Abstract
Purpose
To examine whether children with specific language impairment (SLI) differ from normally developing peers in motor skills, especially those related to timing.
Method
Standard measures of gross and fine motor development were obtained. Furthermore, finger and hand movements were recorded while children engaged in four different timing tasks, including tapping and drawing circles in time with a metronome or a visual target. Fourteen children with SLI (aged 6- to 8-years) and 14 age-matched peers who were typically developing participated.
Results
As expected, children with SLI showed poorer performance on a standardized test of gross and fine motor skill than their normally developing peers. However, timing skill in the manual domain was equivalent to typically developing children.
Conclusions
Consistent with earlier findings, relatively poor gross and fine motor performance is observed in children with SLI. Surprisingly, rhythmic timing is spared.
Keywords: SLI, motor control, timing, language disorders
Language production, whether spoken, signed, or written, is a motor activity. For example, speech requires the coordination between linguistic, articulatory, phonatory, respiratory and laryngeal systems (Smith, 1992). Difficulties in spoken language can reside in several of these systems, or in the coordination of activities between systems. Many recent investigations of both normal and disordered language production show that the neural anatomical structures for language and movement overlap (e.g., Arbib, 2006; Kent, 2004). Compatible with the idea of shared neural organization, there is a high degree of co-occurrence of cognitive-linguistic and motor deficits in many tasks (e.g., Diamond, 2000; Hill, 2001). Theorists in speech development have noticed the importance of manual gestures as a precursor of language (MacNeilage, 2008). The observed co-occurrence of language and motor deficits is only beginning to become important in accounts of specific language impairment, SLI (e.g., Alcock et al., 2000; Hill, 2001; Jancke et al., 2006; Ullman & Pierpont, 2005).
SLI is defined as a deficit in language that is not explained by non-verbal cognitive, auditory, oral motor, or other neurological factors (Leonard, 1998). Major theories of the core deficit in SLI focus on problems in acquiring the grammatical aspects of language, for example emphasizing the obligatory nature of tense and agreement (Rice & Wexler, 1996) and notions of grammatical movement (van der Lely, 1998).
However, it has become clear that many non-linguistic factors contribute to the expression of SLI, one of these being deficits in limb motor control (see Bishop, 2002; Hill, 2001; Leonard et al., 2007; Ullman & Pierpont, 2005). As measured by general standardized tests, children with SLI show significant delays in motor performance, similar to those observed in children with developmental coordination disorder (e.g., Hill & Bishop, 1998; for a review, see Hill, 2001). Experimental evidence has revealed specific difficulties with fine motor tasks such as timed peg moving (Jancke et al., 2006; Powell & Bishop, 1992) and finger opposition (Stark & Tallal, 1981). In particular, gross motor deficits have been documented in balance (Powell & Bishop, 1992).
Another domain of impaired performance involves praxis. Children with SLI are poorer than their normally developing peers at imitating gestures, such as miming brushing their teeth and saluting (Hill, 1998). Hill, Bishop, and Nimmo-Smith (1998) found that children with SLI produce errors in the manual implementation of a sequence of actions, but not in the conceptualization of those actions.
It is evident that children with SLI exhibit generalized motor deficits. Studies have also focused on oral motor skill and how it relates to language status. Notably, half of the members of the KE family, who initially provided evidence for a genetic basis for grammatical function (Gopnik, 1990) demonstrate oral dyspraxia (Alcock et al., 2000). Affected family members show the expected deficit in tense and agreement, but also are impaired in their ability to produce complex sequences of nonverbal oral movements (Alcock et al, 2000). Clearly, oral dyspraxia is part of the phenotype. Oral motor deficits have been identified in both speech (Goffman, 1999; Goffman, 2004) and nonspeech (Alcock, 2006; Stark & Blackwell, 1997) tasks in children with SLI.
In the present article we consider the role of motor timing in the generalized motor deficits observed in SLI. Several investigators have suggested that a core deficit in SLI involves temporal processing, both in perception (Tallal et al., 1996; Tallal & Stark, 1980) and production (Alcock et al., 2000; Tallal & Stark, 1980; Thomson & Goswami, in press). The production of rhythmic tapping and vocalization has been implicated in SLI (Alcock et al., 2000). Overall, motor deficits are predicted in children with SLI, especially those involving speeded, sequential, and/or timed movements (e.g., Bishop, 2002; Leonard et al., 2007; Ullman & Pierpont, 2005).
In this study we examine the hypothesis that timing deficits underlie some of the motor and language deficits exhibited by children with SLI. Language is rhythmic, and aspects of rhythmic output are known to be difficult for children with SLI. For example, in language production, children with SLI show a weakness in prosody, omitting syllables that are not consistent with a preferred prosodic pattern (McGregor & Leonard, 1994) and producing unstressed syllables with large and long articulatory movements (Goffman, 1999, 2004). Perceptual and motor aspects of rhythm appear to characterize important aspects of SLI.
Bishop (2002) observed that speed of tapping is compromised in children with SLI compared to normally developing children. Leonard et al. (2007) showed that multiple tasks that require speeded information processing, including tapping speed as well as tapping alternation, are affected in language impaired children compared to normally developing controls.
In the present investigation we chose to examine two aspects of motor performance of children with SLI. First, to replicate prior work demonstrating a generalized motor deficit in children with SLI, we used a standardized assessment tool to assess gross and fine motor skill performance. We also experimentally examined timing control. First, we turn our attention to generalized motor performance.
One way to view motor performance is to compare control of gross and fine motor skills against standard normative data. One of the gold-standards for motor development is the Bruininks-Oseretsky Test of Motor Proficiency (Hattie & Edwards, 1987; Riggen, Ulrich, & Ozmun, 1990; Tan, 2001). This is a standardized and norm-referenced test of motor development that is widely used by clinicians (especially occupational and physical therapists) and researchers to evaluate motor performance and to discriminate children with motor disorders from those who are developing normally. The Bruininks-Oseretsky test (Bruininks, 1978) evaluates motor performance in fine manual control, manual coordination, body coordination, balance, strength, and agility. Children with motor difficulties score poorly on the Bruininks-Oseretsky test (Missiuna, 2001).
There are limitations of relying on a general standardized motor test, especially since motor performance has been viewed as being comprised as a set of modular computations (Keele & Ivry, 1987). In other words, certain aspects of performance are the responsibility of processes not embodied within the skill itself. One of the most studied of these processes is motoric timing (Ivry & Spencer, 2004).
