Abstract
Worldwide lung cancer incidence is decreasing or leveling off among men, but rising among women. Sex differences in associations of tobacco carcinogens with lung cancer risk have been hypothesized, but the epidemiologic evidence is conflicting. We tested sex-smoking interaction in association with lung cancer risk within a population-based case-control study, the Environment and Genetics in Lung Cancer Etiology (EAGLE) Study (Lombardy, Italy, 2002–2005). Detailed lifetime smoking histories were collected by personal interview in 2,100 cases with incident lung cancer and 2,120 controls. Odds ratios and 95% confidence intervals for pack-years of cigarette smoking were estimated by logistic regression, adjusted for age, residence area, and time since quitting smoking. To assess sex-smoking interaction, we compared the slopes of odds ratios for logarithm of pack-years in a model for men and women combined. Overall, the slope for pack-years was steeper in men (odds ratio for female-smoking interaction = 0.39, 95% confidence interval: 0.24, 0.62; P < 0.0001); after restriction to ever smokers, the difference in slopes was much smaller (odds ratio for interaction = 0.63, 95% confidence interval: 0.29, 1.37; P = 0.24). Similar results were found by histological type. Results were unchanged when additional confounders were evaluated (e.g., tobacco type, inhalation depth, Fagerström-assessed nicotine dependence). These findings do not support a higher female susceptibility to tobacco-related lung cancer.
Keywords: case-control studies, lung cancer, sex differences, smoking
Editor's note: An invited commentary on this article appears on page 613.
Lung cancer is the leading cause of cancer mortality worldwide, with almost 1.4 million deaths per year (18% of total cancer mortality) (1). Among men, it is still the most common cancer (1.1 million cases, 16.5% of the total); among women, it is fourth in frequency (513,000 cases, 8.5% of all cancers) but second in the number of deaths (427,000 deaths, 12.8% of the total) (1). Tobacco smoking is the major cause, accounting for 80% of the worldwide lung cancer burden in males and at least 50% in females (1).
In the last 3 decades, lung cancer incidence rates worldwide have decreased or leveled off among men, but have risen among women (1). This increase (by 600% in the last 50 years) has been defined as a “contemporary epidemic” (2). Additionally, women show a different clinical pattern of lung cancer than men: They tend to be younger, to be never smokers, to have the adenocarcinoma histological type, and to have improved survival rates for all stages at diagnosis (2, 3).
Biologically, a number of explanations have been proposed for sex differences in lung cancer susceptibility. Estrogen receptors are present in both normal and neoplastic lung tissues and could accelerate the metabolism of smoke-related carcinogens in a dose-dependent way, as suggested by higher levels of polycyclic aromatic hydrocarbons–DNA adducts in female smokers compared with males (4). Inherited genetic polymorphisms affecting activating and detoxifying enzymes could explain a different susceptibility between the sexes to tobacco carcinogens (5). In addition, several lifestyle and behavioral factors related to smoking habits or environmental and occupational exposures could account for some sex differences (3, 6).
Epidemiologic studies are conflicting. Several case-control studies (7–10), but not all (11–13), have found a higher relative risk among women compared with men for the same level of smoking exposure. On the contrary, the majority of cohort studies (14–23), with a few exceptions (24, 25), and a recent meta-analysis including a Japanese cohort and case-control studies (26) found no difference between the sexes or even a higher rate ratio in men. The issue is vigorously debated because of the potential impact on health policy (24). Debate still exists about the best study design (cohort vs. case-control), risk estimate measure (absolute vs. relative), model of interaction (additive vs. multiplicative), treatment of never smokers (inclusion vs. exclusion), and potential confounders (e.g., depth of inhalation, tobacco type) with which to answer this question (27–31).
To address these issues, we took advantage of data from the Environment And Genetics in Lung cancer Etiology (EAGLE) Study, a large population-based case-control study conducted in the Lombardy region of Italy between 2002 and 2005. The EAGLE Study, which was designed to explore the role of tobacco smoking in lung cancer risk in combination with other genetic and environmental factors (32), allowed us to exploit very detailed lifetime smoking histories collected by personal interview with the index subjects.
Our aim was to evaluate the interaction between female sex and tobacco smoking in association with lung cancer risk using different exposure-response models and taking into account several potential confounders and effect modifiers. Moreover, the large sample size allowed us to perform analyses according to the main histological types of lung cancer.
MATERIALS AND METHODS
Study design
The EAGLE Study (32) included 2,100 incident lung cancer cases (448 women and 1,652 men) and 2,120 population controls (500 women and 1,620 men). The subjects were enrolled in April 2002–June 2005 in 216 municipalities (including the cities of Milan, Monza, Brescia, Pavia, and Varese) in Lombardy, the most developed and populated (over 9 million inhabitants) region of Italy. Subjects were 35–79 years of age at diagnosis (cases) or at sampling/enrollment (controls). Response rates (participants/eligible subjects) were 86.6% (cases) and 72.4% (controls).
Cases were persons with newly diagnosed primary cancer of the trachea, bronchus, or lung, of any stage and morphology, verified by means of tissue pathology (67.0%), cytology (28.0%), or review of clinical records (5.0%). They were recruited in 13 hospitals which cover over 80% of the lung cancer cases from the study area. Controls were randomly sampled from population databases of the area, frequency-matched to cases by residence (5 areas), sex, and age (5-year categories), and contacted through family physicians. The study was approved by local and US National Cancer Institute institutional review boards, and all participants signed an informed consent form.
Data collection
All subjects underwent a computer-assisted personal interview for collection of extensive information on the major risk factors for lung cancer and completed a self-administered questionnaire on aspects of behavior possibly associated with smoking persistence. In particular, information on lifetime tobacco smoking was collected, including numbers of cigarettes, cigars, pipes, and cigarillos smoked per day; age at initiation/quitting; number of quitting attempts and time between attempts; inhalation pattern; percentage of each cigarette smoked; and secondhand smoke (SHS) exposure during childhood, at the workplace, and at home during adulthood. The 6-item Fagerström Test for Nicotine Dependence (FTND) was administered to assess nicotine dependence (33).
