a036301. [DOI] [PubMed] [Google Scholar]
- 13.World Cancer Research Fund International. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: World Cancer Research Fund/American Institute for Cancer Research; 2007. [Google Scholar]
- 14.Nelson DE, Davis RM, Chrismon JH, et al. Pipe smoking in the United States, 1965–1991: prevalence and attributable mortality. Prev Med. 1996;25(2):91–99. doi: 10.1006/pmed.1996.9999. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. State-specific prevalence of current cigarette and cigar smoking among adults—United States, 1998. MMWR Morb Mortal Wkly Rep. 1999;48(45):1034–1039. [PubMed] [Google Scholar]
- 16.Olsson AC, Gustavsson P, Zaridze D, et al. Lung cancer risk attributable to occupational exposures in a multicenter case-control study in Central and Eastern Europe. J Occup Environ Med. 2011;53(11):1262–1267. doi: 10.1097/JOM.0b013e318234e2d2. [DOI] [PubMed] [Google Scholar]
- 17.Vital signs: nonsmokers’ exposure to secondhand smoke—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2010;59(35):1141–1146. Centers for Disease Control and Prevention. [PubMed] [Google Scholar]
- 18.Sisti J, Boffetta P. What proportion of lung cancer in never-smokers can be attributed to known risk factors? Int J Cancer. 2012;131(2):265–275. doi: 10.1002/ijc.27477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Prevalence of fruit and vegetable consumption and physical activity by race/ethnicity—United States, 2005. MMWR Morb Mortal Wkly Rep. 2007;56(13):301–304. [PubMed] [Google Scholar]
- 20.Wakelee HA, Chang ET, Gomez SL, et al. Lung cancer incidence in never smokers. J Clin Oncol. 2007;25(5):472–478. doi: 10.1200/JCO.2006.07.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med. 2008;5(9):e185. doi: 10.1371/journal.pmed.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siegfried JM. Women and lung cancer: does oestrogen play a role? Lancet Oncol. 2001;2(8):506–513. doi: 10.1016/S1470-2045(01)00457-0. [DOI] [PubMed] [Google Scholar]
- 23.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol. 2009;36(6):524–531. doi: 10.1053/j.seminoncol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greiser CM, Greiser EM, Doren M. Menopausal hormone therapy and risk of lung cancer—systematic review and meta-analysis. Maturitas. 2010;65(3):198–204. doi: 10.1016/j.maturitas.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Chlebowski RT, Anderson GL, Manson JE, et al. Lung cancer among postmenopausal women treated with estrogen alone in the Women's Health Initiative randomized trial. J Natl Cancer Inst. 2010;102(18):1413–1421. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slatore CG, Chien JW, Au DH, et al. Lung cancer and hormone replacement therapy: association in the Vitamins and Lifestyle Study. J Clin Oncol. 2010;28(9):1540–1546. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegfried JM. Early changes in pulmonary gene expression following tobacco exposure shed light on the role of estrogen metabolism in lung carcinogenesis. Cancer Prev Res. 2010;3(6):692–695. doi: 10.1158/1940-6207.CAPR-10-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin ER. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol Endocrinol. 2003;17(3):309–317. doi: 10.1210/me.2002-0368. [DOI] [PubMed] [Google Scholar]
- 29.Siegfried JM, Gubish CT, Rothstein ME, et al. Combining the multitargeted tyrosine kinase inhibitor vandetanib with the antiestrogen fulvestrant enhances its antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2012;7(3):485–495. doi: 10.1097/JTO.0b013e31824177ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen H, Yuan Y, Sun J, et al. Combined tamoxifen and gefitinib in non-small cell lung cancer shows antiproliferative effects. Biomed Pharmacother. 2010;64(2):88–92. doi: 10.1016/j.biopha.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Ren J-H, He W-S, Yan G-L, et al. EGFR mutations in non-small-cell lung cancer among smokers and non-smokers: a meta-analysis. Environ Mol Mutagen. 2012;53(1):78–82. doi: 10.1002/em.20680. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi T, Ando M, Kubo A, et al. Long exposure of environmental tobacco smoke associated with activating EGFR mutations in never-smokers with non-small cell lung cancer. Clin Cancer Res. 2011;17(1):39–45. doi: 10.1158/1078-0432.CCR-10-1773. [DOI] [PubMed] [Google Scholar]