Keele and colleagues have championed the notion that much information processing can be considered to be accomplished by self-encapsulated procedures, often called modules, or computations (Fodor, 1983; Keele & Ivry, 1987). These procedures are shareable across a variety of tasks, such that when presented with a new task, the person can orchestrate a collection of these procedures to solve the specific procedural task demands.
Keele and colleagues (Keele & Ivry, 1987) began a research program that led to the strong claim that timing was an elementary perceptual and motor process that could be shared by a variety of motor tasks. Furthermore, Ivry (1997) has posited that the cerebellum’s main role is in movement as well as perceptual timing processes in the millisecond range.
Adults with cerebellar damage show clear timing deficits (Ivry, Keele & Diener, 1988). Speech is obviously an activity that requires precise timing, such as perceiving and producing differences in voice onset time. Individuals with cerebellar lesions are deficient in this discrimination compared to intact age-matched controls (see Ivry, Spencer, Zelaznik & Diederichsen, 2002, for a description of this work). Critically, children with SLI have also shown reduced performance on perceptual tasks that require the processing of rapidly changing auditory stimuli (Tallal et al., 1996). Furthermore, Williams, Woollacott and Ivry (1992) have shown that children classified as clumsy show timing deficits compared to age-matched typically developing children.
The motor production task that has been used to capture this amodal timing computation has been the timing in tapping task. In the tapping task a person flexes and extends a finger at the metacarpophalangeal joint, normally resting the hand on a desktop and attempting to touch down (flexion) with the fingertip coincident with a metronome beat, called synchronization. After a series of synchronization intervals, the metronome is disengaged and the person attempts to continue to produce temporal intervals as accurately and as consistently as possible. This latter phase is called continuation.
Robertson, Zelaznik, Spencer and colleagues (Robertson et al., 1999) compared the timing behavior of the tapping task, as described above, with a continuous circle drawing timing task. In this latter task, a 5 to 8 cm diameter circle is placed on a desktop. At the 12 O’clock position (from the participant’s perspective) a smaller 0.5 to 1.0 cm circle is placed so that it intersects the larger circle circumference. The person does not trace the larger circle, but uses it as a guide to move the finger around it, having the tip of the finger enter into the 12 O’clock location coincident with the metronome beat, and then to continue accurately and precisely during continuation.
Robertson et al. (1999) reasoned that if the same timing module was being used to control time in circle drawing as in tapping, then individual differences in timing precision in tapping should be related to individual differences in timing precision in circle drawing. Such was not the case. There were no significant correlations between circle drawing tasks and timing in tapping tasks. Following this original set of experiments, Spencer, Ivry, Zelaznik and colleagues documented the robustness of the finding that timing processes in tapping are distinct and separable from timing processes in circle drawing (see Ivry et al., 2002 and Zelaznik, et al., 2008 for thorough reviews).
Spencer, Zelaznik, Ivry and Diederichsen (2003) and Spencer, Ivry and Zelaznik (2005) showed that the cerebellum is crucial for the control of timing in the tapping task (this is the archetypal Keele and Ivry task to capture the timing module), but is not crucial for timing control in continuous circle drawing. Individuals with cerebellar lesions did not exhibit any timing deficits for the continuous circle drawing timing task, but did exhibit timing deficits on the tapping timing task as well as on an intermittent circle drawing timing task (Spencer et al., 2003). The neurophysiological dissociation for discrete-like timing requiring cerebellar processes, and continuous timing not requiring cerebellar resources provided strong support for the event (discrete-like) — emergent (continuous) timing distinction (Zelaznik et al., 2008). Furthermore, the distinction is not due to the difference between finger timing (tapping) and arm timing (circle drawing), as Spencer et al. (2003) observed that smooth continuous finger timing was not compromised when performance was controlled via a damaged cerebellar hemisphere. Finally, this distinction was not due to just moving the finger but the actual demand to control duration (Spencer et al., 2005). These experiments from a cognitive neuroscience perspective as well as other behavioral work (see Zelaznik et al., 2008), led Ivry, Spencer, Zelaznik and Diederichsen (2002; Spencer et al., 2003) to propose the event-emergent timing framework.
The tapping task, which requires timing computations, uses an event timer and requires cerebellar involvement, whereas the circle drawing timing task does not need timing computations; timing emerges from dynamical control processes. Recently, Huys, Studenka, Rheaume, Zelaznik and Jirsa (2008) have shown that these two types of movements, smooth and continuous, versus discrete-like are two topologically distinct descriptions. This latter work adds theoretical and empirical support for the event-emergent timing distinction.
In the present study we examined whether children with SLI exhibit deficits in non-speech timing. Furthermore, circle drawing is thought not to rely upon the Keele and Ivry timing module, called event timing. Therefore, if children with SLI show a deficit in event timing, they will not exhibit a deficit in an emergent timing task, circle drawing. We compared any specific timing deficits observed with a more generalized motor deficit captured by the Bruininks-Oseretsky Test of Motor Proficiency, Version 1 (Bruininks, 1978).
Timing with the finger requires much more distal control than circle drawing (controlled with shoulder and elbow oscillation), and we know that development proceeds from proximal to distal (Haywood & Getchell, 2009). Thus, we included a hand tapping task, in which the arm is moved up and down to have the hand tap on the table top. The fourth timing task was a modulated hand tapping task that required the child to generate a large movement followed by a small movement. Goffman (1999, 2004) has found that modulation of strong versus weak syllables in speech is problematic in SLI, with the control of amplitude as well as duration implicated. Manual modulation might mirror such a deficit. We hypothesized that prior results demonstrating that children with SLI will exhibit a generalized motor impairment, as captured by the BOT, will be replicated. Furthermore, based upon the role of the cerebellum in event timing, we hypothesized that children with SLI will exhibit impaired timing performance on each of the timing tasks, except the emergently-timed circle drawing task. This latter task does not require cerebellar timing processes.
Methods
Participants
Twenty eight children served as subjects. Fourteen were children with SLI, age 6- to 8-years (M = 7;5 (years; months), SD = 13 months, range = 5;11–8;11). Fourteen age matched children served as controls (M = 7;5, SD = 13, range 6;1 – 8;11). Half of the children in each group were girls.
Thirteen of the 14 children with SLI had received a clinical diagnosis when they were 4- to 6-years old and had participated or were presently participating in therapy and in research studies in Purdue University’s M. D. Steer clinic. All of these children met the criteria for SLI (Leonard, 1998) at the time of diagnosis. One child was newly recruited for this study. She met the same diagnostic criteria. All of the children were monolingual speakers of English. At the time of entry, all scored less than 1.5 SD below the mean for their age on the Structured Photographic Expressive Language Test-II (Werner & Krescheck, 1983). The children in the ND group all performed within expected limits (M=113, SD=8, range=90–149) on the Structured Photographic Expressive Language Test-III (Dawson & Stout, 2003).