Statistical analysis
We calculated odds ratios and 95% confidence intervals for cigarette smoking exposure, separately for males and females, in unconditional multiple logistic regression models, adjusted for residential area (5 categories, including the 5 cities and their surrounding municipalities), age (5-year categories) and time since quitting smoking (categorical: 0 for never/current smokers; otherwise, 0.5–0.9, 1–1.9, 2–4.9, 5–9.9, 10–19.9, 20–29.9, or ≥30 years). We defined as never smokers subjects who had smoked fewer than 100 cigarettes in their lifetime.
The main exposure-response models assessed cumulative cigarette exposure (pack-years) treated as either a categorical (0 for never smokers; otherwise, 1–19, 20–39, or ≥40 pack-years) or a continuous (log10 (1 + pack-years/5)) variable (9). We first fitted models separately for men and women. However, although they are useful for showing exposure-response patterns within sexes, the odds ratios from separate models cannot be safely compared between the sexes because they are obtained using different intercepts (reference categories). Therefore, to formally assess interaction (on the multiplicative scale), we fitted models for men and women combined including sex-smoking product terms. We evaluated the latter using the likelihood ratio test for models containing categorical pack-years, and Wald-based 95% confidence intervals and tests when fitting models estimating the odds ratio-smoking slope using continuous pack-years. We used the male sex as the reference group, so a positive or negative interaction corresponds to higher or lower odds ratios in women, respectively.
We used the “floating trend” approach to visualize the relationship between adjusted odds and pack-year categories in the two sexes. In the floating trend approach, the lack of dependence of point and confidence interval estimates in different exposure categories on the reference category provides a reference-free representation of the dose-response relationship (34); this is especially advantageous in our study for men, because almost all male cases were smokers. The odds represent not incidence odds but simply arbitrary case-control ratios that can be compared to visualize patterns and test for trends (34). We plotted the odds on a logarithmic scale for never/current smokers aged 65–69 years residing in area 1 (Milan), the largest category in our study.
The same analyses were repeated among ever smokers only, by smoking status, and with adjustment for smoking of other types of tobacco (i.e., pipes, cigars, and cigarillos; dichotomous variable: ever/never), inhalation depth (4 categories: none, slight, moderate, or deep), and FTND score (3 categories: score of 0–3, no dependence; 4 or 5, dependence; 6–10, severe dependence). We also explored the role of SHS exposure (any exposure in childhood, or adulthood exposure at home and at work) either as a confounder (by adjusting for SHS exposure) or as an effect modifier (by analyzing sex-smoking interactions separately among those ever and never exposed to SHS exposure). In further analyses, we also adjusted for education as a surrogate for socioeconomic status (4 categories: none, elementary school, middle school, or high school/higher degree) and occupations known or suspected to be associated with lung cancer risk (35) (dichotomous variable: ever/never). Among ever smokers, we also created a surrogate for cumulative cigarette “dose,” by multiplying the cumulative cigarette exposure (pack-years) by the reported percentage of each cigarette smoked (25%, 50%, or ≥75%). We used this variable both as a continuous variable (log10 (1 + dose/5)) and as a categorical variable (0 for never smokers; otherwise 1–19, 20–29, or ≥40 pack-years).
Separate analyses were performed for the main lung cancer histological types (adenocarcinoma, squamous-cell carcinoma, and small-cell carcinoma) in a multinomial logistic regression model. We restricted the analyses for squamous- and small-cell carcinomas to ever smokers only, given the extreme paucity of never smokers among cases with these histological types. All P values were 2-sided. Analyses were carried out using Stata, version 11 (StataCorp LP, College Station, Texas).
RESULTS
Of the 2,100 cases and 2,120 controls enrolled in our study, 1,943 (92.5%) and 2,116 (99.8%) were interviewed, respectively. In particular, interviews were obtained from 406 women and 1,537 men among cases and 499 women and 1,617 men among controls (Table 1). Two-thirds of the subjects came from the area of Milan (Lombardy's capital). Among men, controls had a higher educational level. Male cases had held more jobs associated with lung cancer risk during their working lives than controls. Approximately 14%–15% of cases and 6%–7% of controls had previously or newly diagnosed primary cancer(s) other than lung cancer. The majority of lung cancers were adenocarcinomas (>50% in women).
Table 1.
Selected Characteristics of Lung Cancer Cases and Controls With Interview Data Available, by Sex, the EAGLE Study, Lombardy, Italy, 2002–2005a,b
| Women |
Men |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases |
Controls |
Cases |
Controls |
|||||||||
| No.c | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Enrolled | 448 | 500 | 1,652 | 1,620 | ||||||||
| Interviewed | 406 | 90.6 | 499 | 99.8 | 1,537 | 93.0 | 1,617 | 99.8 | ||||
| Area of residence | ||||||||||||
| Milan | 288 | 70.9 | 349 | 69.9 | 987 | 64.2 | 1,089 | 67.3 | ||||
| Monza | 24 | 5.9 | 23 | 4.6 | 109 | 7.1 | 94 | 5.8 | ||||
| Brescia | 47 | 11.6 | 53 | 10.6 | 203 | 13.2 | 194 | 12.0 | ||||
| Pavia | 21 | 5.2 | 37 | 7.4 | 107 | 7.0 | 92 | 5.7 | ||||
| Varese | 26 | 6.4 | 37 | 7.4 | 131 | 8.5 | 148 | 9.2 | ||||
| P value | 0.55 | 0.17 | ||||||||||
| Age, years | 64.8 (10.1) | 64.1 (10.1) | 66.8 (7.9) | 65.8 (8.1) | ||||||||
| P value | 0.32 | < 0.001 | ||||||||||
| Educational level | ||||||||||||
| None | 21 | 5.2 | 24 | 4.8 | 91 | 5.9 | 66 | 4.1 | ||||
| Elementary school | 128 | 31.5 | 143 | 28.7 | 625 | 40.7 | 431 | 26.7 | ||||
| Middle school | 134 | 33.0 | 158 | 31.7 | 424 | 27.6 | 455 | 28.1 | ||||
| High school | 104 | 25.6 | 135 | 27.1 | 314 | 20.4 | 441 | 27.3 | ||||
| University | 19 | 4.7 | 39 | 7.8 | 83 | 5.4 | 224 | 13.9 | ||||
| P value | 0.35 | < 0.001 | ||||||||||
| Employed in an occupation associated with lung cancer | ||||||||||||
| Never | 379 | 93.3 | 471 | 94.4 | 1,015 | 66.0 | 1,171 | 72.4 | ||||
| List B (exposure to suspected carcinogens) | 24 | 5.9 | 26 | 5.2 | 345 | 22.5 | 346 | 21.4 | ||||
| List A (exposure to known carcinogens) | 3 | 0.7 | 2 | 0.4 | 177 | 11.5 | 100 | 6.2 | ||||
| P value | 0.71 | <0.001 | ||||||||||
| Other cancer(s)d | ||||||||||||
| No | 336 | 82.8 | 448 | 89.8 | 1,306 | 85.0 | 1,473 | 91.1 | ||||
| Yes | 70 | 17.2 | 51 | 10.2 | 231 | 15.0 | 144 | 8.9 | ||||
| P value | 0.002 | <0.001 | ||||||||||
| Lung cancer morphology (histological type) | ||||||||||||
| Adenocarcinoma | 220 | 54.2 | 582 | 37.9 | ||||||||
| Squamous-cell carcinoma | 45 | 11.1 | 459 | 29.9 | ||||||||
| Large-cell carcinoma | 28 | 6.9 | 61 | 4.0 | ||||||||
| Non-small-cell carcinoma NOS | 34 | 8.4 | 142 | 9.2 | ||||||||
| Small-cell carcinoma | 38 | 9.4 | 157 | 10.2 | ||||||||
| Other | 26 | 6.4 | 65 | 4.2 | ||||||||
| Data not available | 15 | 3.7 | 71 | 4.6 | ||||||||
| P value | <0.001 | |||||||||||
Abbreviations: EAGLE, Environment and Genetics in Lung Cancer Etiology; NOS, not otherwise specified; SD, standard deviation.
a P values were calculated from the χ2 test (categorical variables) or Student's t test (continuous variables) for comparison between cases and controls of the same sex or between cases of different sexes (for lung cancer morphology only).
b Percentages may not add to 100.0 because of rounding.
c Number of participants.
d Primary cancer(s) (previously or newly diagnosed) other than lung cancer.
Among cases, one-fourth of women were never smokers as compared with only 2% of men; among controls, 57% of women were never smokers as compared with 25% of men (Table 2). In both sexes, current smokers comprised approximately 50% of cases and less than 30% of controls. Almost half of men (cases or controls) were former smokers (i.e., they had quit smoking ≥6 months previously), as compared with less than 30% of women. The cumulative exposure, duration, and average intensity of cigarette smoking were substantially higher in men and, within both sexes, for lung cancer cases. Men and controls had refrained from smoking for a longer period of time but also had more frequently smoked 100% of each cigarette. Inhalation depth was greater among cases and men. Very few women had smoked other types of tobacco, compared with almost 20% of both male cases and male controls. Nicotine dependence, as assessed with the FTND, was higher for men and for cases. The smoking pattern differed (i.e., was more dependent) between cases and controls for all 6 items on the Fagerström test and between men and women for almost all items (see Web Table 1, available at http://aje.oxfordjournals.org/).
Table 2.
Smoking Habits of Lung Cancer Cases and Controls, by Sex, the EAGLE Study, Lombardy, Italy, 2002–2005a,b
| Women |
Men |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases |
Controls |
Cases |
Controls |
|||||||||
| No.c | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| No. interviewed | 406 | 499 | 1,537 | 1,617 | ||||||||