At the time this study was conducted, each child with SLI was given the non-word repetition task (Dollaghan & Campbell, 1998). The purpose of this task was to confirm SLI diagnostic status. The children with SLI exhibited a mean score of 51% (SD=11) correct on the four-syllable non-words and 75% (SD=8) correct overall. This was consistent with Dollaghan and Campbell’s (1998) results for children with SLI (50% (16) on 4-syllable non-words and 66% (12) overall).
All 28 of the children demonstrated nonverbal intelligence scores greater than 85 as assessed using the Columbia Mental Maturity Scale (Burgemeister, Blum, & Lorge, 1972)(ND, M = 123, SD = 12; SLI, M = 113, SD = 15), with no differences between groups, t(26)=1.57, p = .07. In addition, each child passed a hearing screening, responding at 20 dB at 500 Hz, 1000 Hz, 2000 Hz, and 4000 Hz. Normally developing children were recruited via advertisements in local newspapers and referral from friends who had participated. All experimental, assessment activities and consent procedures were approved by the Purdue University IRB.
Apparatus
For each of the four timing tasks, an infrared light emitting diode (IRED) was affixed to the fingertip of the dominant hand index finger of the participant. The three dimensional location of the IRED was sampled by an Optotraktm infrared motion capture system at 250 Hz. A participant was seated in a child-sized stable chair, and rested her arms on a table directly in front of her.
Tasks
In the tapping and circle drawing timing tasks, the child was required to attempt to complete a cycle of movement coincident with a clearly audible metronome beat (1600 Hz, 10 ms duration tone), set to a 600 ms period (10 ms duration tone, followed by 590 ms of silence). The synchronization portion of the trial produced fourteen tones, and thus 13 cycles of movement. The metronome disengaged and the child continued to produce intervals at the prescribed rate for an additional thirty to thirty three intervals. For finger tapping, the movement consisted of flexion and extension of the index finger (at the metacarpophalangeal joint) situated so that the finger moved up and down with reference to the table top. The child rested the heel of the palm of the hand on the table top, formed a bridge with the other four fingers (as though playing billiards), and then oscillated the index finger in flexion and extension so that the finger tip touched the table top coincident with the metronome beat during synchronization and attempted to maintain the timing during the continuation phase of the trial, when the metronome was no longer engaged.
The circle drawing timing task required the child to smoothly and repetitively move their finger around a 7.5 cm diameter circle, placed on the table top. At the top of this circle, at the 12 O’clock position (relative to the child), a 1 cm diameter circle was placed. This smaller diameter circle served as the timing target. The task required the child to have the index finger at the timing target coincident with the metronome beat, and then to continue at that rate after the metronome disengaged. The task for the child was not to accurately trace the larger 7.5 cm diameter circle, but to use the larger circle as a guide to get the approximate size of the circle. When performing the circle drawing movements, the child used elbow and shoulder joint motion to produce the circular trajectory at the index finger.
The hand tapping tasks were conducted using visual rather than auditory timing cues. In pilot testing, we found that children had difficulty learning the hand modulated tapping task. This was especially the case because we wanted to avoid the use of language cues (e.g., big vs. small). We found that visual cues were more effective in learning this task. We recruited a child to perform the task to the metronome using the same 600 ms target as in tapping and circle drawing. With a videotape analysis we confirmed that she was accurate and consistent in producing intervals at the target frequency. This video was then used to elicit the hand tapping tasks.
The hand tapping task required the child to move her hand perpendicular to the plane of the table top, primarily with shoulder flexion and some elbow extension to touch down on the table top coincident with the visual target. The hand modulated tapping task was identical to hand tapping, with the additional requirement that the distance/extent of the hand movement alternate between a large extent and a small extent but keeping a constant period between touch-downs.
The Bruininks-Oseretsky motor tasks (Bruininks, 1978) were implemented as described in the test manual. Five sub-tests from the Bruininks-Oseretsky Test of Motor Proficiency were employed. Each sub-test yields a standard score (Mean = 15, Standard Deviation = 5). The sub-tests implemented and sample items are as follows:
Balance (e.g., standing on preferred leg on floor; walking forward on balance beam)
Bilateral Coordination (e.g., tapping foot and finger on same side; tapping foot and finger on opposite side; jumping up and clapping hands)
Upper-Limb Coordination (e.g., catching a bounced ball with both hands; touching nose with index fingers with eyes closed)
Visual-Motor Control (e.g., cutting out circles with preferred hand; copying a triangle with preferred hand)
Upper-Limb Speed and Dexterity (e.g., stringing beads with preferred hand; rapidly making dots inside circles with preferred hand)
Participants performed the finger tapping and the circle drawing timing task in one session and the hand tapping and the modulated hand tapping in a second session. The finger tapping and circle drawing tasks were completed in the first session. Standardized motor, language, and cognitive testing activities followed the experimental tasks within each session. Because this data collection was part of a larger project, some of the standardized testing was extended into later sessions.
Procedures
For each of the four timing tasks, a trial began with the experimenter asking the child if she was ready. The metronome or video then engaged and the child attempted to synchronize. The metronome or video disengaged, and the child continued to produce intervals (sometimes the child needed a reminder to continue). This task was performed for a maximum of eight trials, which guaranteed that there would be at least four usable trials. A trial was unusable when the IRED was shielded from the camera (because the child rotated the index finger), or when the child stopped moving during the continuation phase of the trial. The tasks on the Bruininks-Oseretsky Test of Motor Proficiency (Bruininks, 1978) were performed according to the standardization procedures described in the manual.
Data Collection and Reduction
Three dimensional kinematic data for the timing tasks were collected at 250 Hz. These data were filtered (Butterworth, 5th order, low-pass filter with a 25 Hz cutoff, implemented in the forward and backward directions) so as not to lose the abrupt changes in position for the finger and hand tapping tasks when the finger or the hand contacted the table. For the three tapping timing tasks the superior-inferior (z) dimension of movement was used to determine cycle duration. For circle drawing, the anterior-posterior dimension (y) was used. This is equivalent to moving back and forth in a straight line from 12 O’clock to 6 O’clock along the 7.5 diameter of the circle.