| Cigarette smoking status | ||||||||||||
| Never smoker | 103 | 25.4 | 282 | 56.5 | 29 | 1.9 | 397 | 24.6 | ||||
| Former smoker (quit >6 months previously) | 116 | 28.6 | 110 | 22.0 | 723 | 47.0 | 799 | 49.4 | ||||
| Current smoker | 187 | 46.1 | 107 | 21.4 | 785 | 51.1 | 420 | 26.0 | ||||
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.1 | ||||
| P for cases vs. controls | <0.001 | <0.001 | ||||||||||
| P for women vs. men | <0.001 | <0.001 | ||||||||||
| Pack-years of smokingd | 32.6 (21.1) | 16.4 (16.4) | 51.8 (28.1) | 29.3 (22.4) | ||||||||
| P for cases vs. controls | <0.001 | <0.001 | ||||||||||
| P for women vs. men | <0.001 | <0.001 | ||||||||||
| Duration of smoking, yearsd | 38.5 (12.6) | 28.1 (15.2) | 44.0 (11.3) | 32.7 (14.9) | ||||||||
| P for cases vs. controls | <0.001 | <0.001 | ||||||||||
| P for women vs. men | <0.001 | <0.001 | ||||||||||
| Intensity of smoking, packs/dayd | 0. 8 (0.4) | 0.5 (0.4) | 1.2 (0.6) | 0.8 (0.5) | ||||||||
| P for cases vs. controls | <0.001 | <0.001 | ||||||||||
| P for women vs. men | <0.001 | <0.001 | ||||||||||
| Years since quitting smokingd | 4.2 (8.4) | 9.2 (12.8) | 5.9 (9.5) | 14.1 (14.5) | ||||||||
| P for cases vs. controls | <0.001 | <0.001 | ||||||||||
| P for women vs. men | <0.001 | <0.001 | ||||||||||
| Percentage of each cigarette smokedd | ||||||||||||
| 25 | 5 | 1.7 | 1 | 0.5 | 1 | 0.1 | 3 | 0.2 | ||||
| 50 | 17 | 5.6 | 11 | 5.1 | 62 | 4.1 | 36 | 3.0 | ||||
| 75 | 113 | 37.3 | 53 | 24.4 | 449 | 29.8 | 295 | 24.2 | ||||
| 100 | 168 | 55.4 | 152 | 70.0 | 995 | 66.0 | 885 | 72.6 | ||||
| Unknown | 1 | 0.1 | ||||||||||
| P for cases vs. controls | 0.006 | 0.002 | ||||||||||
| P for women vs. men | <0.001 | 0.39 | ||||||||||
| Inhalation patternd | ||||||||||||
| None | 21 | 6.9 | 36 | 16.6 | 24 | 1.6 | 75 | 6.1 | ||||
| Slight (back to throat) | 59 | 19.5 | 53 | 24.4 | 123 | 8.2 | 130 | 10.7 | ||||
| Moderate (partly into chest) | 91 | 30.0 | 92 | 42.4 | 389 | 25.8 | 479 | 39.3 | ||||
| Deep (deeply into chest) | 122 | 40.3 | 34 | 15.7 | 949 | 62.9 | 534 | 43.8 | ||||
| Unknown | 10 | 3.3 | 2 | 0.9 | 23 | 1.5 | 2 | 0.2 | ||||
| P for cases vs. controls | <0.001 | <0.001 | ||||||||||
| P for women vs. men | <0.001 | <0.001 | ||||||||||
| FTND scored | 4.0 (2.4) | 1.9 (2.4) | 4.8 (2.4) | 2.9 (2.6) | ||||||||
| P for cases vs. controls | <0.001 | <0.001 | ||||||||||
| P for women vs. men | 0.004 | <0.001 | ||||||||||
| Smoking of pipes, cigars, or cigarillos | ||||||||||||
| Never | 401 | 98.8 | 497 | 99.6 | 1,270 | 82.6 | 1,308 | 80.9 | ||||
| Ever | 5 | 1.2 | 2 | 0.4 | 267 | 17.4 | 309 | 19.1 | ||||
| P for cases vs. controls | 0.16 | 0.21 | ||||||||||
| P for women vs. men | <0.001 | <0.001 | ||||||||||
Abbreviations: EAGLE, Environment and Genetics in Lung Cancer Etiology; FTND, Fagerström Test for Nicotine Dependence; SD, standard deviation.
a P values were calculated from the χ2 test (categorical variables) or Mann-Whitney test (continuous variables) for comparison between cases and controls of the same sex or between cases of different sexes (for lung cancer morphology only).
b Percentages may not add to 100.0 because of rounding.
c Number of participants.
d Ever cigarette smokers only.
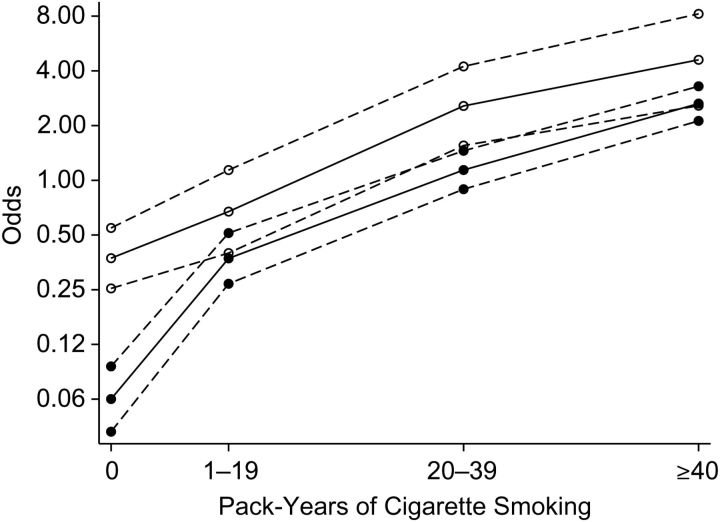
Lung cancer odds ratios for pack-years (categorical) were higher in men than in women, with a negative female sex-smoking interaction (P = 0.0009) (Table 3). When we restricted analyses to ever smokers, the odds ratios for women were slightly higher than those for men, but there was no evidence of an interaction (P = 0.55) (Table 3). The floating trend graph (Figure 1) shows a higher increase in odds from never smokers to the category 1–19 pack-years in men. Conversely, for medium (20–29 pack-years) and high (≥40 pack-years) categories, the lines appear substantially parallel across the two sexes. Similar results were obtained for pack-years as a log-transformed continuous variable: a negative female sex-smoking interaction (odds ratio = 0.39, 95% confidence interval: 0.24, 0.62; P < 0.0001) in all subjects and no interaction (odds ratio = 0.63, 95% confidence interval: 0.29, 1.37; P = 0.24) among ever smokers (Table 3). We also explored the association within former and current smokers separately and found no major difference (Table 4). Odds ratios were slightly higher in men for the highest category of pack-years in both subgroups and for the continuous variable among current smokers only, but there was no sex-smoking interaction (Table 4).
Table 3.