The relevant displacement record was differentiated with a three-point central-difference technique. We then used an in-house developed algorithm to define the initiation/termination of a cycle. For the three tapping tasks, we calculated the maximum downward velocity for each downward movement of the finger/hand and then determined when after that landmark did the IRED velocity drop below three percent of that value (in absolute value). That sample value defined a touch down, and thus marked the end of one interval and the beginning of the next. For circle drawing the same three-percent velocity criterion was employed. This criterion, basically, captured the reversal sample of the finger in the anterior-posterior dimension. Biberstine, Zelaznik, Kennedy and Whetter (2005) showed that this algorithm worked equally well as a criterion based upon the exact location of a digit in the timing circle.
The sub-tests on the Bruininks-Oseretsky Test of Motor Proficiency (Bruininks, 1978) were scored in accordance with the procedures described in its manual. Standard scores obtained within each sub-test were subjected to statistical analysis.
Results
Bruininks-Oseretsky Tasks
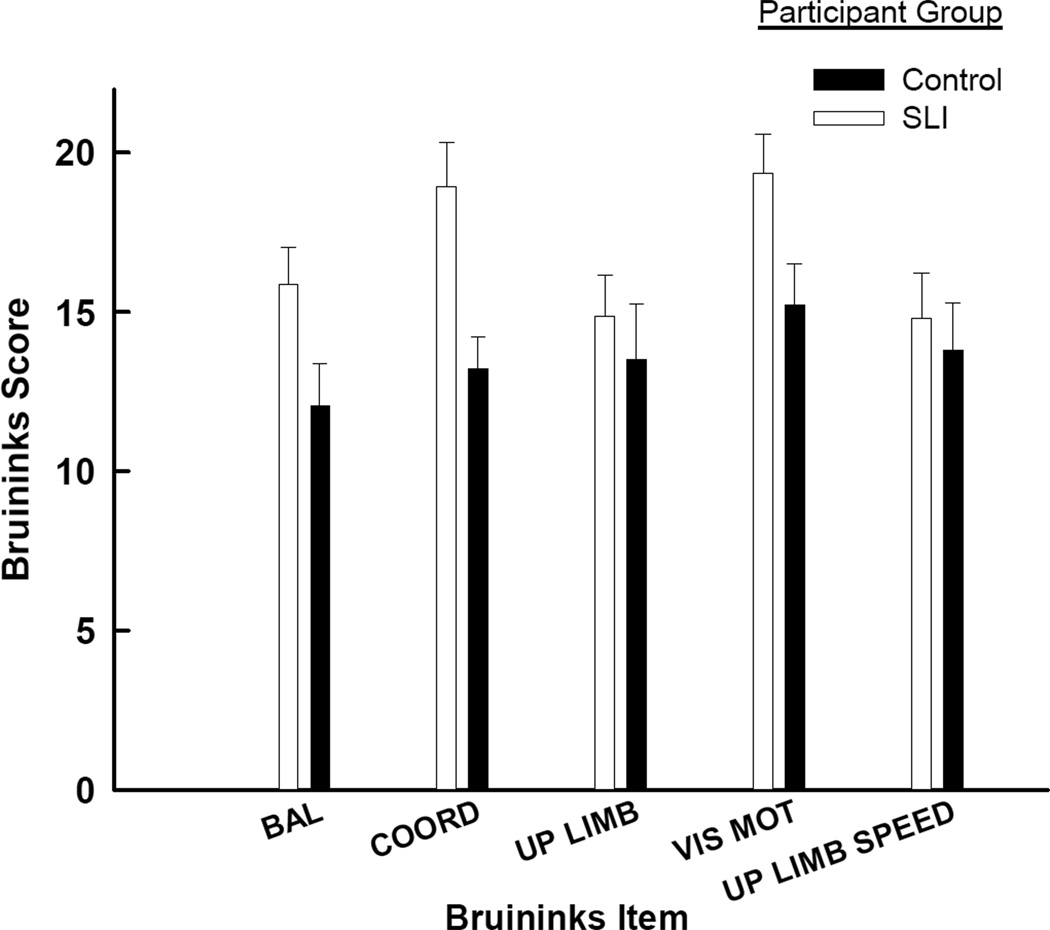
Figure 1 presents the aggregate scores on the five sub-tests. First, note that for each of the five tests, the children with SLI scored less than the control counterparts. This observation was supported with a groups by subtest (2 × 5) ANOVA. There was a significant effect of group, F(1,26) = 7.49, p = .01, MSe = 47.82, as well as an effect of subtest, F(4,104) = 2.98, p = .02, MSe = 20.00. The interaction of subtest and group was not significant, F(4,104) = 1.38, p = .25. It is also important to recognize that SLI and typically developing children exhibited mean values on the Bruininks that were within one SD of the normative mean of 15. We also note that the typically developing children scored slightly higher than the normative mean of 15.
Figure 1.
Average scores on the five subtests used from the Bruininks-Oseretsky test as a function of participant group. All variability lines are standard errors.
In order to determine whether the typically developing children were different from the normative population we conducted independent Student t tests, testing the null hypothesis that the true value of BOT scale was 15. For the normally developing the values of t(13) were .72, 2.84, −0.11, 3.57 and −0.15, for the balance, bilateral coordination, upper limb coordination, visuo-motor control and upper-limb speed and dexterity measures, respectively. Only the bilateral coordination as well as the visuo-motor control tasks significantly differed from 15 (p < .05). For the SLI children the values of t(13) were −2.26, −1.78 −0.85 0.17 −0.81, for the same five tests. Only the balance test was significantly different from 15 (p < .05).