Cumulative Exposure to Cigarette Smoking and Associated Odds Ratios for Lung Cancer Among All Subjects and Ever Smokers, by Sex, the EAGLE Study, Lombardy, Italy, 2002–2005
| Exposure Measure | Odds of Lung Cancer |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cigarette Smoking Exposure |
All Subjects |
Ever Smokers |
||||||||||||||
| Women |
Men |
Women |
Men |
Women |
Men |
|||||||||||
| Cases |
Controls |
Cases |
Controls |
ORa | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |||||
| No. | % | No. | % | No. | % | No. | % | |||||||||
| Categorical variable, pack-years | ||||||||||||||||
| Never smoker | 103 | 25.4 | 282 | 56.5 | 29 | 1.9 | 397 | 24.6 | 1.0 | Referent | 1.0 | Referent | ||||
| 1–19 | 87 | 21.4 | 147 | 29.5 | 118 | 7.7 | 464 | 28.7 | 1.8 | 1.1, 2.9 | 5.9 | 3.7, 9.5 | 1.0 | Referent | 1.0 | Referent |
| 20–39 | 124 | 30.5 | 49 | 9.8 | 420 | 27.3 | 413 | 25.5 | 6.9 | 4.4, 10.7 | 18.2 | 12.0, 27.7 | 4.0 | 2.5, 6.4 | 3.1 | 2.3, 4.0 |
| ≥40 | 92 | 22.7 | 21 | 4.2 | 967 | 62.9 | 341 | 21.1 | 12.3 | 7.2, 21.2 | 42.2 | 28.1, 63.4 | 7.2 | 3.9, 13.4 | 7.1 | 5.4, 9.4 |
| Data not available | 0 | 0 | 0 | 0 | 3 | 0.2 | 2 | 0.1 | ||||||||
| Pinteractionb | 0.0009c | 0.55 | ||||||||||||||
| Continuous variable (log10 (1 + pack-years/5)) | 11.3 | 7.6, 16.8 | 28.4 | 21.2, 38.1 | 37.5 | 15.3, 92.0 | 27.4 | 18.0, 41.7 | ||||||||
| Female sex-smoking interaction | 0.39 | 0.24, 0.62 | 0.63 | 0.29, 1.37 | ||||||||||||
| Pinteractionb | <0.0001d | 0.24 | ||||||||||||||
Abbreviations: CI, confidence interval; EAGLE, Environment and Genetics in Lung Cancer Etiology; OR, odds ratio.
a ORs and 95% CIs from unconditional logistic regression models, adjusted for residential area, age, and years since quitting smoking.
b P values were calculated from the likelihood ratio test or Wald test for the product of sex and pack-years (pack-years as a categorical or continuous (log10 (1 + pack-years/5)) variable, respectively).
c Negative female sex-smoking interaction coefficients for all of the pack-year categories evaluated.
d Negative female sex-smoking interaction coefficient.
Figure 1.
Floating trends in the odds of lung cancer on a logarithmic scale according to pack-years of cigarette smoking, adjusted for residence area, age, and years since quitting smoking, in females (○) and males (•), the EAGLE Study, Lombardy, Italy, 2002–2005. Estimates shown are for never smokers (0 pack-years) and current smokers aged 65–69 years residing in area 1 (Milan). Dashed lines, 95% confidence interval. EAGLE, Environment and Genetics in Lung Cancer Etiology.
Table 4.
Cumulative Exposure to Cigarette Smoking and Associated Odds Ratios for Lung Cancer Among Former Smokers and Current Smokers, by Sex, the EAGLE Study, Lombardy, Italy, 2002–2005
| Exposure Measure | Odds of Lung Cancer |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cigarette Smoking Exposure |
Former Smokers |
Current Smokers |
||||||||||||||
| Women |
Men |
Women |
Men |
Women |
Men |
|||||||||||
| Cases |
Controls |
Cases |
Controls |
ORa | 95% CI | ORa | 95% CI | ORb | 95% CI | ORb | 95% CI | |||||
| No. | % | No. | % | No. | % | No. | % | |||||||||
| Categorical variable, pack-years | ||||||||||||||||
| Never smoker | 103 | 25.4 | 282 | 56.5 | 29 | 1.9 | 397 | 24.6 | ||||||||
| 1–19 | 87 | 21.4 | 147 | 29.5 | 118 | 7.7 | 464 | 28.7 | 1.0 | Referent | 1.0 | Referent | 1.0 | Referent | 1.0 | Referent |
| 20–39 | 124 | 30.5 | 49 | 9.8 | 420 | 27.3 | 413 | 25.5 | 3.1 | 1.5, 6.4 | 2.8 | 2.0, 3.8 | 5.2 | 2.8, 9.8 | 4.2 | 2.5, 7.0 |
| ≥40 | 92 | 22.7 | 21 | 4.2 | 967 | 62.9 | 341 | 21.1 | 4.1 | 1.0, 17.0 | 4.7 | 3.3, 6.6 | 9.1 | 4.4, 18.8 | 13.0 | 7.7, 21.6 |
| Data not available | 0 | 0 | 0 | 0 | 3 | 0.2 | 2 | 0.1 | ||||||||
| Pinteractionc | 0.95 | 0.32 | ||||||||||||||
| Continuous variable (log10 (1 + pack-years/5)) | 25.0 | 6.3, 99.1 | 14.1 | 8.4, 23.8 | 60.9 | 17.4, 213.0 | 77.6 | 37.5, 160.7 | ||||||||
| Female sex-smoking interaction | 0.83 | 0.27, 2.57 | 0.78 | 0.20, 3.07 | ||||||||||||
| Pinteractionc | 0.74 | 0.73 | ||||||||||||||
Abbreviations: CI, confidence interval; EAGLE, Environment and Genetics in Lung Cancer Etiology; OR, odds ratio.
a ORs and 95% CIs from unconditional logistic regression models, adjusted for residential area, age, and years since quitting smoking.
b ORs and 95% CIs from unconditional logistic regression models, adjusted for residential area and age.
c P values were calculated from the likelihood ratio test or Wald test for the product of sex and pack-years (pack-years as a categorical or continuous (log10 (1 + pack-years/5)) variable, respectively).
In the analyses for the main lung cancer histological types, odds ratios for pack-years (categorical) among adenocarcinoma cases were higher in men than in women, with a negative female sex-smoking interaction (P = 0.005) (Table 5). In the analyses restricted to former and current smokers, there was no evidence of interaction (P = 0.76 and P = 0.47, respectively). These results were confirmed using pack-years as a log-transformed continuous variable (Table 5). Similarly, although the findings were based on a smaller number of subjects, no significant sex-smoking interaction was found for squamous- and small-cell carcinoma cases, either treating pack-years as a categorical variable or treating it as continuous (Web Tables 2 and 3).
Table 5.