Timing Tasks
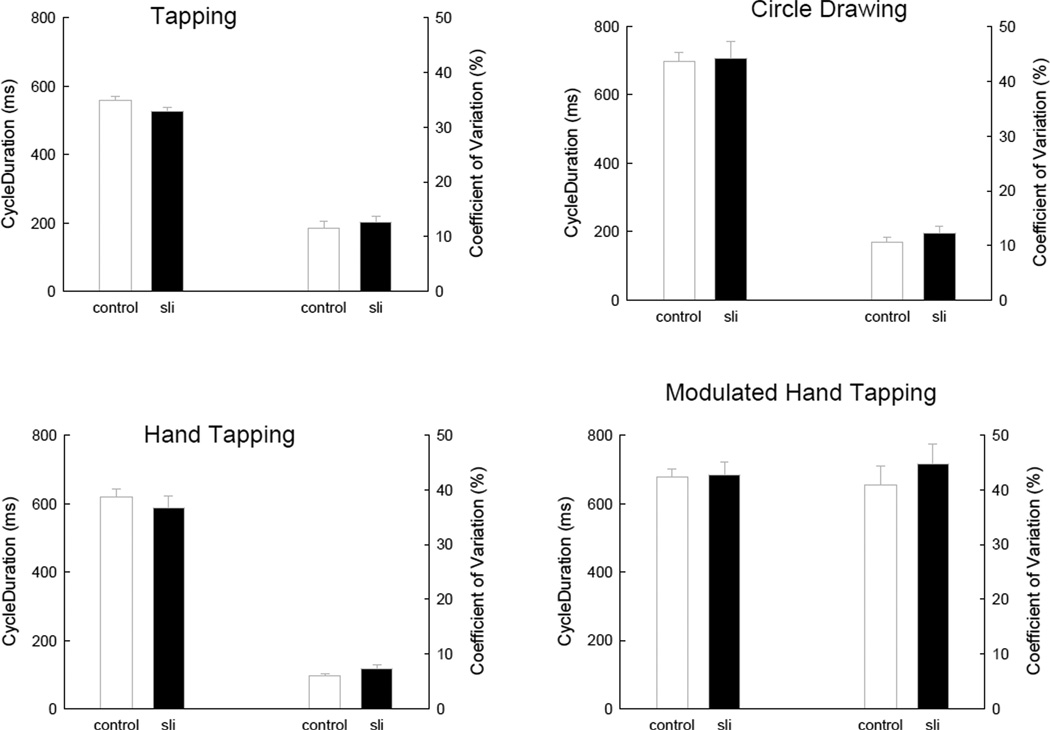
Figure 2 presents the average cycle duration as well as the coefficient of variation for each of the four tasks (one task per panel) as a function of participant group. The coefficient of variation is the within-subject within trial standard deviation in the interval duration time series (after detrending1) divided by the average interval (prior to detrending) and converted to a percentage. These values were then averaged across trials and participant within each group. These calculations were done on the time series on the third interval after the metronome disengaged until the next to last interval of the time series.
Figure 2.
Descriptive data (mean cycle duration and coefficient of variation) on each of the four timing tasks as function of participant group. All variability lines are standard errors.
As the reader can see in Figure 2, the hand tapping and modulated hand tapping tasks were on average around the 600 ms goal duration. Whereas in finger tapping the average cycle duration was less than 600 ms and for circle drawing the average duration was more than the 600 ms goal. A group by task ANOVA supported these observations. There was a significant effect of task, F(3,26) = 13.73, p < .0001, MSe = 11183. There were no effects of participant groups or interactions between group and task, Fs < 1.
In terms of timing precision, as captured by the coefficient of variation, there also were no differences between groups, F(1,26) = 1.06, p = .31, nor an interaction between group and task, F < 1. These data also are presented in Figure 2. The right hand y axis and bars on the right of the graph show these results. There was a clear effect of task F(3,78) = 185.20, p < .0001, MSe = 41.3. Finger tapping and circle drawing exhibited equal coefficient of variation values, whereas hand tapping was almost twice as precise as circle drawing and finger tapping and as expected the modulated tapping task.
Modulated task analysis
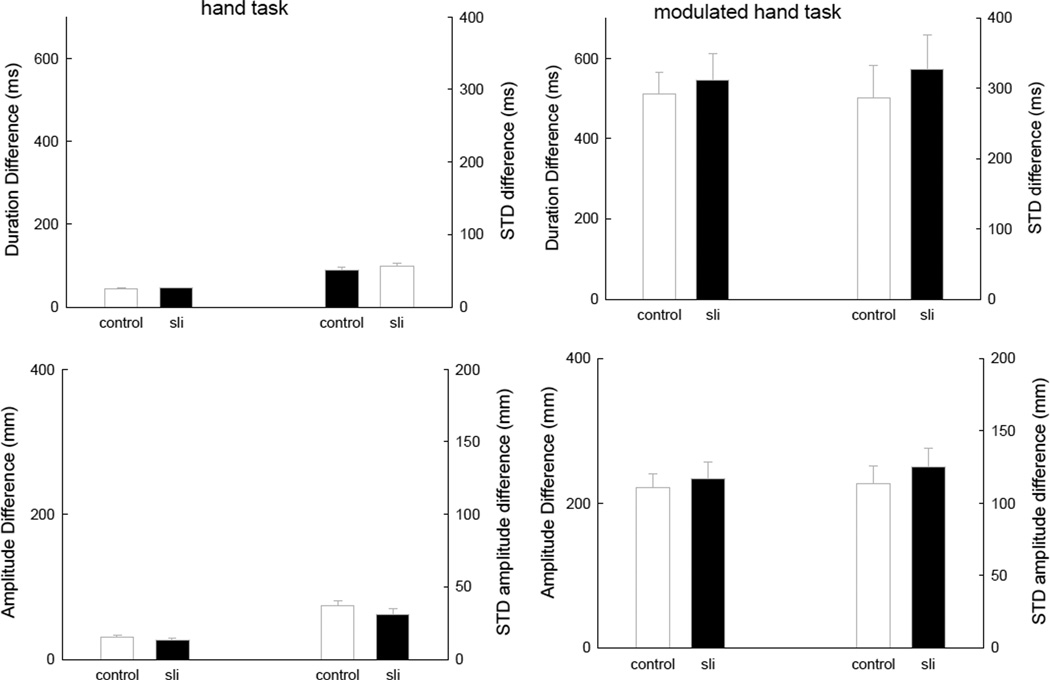
The modulated hand task gives us an opportunity to examine how well children modulated the amplitude of their movements. We compared the hand tapping with the modulated hand tapping task. The continuation time series was split into a set of odd-numbered and even-numbered intervals. The amplitude of the odds and evens, duration of the odds and evens and the concomitant within odd and even series variability was calculated. Amplitude was defined as the maximum displacement away from the table top. Figure 3 presents the results of these calculations. The duration difference presented is the absolute value of the difference, computed within a trial and then averaged across trials. First notice that the hand tapping task shows a very small difference between the odd and even intervals, and a small standard deviation for both the amplitude and the duration difference and the difference standard deviation. However, for the modulated task children could not maintain an equal period for the odd and even intervals and thus the difference was large, and of course the standard deviation of the difference was large. Note that for the duration difference, the data have extremely similar values as the duration data for the modulated hand task, when we did not separate the odds from the evens (see Figure 2). However, in terms of amplitude modulation, we have the first indication of a decrement in performance for the SLI children compared to the other two groups. The standard deviation values for the timing difference as well as the amplitude difference were numerically greater than the other two groups, albeit not significantly so, with all of the F ratio values for group differences being less than one. However, as seen in the Figure the task differences all were significant. There was an effect of task on response duration difference, F(1,26) = 139.23, p < .001, MSe = 23710, and there was an effect on the standard deviation of this difference, F(1,26) = 60.43, p < .001, MSe = 15044. The amplitude difference between odds and evens showed a significant task effect, F(1,26) = 80.33, p < .001, MSe = 2792, and the task effect on the standard deviation was also significant, F(1,26) = 80.29, p < .001, MSe = 1273.
Figure 3.
The two top panels show the average duration difference between odd and even intervals and the standard deviation of the difference for the hand task and modulated hand task. The two bottom panels show the average amplitude difference as well as the standard deviation of the difference for the two hand timing tasks. Please note, that in order to show the real effects of modulation compared to no-modulation the scaling of the hand task is matched to the scaling of the modulated hand task. All variability lines are standard errors.
Discussion
Children with SLI show no apparent deficit in motor timing performance that requires very simple repetitive actions compared with age matched controls. Thus, we provide no evidence in support of the hypothesis that children with SLI will show selective timing impairments in tasks that require event timing. Furthermore, modulating movement extent in the modulated hand timing task showed slight numerical deficits in performance for children with SLI, but these effects did not even approach statistical differences. On the other hand, more gross measures of motor development as well as finer distal control measures from the Bruininks-Oseretsky Test of Motor Proficiency (Bruininks, 1978) showed real differences between children with SLI and their normally developing peers. However, we must view these differences with a bit of caution.
According to the published norms of the BOT, the normally developing children and the children with SLI generally performed in the performance range considered as normal. Thus, the “deficit” in the SLI children is not the result of performing below the normative expectation. Our normally developing children performed, on average, numerically above the normative values and the children with SLI below.
Hattie and Edwards (1987) note that children with verbal deficiencies might not comprehend the instructions of the Bruninks-Oseretsky tests, and as such their performance suffers because individuals do not understand what to do. In fact several classical theories of motor learning (Adams, 1971; Fitts & Posner, 1967; Gentile, 1972) believe that the early stages of practice require cognitive processes to understand the nature of the task. Fitts and Posner (1967) called this early stage of practice the cognitive stage. It is quite possible that the Bruininks-Oseretsky tasks load heavily on verbal-cognitive processes. Thus, children with SLI performed less adequately compared to their normally developing counterparts due to verbal-linguistic deficiencies.
Why did we not see a group difference on the motor timing tasks? These tasks were deliberately chosen because research with adults has shown that the tapping as well as the circle drawing timing tasks do not show benefits of practice (Spencer and Zelaznik, unpublished observations), and as such it is assumed that research subjects quickly bypass the early cognitive-verbal practice processes. Thus, our motor timing tasks are assumed to capture more “motor” processes.
Overall, the pattern of results seems not to favor the recently proposed procedural memory deficit hypothesis (Ullman & Pierpont, 2005). Ullman and Pierpont (2005) postulated that the production of the rules of grammar, a hallmark behavior of the SLI deficit, depends upon procedural processes. These are the implicit procedures and knowledge that individuals possess. For example, tying one’s shoelaces is procedural knowledge. We are not explicitly aware of these processes. Patient HM who showed classic short-term memory deficits was still able to show “memory” of practiced motor (procedural) tasks (see Milner, Squire, & Kandel, 1998). Thus, Ullman and Pierpont postulated that children with SLI lack an integral procedural system.
On face validity the repetitive tapping and circle drawing timing task appear to be very procedural, but we found close to identical performance between the children with SLI and their age-matched ND peers on all measures of timing behavior. If children with SLI possess a robust deficit in a set of procedures and/or computations shared across language and motor domains, the tapping task in particular should have been diagnostic. For example, Williams et al. (1992) found that children with developmental coordination disorder display timing deficits. More importantly, Gilden and Hancock (2007) showed timing performance differences between children with ADHD and typically performing controls. As timing in tapping clearly requires cerebellar involvement, and other diagnostic schemes are related to timing deficits, the support for the procedural deficit hypothesis is not present. The lack of support for the procedural deficit hypothesis, of course, is not a disproof of this idea. Future work should examine this issue in greater detail.
Our results are neutral to the speed of processing and size of working memory hypotheses about SLI (Kail, 1994; Miller et al., 2001; Leonard et al., 2007). All of our timing tasks are “graded”, so that the control of time is of importance, not how fast a task can be completed. Furthermore, we know that tasks requiring people to move as fast as possible are not related to tasks requiring temporal control (Keele & Hawkins, 1982; Robertson et al., 1999).
The present findings replicate those reported previously (e.g., Bishop, 2002; Hill, 2001); children with SLI clearly show lower motor performance than their age-matched peers, as captured by the BOT. We had hypothesized that these deficits would be observed in the simple timing tasks included here. Difficulties in the tapping (but not the circle drawing) tasks would provide a motor analog to earlier observations that children with SLI demonstrate perceptual processing deficits (Tallal et al., 1996). Finally, timing deficits are implicated in speech production. Children with SLI show difficulty in producing the small and short articulatory movements required for the production of well formed unstressed syllables (Goffman, 1999, 2004; Goffman et al., 2006). Spatial and temporal deficits are observed in articulation and we predicted analogous difficulties in the manual domain.
Apparently, however, children with SLI do not have particular difficulty with these simple timing tasks. This result is similar to the work of Zelaznik, Smith and Franz (1994) and Hulstijn, Summers, van Lieshout and Peters (1992) with adults who stutter. Each of these studies found that on simple tapping timing tasks, individuals who stutter performed as well as non-stuttering individuals. However, Zelaznik, Smith, Franz and Ho (1997) found that on a coordinated, bimanual timing task, adults who stutter were more variable on their phase relations than those who do not stutter. Zelaznik at al. (1997) hypothesized that this deficit was due to the intuition that the bimanual finger oscillation captured an elementary coordination process necessary in speech, which also involves coordination across different effectors.
Recently, Mechsner, Kerzel, Knoblich, and Prinz (2001) and Franz, Zelaznik, Swinnen and Walter (2001) have shown that coordination tasks have a clear conceptual and/or perceptual component. This aspect of coordinative tasks might be related to linguistic-verbal processes, and as such would be then diagnostic of SLI. We deliberately did not include any of these types of tasks in the present investigation.
It is highly informative to find tasks in which children with SLI perform similarly to their age matched peers. It is now evident that children with SLI have numerous deficits that fall outside of the domain of language. Most explanations for these deficits have been quite broad, for example implicating generalized slowing (Kail, 1994; Miller et al., 2001) or global motor deficits (Bishop, 2002; Hill, 2001). Presumably, however, these children are not simply broadly impaired. Although it is clear that the construct of SLI needs to be extended outside of the language domain, it still should remain “specific.” Such specificity implies that there are aspects of development that are unaffected. It may be expected that particular domains of cognitive, language, and motor processing should be related in children diagnosed with SLI. To begin the process of understanding mechanisms underlying the deficit and, ultimately, to develop an appropriate approach to treatment, it is critical to specify these cross domain relations.