Cumulative Exposure to Cigarette Smoking and Associated Odds Ratios for Lung Adenocarcinoma Among All Subjects, Former Smokers, and Current Smokers, by Sex, the EAGLE Study, Lombardy, Italy, 2002–2005
| Exposure Measure | Odds of Lung Adenocarcinoma |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cigarette Smoking Exposure |
All Subjects |
Former Smokers |
Current Smokers |
|||||||||||||||||
| Women |
Men |
Women |
Men |
Women |
Men |
Women |
Men |
|||||||||||||
| Cases |
Controls |
Cases |
Controls |
ORa | 95% CI | ORa | 95% CI | ORa | 95% CI | ORa | 95% CI | ORb | 95% CI | ORb | 95% CI | |||||
| No. | % | No. | % | No. | % | No. | % | |||||||||||||
| Categorical variable, pack-years | ||||||||||||||||||||
| Never smoker | 77 | 35.0 | 282 | 56.5 | 18 | 3.1 | 397 | 24.6 | 1.0 | Referent | 1.0 | Referent | ||||||||
| 1–19 | 55 | 25.0 | 147 | 29.5 | 56 | 9.6 | 464 | 28.7 | 1.3 | 0.7, 2.2 | 4.2 | 2.3, 7.5 | 1.0 | Referent | 1.0 | Referent | 1.0 | Referent | 1.0 | Referent |
| 20–39 | 53 | 24.1 | 49 | 9.8 | 171 | 29.4 | 413 | 25.5 | 3.8 | 2.6, 6.5 | 11.4 | 6.7, 19.3 | 2.9 | 1.1, 7.7 | 2.6 | 1.7, 4.1 | 4.1 | 1.8, 9.2 | 3.4 | 1.7, 6.8 |
| ≥40 | 35 | 15.9 | 21 | 4.2 | 335 | 57.6 | 341 | 21.1 | 6.7 | 3.6, 12.4 | 23.2 | 14.0, 38.6 | 2.6 | 0.4, 15.2 | 3.8 | 2.4, 6.1 | 7.8 | 3.1, 19.2 | 9.2 | 4.7, 18.2 |
| Data not available | 0 | 0 | 0 | 0 | 2 | 0.3 | 2 | 0.1 | ||||||||||||
| Pinteractionc | 0.005d | 0.76 | 0.47 | |||||||||||||||||
| Continuous variable (log10 (1 + pack- years/5)) | 6.1 | 3.9, 9.8 | 16.8 | 11.6, 24.2 | 12.6 | 2.4, 65.6 | 9.1 | 4.6, 17.9 | 45.5 | 9.7, 214.5 | 39.2 | 15.9, 96.7 | ||||||||
| Female sex- smoking interaction | 0.34 | 0.19, 0.59 | 0.63 | 0.16, 2.40 | 0.70 | 0.14, 3.49 | ||||||||||||||
| Pinteractionc | <0.0001e | 0.50 | 0.66 | |||||||||||||||||
Abbreviations: CI, confidence interval; EAGLE, Environment and Genetics in Lung Cancer Etiology; OR, odds ratio.
a ORs and 95% CIs from unconditional logistic regression models, adjusted for residential area, age, and years since quitting smoking.
b ORs and 95% CIs from unconditional logistic regression models, adjusted for residential area and age.
c P values were calculated from the likelihood ratio test or Wald test for the product of sex and pack-years (pack-years as a categorical or continuous (log10 (1 + pack-years/5)) variable, respectively).
d Negative female sex-smoking interaction coefficients for all of the pack-year categories evaluated.
e Negative female sex-smoking interaction coefficient.
We performed further analyses, individually adjusting the same logistic regression models for education, having ever worked in an occupation associated with lung cancer, smoking of other tobacco products, inhalation depth, SHS exposure, or FTND score; using cumulative cigarette “dose,” as both a continuous and a categorical variable; or exploring subgroups of “low-exposed” subjects (current smokers of fewer than 10 cigarettes/day) and long-term quitters (persons who had refrained from smoking for ≥10 years). We also conducted analyses stratified by age or SHS exposure. Results were always virtually unchanged (data not shown).
DISCUSSION
In a large population-based case-control study with detailed information on lifetime smoking habits, direct interviews with cases, and a high response rate in both cases and controls, we found a clear negative interaction between female sex and tobacco smoking when using never smokers as the reference category, corresponding to higher odds ratios in men for a given level of lifetime cumulative smoking exposure (measured in pack-years). In contrast, the analyses restricted to ever smokers, based on the more stable reference of light smokers, showed no significant sex-smoking interaction. This finding was also depicted by the floating trend graph (Figure 1), which showed that the exposure-response curves in men and women were substantially parallel. Even assuming a worst-case scenario for women, the relatively tight confidence interval for sex-smoking interaction in ever smokers (e.g., for pack-years as a continuous variable, 95% CI: 0.29, 1.37), resulting from the large sample size of the EAGLE Study, allows us to conclude that our results are incompatible (chance of <2.5%) with the actual relative risk of lung cancer being 1.37-fold higher in women than in men. The lack of sex-smoking interaction was confirmed in all models adjusting for several potential confounders, stratifying by smoking status, or taking into account possible effect modifiers. These findings were consistent across the 3 main lung cancer subtypes evaluated.
Some case-control studies (7–10), but not all (11–13), have found a higher relative risk of lung cancer among women. On the contrary, most (14–23) but not all (24, 25) cohort studies have found either no sex difference or a higher rate ratio among men. Discrepancies among studies might be attributable to variation in study design, the definition of smoking exposure (which also depends on the accuracy of information collection), estimation of relative risks versus absolute risks, and the use of never smokers or light smokers as the reference category in the analysis (27–31). True sex differences in the underlying risk, such as differences in occupational exposure, secular changes in cigarette smoking and rates of smoking, SHS exposure, and changes in lung cancer itself, may also contribute to the discrepant results (3, 6).
Unlike the majority of previous case-control studies, we were able to assess an accurate individual lifetime smoking history for all of the interviewed subjects. For example, the study by Risch et al. (9), which initiated the hypothesis of higher female susceptibility to tobacco-related lung cancer, relied mainly on next-of-kin exposure assessment of cases. In addition, we addressed this issue in a modern, developed social context where smoking by women was no longer stigmatized, making a sex-specific response bias unlikely. In addition, we accounted for several potential confounders, including different smoking features between the sexes (e.g., inhalation depth, type of tobacco, percentage of each cigarette smoked), nicotine dependence, SHS exposure, and work-related exposure to lung carcinogens, that could affect the baseline risk among never smokers in a sex-specific way.
Our findings are consistent with the majority of previous prospective studies (14–16, 18–23), including the large recent cohort study conducted by Freedman et al. (17). Notably, the latter study had a less accurate assessment of smoking exposure and lacked information on age at smoking initiation, which prevented calculation of smoking duration and thus pack-years.
Examining the previous studies (7–26), the frequency of never smoking among cases was always higher in women than in men, even with higher sex disproportion than we had in our study (e.g., 2 large multicenter European case-control studies found 31.8% vs. 2.1% (11) and 29.6% vs. 1.9% (13) for women and men, respectively). Additionally, the prevalence of current smoking in our study base was not dissimilar to that reported for the same time period in Europe (about 40% among males and 18% among females) (36), in Italy (about 29% and 22%, respectively) (37), or in northern Italy, where the Lombardy region is located (about 27% and 19%, respectively) (38).
In our study, the analyses restricted to the main lung cancer histological types showed no positive female sex-smoking interaction, in accordance with most previous case-control and cohort studies, with a few exceptions (9, 17).
Our study had several key strengths: enrollment of incident cases and randomly sampled population controls; participation rates of 86.6% among cases and 72.4% among controls; and face-to-face collection of detailed information with a structured questionnaire by trained interviewers. Moreover, we had a large sample size that allowed testing for interaction effects and evaluation of risk by histological type. In the main analyses on all lung cancers, the narrow confidence interval for the sex-smoking interaction in ever smokers (95% CI: 0.29, 1.37) indicates that in this study we had sufficient statistical power to detect relatively small (compared with the large smoking main effects) positive or negative interactions. In the analyses within histological subgroups, the power to detect modest-to-high sex-smoking odds ratio interactions was high for adenocarcinoma but not for squamous- and small-cell carcinoma.
Despite our accurate individual exposure assessment, inadequate control for confounders of smoking effect as well as recall bias for smoking are possible in any retrospective study on lung cancer, but this should not be different in males and females.
This analysis, in accord with previous high-quality studies, used logistic regression to estimate the association between pack-years of smoking and risk of lung cancer. In future analyses of sex-specific differences in lung cancer susceptibility, researchers might examine different smoking metrics and alternatives to logistic regression modeling.
In conclusion, our findings do not support the controversial hypothesis that women have a higher relative risk of lung cancer than men from the same amount of tobacco exposure. Thus, as far as lung cancer is concerned, equally vigorous health policy interventions should continue to focus on eliminating smoking in both sexes.
ACKNOWLEDGMENTS
Author affiliations: Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Sara De Matteis, Andrew W. Bergen, Neil E. Caporaso, Jay H. Lubin, Sholom Wacholder, Maria Teresa Landi); Unit of Epidemiology, Department of Preventive Medicine, Fondazione IRCCS Ca’ Granda–Ospedale Maggiore Policlinico, Milan, Italy (Sara De Matteis, Dario Consonni, Angela C. Pesatori, Pier Alberto Bertazzi); Department of Clinical Sciences and Community Health, Università degli Studi di Milano, Milan, Italy (Sara De Matteis, Angela C. Pesatori, Pier Alberto Bertazzi); and Molecular Genetics Program, Center for Health Sciences, SRI International, Menlo Park, California (Andrew W. Bergen).
This work was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics, Bethesda, Maryland; the Lombardy Region, Direzione Generale Sanità, Environmental Epidemiology Program, Milan, Italy (grants 14013-1/5/2010 and 8956-7/6/2006); the CARIPLO Foundation, Milan, Italy; and the Ministero della Salute and Istituto Nazionale per l'Assicurazione contro gli Infortuni sul Lavoro, Rome, Italy (grant PMS/42/06).
We thank Drs. Hormuzd Katki and Stephanie Kovalchik for their helpful comments.
Conflict of interest: none declared.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Patel JD, Bach PB, Kris MG. Lung cancer in US women: a contemporary epidemic. JAMA. 2004;291(14):1763–1768. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 3.Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med. 2010;7(5):381–401. doi: 10.1016/j.genm.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Cote ML, Yoo W, Wenzlaff AS, et al. Tobacco and estrogen metabolic polymorphisms and risk of non-small cell lung cancer in women. Carcinogenesis. 2009;30(4):626–635. doi: 10.1093/carcin/bgp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollerup S, Berge G, Baera R, et al. Sex differences in risk of lung cancer: expression of genes in the PAH bioactivation pathway in relation to smoking and bulky DNA adducts. Int J Cancer. 2006;119(4):741–744. doi: 10.1002/ijc.21891. [DOI] [PubMed] [Google Scholar]
- 6.Subramanian J, Govindan R. Lung cancer in never smokers: a review. J Clin Oncol. 2007;25(5):561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 7.Brownson RC, Chang JC, Davis JR. Gender and histologic type variations in smoking-related risk of lung cancer. Epidemiology. 1992;3(1):61–64. doi: 10.1097/00001648-199201000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Harris RE, Zang EA, Anderson JI, et al. Race and sex differences in lung cancer risk associated with cigarette smoking. Int J Epidemiol. 1993;22(4):592–599. doi: 10.1093/ije/22.4.592. [DOI] [PubMed] [Google Scholar]
- 9.Risch HA, Howe GR, Jain M, et al. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. 1993;138(5):281–293. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 10.Zang EA, Wynder EL. Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst. 1996;88(3-4):183–192. doi: 10.1093/jnci/88.3-4.183. [DOI] [PubMed] [Google Scholar]