It appears that a common thread underlying the sorts of deficits observed in motor skill may be integration across components of processing. In an experiment conducted by Tomblin et al. (2007), children had to implicitly learn and retain a sequential motor task. Children with SLI performed more poorly than their normally developing peers on this task. On the standardized tasks included in the Bruininks-Oseretsky Test of Motor Proficiency (Bruininks, 1978), many of the items required coordination, for example between effectors in bimanual tasks or between a cognitive and a motor goal in tasks such as following a maze or placing pegs in a pegboard. In future work, it seems critical to begin to specify processes that are shared between speech, language and motor behavior, and then develop motor tasks that isolate these processes, akin to the work of Leonard and colleagues on speed of processing (Leonard et al., 2007). We hypothesize that the capacity to coordinate timing across effectors (e.g., two hands or the lips, the tongue, and the jaw) may be one such process. It seems likely that increased cognitive load, such as that required in a complex manual or speech task, is another. It is important to continue to explore these language and motor relationships.
Acknowledgements
This work represents an equal contribution of the two authors. HNZ and LG were involved in all aspects of the project, and jointly wrote the paper. This research was supported by the National Institutes of Health (National Institute of Deafness and other Communicative Disorders) grant DC04826. HNZ was also supported by NSF-ITR 042760. We are grateful to Kelsey Pithoud, Jenn Tsai, Rahul Chakraborty, and Janna Berlin for their wonderful contributions to data collection and analysis.
Footnotes
Because participants can sometimes drift off the prescribed rate, variance in timing will be elevated due to the changing overall rate. This source of variability is usually not of interest to theorists in timing and needs to be removed. Linear detrending of the time series removes any linear tendency of the time series to increase or decrease. The variance of the residuals from this detrending, the detrended variance, is a purer measure of time keeping variance for the questions at hand (see Keele & Hawkins, 1982; Zelaznik et al., 2008).
Contributor Information
Howard N. Zelaznik, Department of Health and Kinesiology, Language and Hearing Science Purdue University
Lisa Goffman, Department of Speech, Language and Hearing Science Purdue University.
References
- Adams JA. A closed-loop theory of motor learning. Journal of Motor Behavior. 1971;3:111–150. doi: 10.1080/00222895.1971.10734898. [DOI] [PubMed] [Google Scholar]
- Alcock KJ. The development of oral motor control and language. Down Syndrome Research and Practice. 2006;10:1–8. doi: 10.3104/reports.310. [DOI] [PubMed] [Google Scholar]
- Alcock KJ, Passingham RE, Watkins K, Vargha-Khadem F. Oral dyspraxia in inherited speech and language impairment and acquired dysphasia. Brain and Language. 2000a;75:17–33. doi: 10.1006/brln.2000.2322. [DOI] [PubMed] [Google Scholar]
- Alcock KJ, Passingham RE, Watkins K, Vargha-Khadem F. Pitch and timing abilities in inherited speech and language impairment. Brain and Language. 2000b;75:34–46. doi: 10.1006/brln.2000.2323. [DOI] [PubMed] [Google Scholar]
- Arbib MA. Action to Language via the Mirror Neuron System. Cambridge, MA: Cambridge University Press; 2006. [Google Scholar]
- Biberstine J, Zelaznik HN, Kennedy L, Whetter E. Timing precision in circle drawing does not depend on spatial precision of the timing target. Journal of Motor Behavior. 2005;37:447–453. doi: 10.3200/JMBR.37.6.447-453. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Motor immaturity and specific speech and language impairment: Evidence for a common genetic basis. American Journal of Medical Genetics. 2002;114:56–63. doi: 10.1002/ajmg.1630. [DOI] [PubMed] [Google Scholar]
- Bruininks RH. The Bruininks-Oseretsky Test of Motor Proficiency. Circle Pines, MN: American Guidance Service; 1978. [Google Scholar]
- Burgemeister B, Blum L, Lorge I. Columbia Mental Maturity Scale. 3rd. New York: Harcourt Brace Jovanovich; 1972. [Google Scholar]
- Dawson J, Stout C. The Structured Photographic Expressive Language Test-3. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Development. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- Dollaghan CA, Campbell TF. Nonword repetition and child language impairment. Journal of Speech, Language, and Hearing Research. 1998;41:1136–1146. doi: 10.1044/jslhr.4105.1136. [DOI] [PubMed] [Google Scholar]
- Fitts PM, Posner MI. Human performance. Belmont, Ca: Brooks/Cole; 1967. [Google Scholar]
- Fodor JA. The modularity of mind: An erssay on faculty psychology. Cambridge, MA: MIT Press; 1983. [Google Scholar]
- Franz EA, Zelaznik HN, Swinnen S, Walter C. Spatial conceptual influences on the coordination of bimanual actions: When a dual task becomes a single task. Journal of Motor Behavior. 2001;33:103–112. doi: 10.1080/00222890109601906. [DOI] [PubMed] [Google Scholar]
- Gentile A. A working model of skill acquisition with application to teaching. Quest. 1972;17:3–23. [Google Scholar]
- Gentile A. Implicit and explicit processes during acquisition of functional skills. Scandinavian Journal of Occupational Therapy. 1998;5:7–16. [Google Scholar]
- Gilden DL, Hancock H. Response variability in attention-deficit disorders. Psychological Science. 2007;18:796–802. doi: 10.1111/j.1467-9280.2007.01982.x. [DOI] [PubMed] [Google Scholar]
- Goffman L. Prosodic influences on speech production in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 1999;42:1499–1517. doi: 10.1044/jslhr.4206.1499. [DOI] [PubMed] [Google Scholar]
- Goffman L. Kinematic differentiation of prosodic categories in normal and disordered language development. Journal of Speech, Language, and Hearing Research. 2004;47:1088–1102. doi: 10.1044/1092-4388(2004/081). [DOI] [PubMed] [Google Scholar]
- Goffman L, Heisler L, Chakraborty R. Mapping of prosodic structure onto words and phrases in children’s and adults’ speech production. Language and Cognitive Processes. 2006;21:25–47. [Google Scholar]
- Gopnik M. Feature-blind grammar and dysphasia. Nature. 1990;344:715. doi: 10.1038/344715a0. [DOI] [PubMed] [Google Scholar]
- Hattie J, Edwards H. A review of the Bruininks-Oseretsky test of motor proficiency. The British journal of educational psychology. 1987;57:104–113. [Google Scholar]