- 11.Kreuzer M, Boffetta P, Whitley E, et al. Gender differences in lung cancer risk by smoking: a multicentre case-control study in Germany and Italy. Br J Cancer. 2000;82(1):227–233. doi: 10.1054/bjoc.1999.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osann KE, Anton-Culver H, Kurosaki T, et al. Sex differences in lung-cancer risk associated with cigarette smoking. Int J Cancer. 1993;54(1):44–48. doi: 10.1002/ijc.2910540108. [DOI] [PubMed] [Google Scholar]
- 13.Simonato L, Agudo A, Ahrens W, et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer. 2001;91(6):876–887. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1139>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Akiba S, Hirayama T. Cigarette smoking and cancer mortality risk in Japanese men and women—results from reanalysis of the six-prefecture cohort study data. Environ Health Perspect. 1990;87:19–26. doi: 10.1289/ehp.908719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bach PB, Kattan MW, Thornquist MD, et al. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95(6):470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 16.Bain C, Feskanich D, Speizer FE, et al. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst. 2004;96(11):826–834. doi: 10.1093/jnci/djh143. [DOI] [PubMed] [Google Scholar]
- 17.Freedman ND, Leitzmann MF, Hollenbeck AR, et al. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9(7):649–656. doi: 10.1016/S1470-2045(08)70154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman GD, Tekawa I, Sadler M, et al. Bethesda, MD:: National Cancer Institute; 1997. Smoking and mortality: the Kaiser Permanente experience; pp. 477–497. Changes in Cigarette-related Disease Risks and Their Implications for Prevention and Control. (Smoking and tobacco control monograph no. 8) (NIH publication no. 97-4213) [Google Scholar]
- 19.Jee SH, Samet JM, Ohrr H, et al. Smoking and cancer risk in Korean men and women. Cancer Causes Control. 2004;15(4):341–348. doi: 10.1023/B:CACO.0000027481.48153.97. [DOI] [PubMed] [Google Scholar]
- 20.Marang-van de Mheen PJ, Smith GD, Hart CL, et al. Are women more sensitive to smoking than men? Findings from the Renfrew and Paisley study. Int J Epidemiol. 2001;30(4):787–792. doi: 10.1093/ije/30.4.787. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson S, Carstensen JM, Pershagen G. Mortality among male and female smokers in Sweden: a 33 year follow up. J Epidemiol Community Health. 2001;55(11):825–830. doi: 10.1136/jech.55.11.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prescott E, Osler M, Hein HO, et al. Gender and smoking-related risk of lung cancer. The Copenhagen Center for Prospective Population Studies. Epidemiology. 1998;9(1):79–83. [PubMed] [Google Scholar]
- 23.Thun MJ, Day-Lally CA, Calle EE, et al. Excess mortality among cigarette smokers: changes in a 20-year interval. Am J Public Health. 1995;85(9):1223–1230. doi: 10.2105/ajph.85.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henschke CI, Yip R, Miettinen OS. Women's susceptibility to tobacco carcinogens and survival after diagnosis of lung cancer. JAMA. 2006;296(2):180–184. doi: 10.1001/jama.296.2.180. [DOI] [PubMed] [Google Scholar]
- 25.Tulinius H, Sigfusson N, Sigvaldason H, et al. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6(11):863–873. [PubMed] [Google Scholar]
- 26.Wakai K, Inoue M, Mizoue T, et al. Tobacco smoking and lung cancer risk: an evaluation based on a systematic review of epidemiological evidence among the Japanese population. Jpn J Clin Oncol. 2006;36(5):309–324. doi: 10.1093/jjco/hyl025. [DOI] [PubMed] [Google Scholar]
- 27.Blot WJ, McLaughlin JK. Are women more susceptible to lung cancer? J Natl Cancer Inst. 2004;96(11):812–813. doi: 10.1093/jnci/djh180. [DOI] [PubMed] [Google Scholar]
- 28.Haugen A. Women who smoke: are women more susceptible to tobacco-induced lung cancer? Carcinogenesis. 2002;23(2):227–229. doi: 10.1093/carcin/23.2.227. [DOI] [PubMed] [Google Scholar]
- 29.Perneger TV. Sex, smoking, and cancer: a reappraisal. J Natl Cancer Inst. 2001;93(21):1600–1602. doi: 10.1093/jnci/93.21.1600. [DOI] [PubMed] [Google Scholar]
- 30.Risch HA, Miller AB. Re: Are women more susceptible to lung cancer? [letter]. J Natl Cancer Inst. 2004;96(20):1560. doi: 10.1093/jnci/djh302. [DOI] [PubMed] [Google Scholar]
- 31.Twombly R. New studies fan controversy over gender risk in lung cancer. J Natl Cancer Inst. 2004;96(12):898–900. doi: 10.1093/jnci/96.12.898. [DOI] [PubMed] [Google Scholar]
- 32.Landi MT, Consonni D, Rotunno M, et al. Environment And Genetics in Lung cancer Etiology (EAGLE) study: an integrative population-based case-control study of lung cancer. BMC Public Health. 2008;8:203. doi: 10.1186/1471-2458-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 34.Greenland S, Michels KB, Robins JM, et al. Presenting statistical uncertainty in trends and dose-response relations. Am J Epidemiol. 1999;149(12):1077–1086. doi: 10.1093/oxfordjournals.aje.a009761. [DOI] [PubMed] [Google Scholar]
- 35.Ahrens W, Merletti F. A standard tool for the analysis of occupational lung cancer in epidemiologic studies. Int J Occup Environ Health. 1998;4(4):236–240. doi: 10.1179/oeh.1998.4.4.236. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization, Regional Office for Europe. The European Tobacco Control Report 2007. Copenhagen, Denmark: World Health Organization: Regional Office for Europe; 2007. http://www.euro.who.int/__data/assets/pdf_file/0005/68117/E89842.pdf. (Accessed February 23, 2012) [Google Scholar]
- 37.Gallus S, Zuccaro P, Colombo P, et al. Smoking in Italy 2005–2006: effects of a comprehensive National Tobacco Regulation. Prev Med. 2007;45(2-3):198–201. doi: 10.1016/j.ypmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Italian National Institute of Statistics. Smoking Habit: Sub National Data. Rome, Italy: 2012. Italian National Institute of Statistics; http://dati.istat.it/?lang=en. (Accessed February 20, 2012) [Google Scholar]