- Haywood KM, Getchell N. Life span motor development - 5th edition. Champaign, IL: Human Kinetics; 2009. [Google Scholar]
- Hill EL. Non-specific nature of specific language impairment: A review of the literature with regard to concomitant motor impairments. International Journal of Language and Communication Disorders. 2001;36:149–171. doi: 10.1080/13682820010019874. [DOI] [PubMed] [Google Scholar]
- Hill EL. A dyspraxic deficit in specific language impairment and developmental language disorder? Evidence from hand and arm movements. Developmental Medicine and Child Neurology. 1998;40:388–395. doi: 10.1111/j.1469-8749.1998.tb08214.x. [DOI] [PubMed] [Google Scholar]
- Hill EL, Bishop D, Nimmo-Smith L. Representational gestures in developmental coordination disorder and specific language impairment: Error-types and the reliability of ratings. Human Movement Science. 1998;17:655–678. [Google Scholar]
- Hulstijn W, Summers JJ, van Lieshout PHM, Peters HFM. Timing in finger tapping and speech: A comparison between stutterers and fluent speakers. Human Movement Science. 1992;11:113–124. [Google Scholar]
- Huys R, Studenka BE, Rheaume NL, Zelaznik HN, Jirsa VK. Distinct timing mechanisms produce discrete and continuous movements. PLoS Computational Biology. 2008;4 doi: 10.1371/journal.pcbi.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry R. Cerebellar timing systems. In: Schmahmann J, editor. The Cerebellum and Cognition. San Diego: Academic Press; 1997. pp. 555–573. [Google Scholar]
- Ivry R, Corcos DM. Slicing the Variability Pie: Component Analysis of Coordination and Motor Dysfunction. In: Newell KM, Corcos M D, editors. Variability and Motor Control. Urbana, IL: Human Kinetics; 1993. pp. 415–447. [Google Scholar]
- Ivry RB, Keele SW, Diener HC. Dissociation of the lateral and medial cerebellum in movement timing and movement execution. Experimental Brain Research. 1988;73:167–180. doi: 10.1007/BF00279670. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Spencer RMC. The neural representation of time. Current Opinion in Neurobiology. 2004;14:225–232. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Ivry R, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. In: Highstein SM, Thach WT, editors. The Cerebellum: Recent developments in cerebellar research. Vol. 978. New York: New York Academy of Sciences; 2002. pp. 302–317. [DOI] [PubMed] [Google Scholar]
- Jancke L, Siegenthaler S, Preis S, Steinmetz H. Decreased white- matter density in a left-sided fronto-temporal network in children with developmental language disorder: Evidence for anatomical anomolies in a motor-language network. Brain and Language. 2006;102:91–98. doi: 10.1016/j.bandl.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Kail R. A method for studying the generalized slowing hypothesis in children with specific language impairment. Journal of Speech and Hearing Research. 1994;37:418–421. doi: 10.1044/jshr.3702.418. [DOI] [PubMed] [Google Scholar]
- Keele SW, Hawkins HL. Explorations of individual differences relevant to high level skill. Journal of Motor Behavior. 1982;14:3–23. doi: 10.1080/00222895.1982.10735259. [DOI] [PubMed] [Google Scholar]
- Keele SW, Ivry RB. Modular analysis of timing in motor skill. In: Bower GH, editor. The psychology of learning and motivation. Vol. 21. New York: Academic Press; 1987. pp. 183–228. [Google Scholar]
- Kent RD. Models of speech motor control: Implications from recent developments in neurophysiological and neurobehavioral science. In: Maasen B, Kent R, Peters H, van Lieshout P, Hulstijn W, editors. Speech motor control in normal and disordered speech. Oxford: Oxford University Press; 2004. pp. 3–28. [Google Scholar]
- Leonard LB. Children with specific language impairment. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Leonard LB, Ellis Weismer S, Miller CA, Francis DJ, Tomblin JB, Kail RV. Speed of Processing, Working Memory, and Language Impairment in Children. Journal of Speech Language and Hearing Research. 2007;50:408–428. doi: 10.1044/1092-4388(2007/029). [DOI] [PubMed] [Google Scholar]
- Lindblom B. Explaining phonetic variation: A sketch of the H & H theory. In: Hardcastle Marchal., editor. Speech production and speech modeling. Kluwer: Dordrecht; 1990. pp. 403–439. [Google Scholar]
- MacNeilage PF. The origin of speech. Oxford, England: Oxford University Press; 2008. [Google Scholar]
- McGregor KK, Leonard LB. Subject pronoun and article omissions in the speech of children with specific language impairment: A phonological interpretation. Journal of Speech and Hearing Research. 1994;37:171–181. doi: 10.1044/jshr.3701.171. [DOI] [PubMed] [Google Scholar]
- Mechsner F, Kerzel D, Knoblich G, Prinz W. Perceptual basis of bimanual coordination. Nature. 2001;414:69–73. doi: 10.1038/35102060. [DOI] [PubMed] [Google Scholar]
- Miller C, Kail R, Leonard L, Tomblin JB. Speech of processing in children with specific language impairment. Journal of Speech, Language, and Hearing Research. 2001;44:416–433. doi: 10.1044/1092-4388(2001/034). [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Missiuna C. Children with developmental coordination disorder: Strategies for success. New York: Haworth Press; 2001. [PubMed] [Google Scholar]
- Powell RP, Bishop DVM. Clumsiness and perceptual problems in children with specific language impairment. Developmental Medicine and Child Neurology. 1992;34:755–765. doi: 10.1111/j.1469-8749.1992.tb11514.x. [DOI] [PubMed] [Google Scholar]
- Rice M, Wexler K. Toward tense as a clinical marker of specific language impairment in English speaking children. Journal of Speech, Language, and Hearing Research. 1996;39:1239–1257. doi: 10.1044/jshr.3906.1239. [DOI] [PubMed] [Google Scholar]
- Riggen KJ, Ulrich DA, Ozmun JC. Reliability and concurrent validity of the test of motor impairment-henderson revision. Adapted Physical Activity Quarterly. 1990;7:249–258. [Google Scholar]
- Robertson S, Zelaznik H, Lantero D, Gadacz K, Spencer R, Doffin J, et al. Correlations for timing consistency among tapping and drawing tasks: Evidence against a single timing process for motor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1316–1330. doi: 10.1037//0096-1523.25.5.1316. [DOI] [PubMed] [Google Scholar]
- Smith A. The control of orofacial movements in speech. Critical Reviews in Oral Biology and Medicine. 1992;3:233–267. doi: 10.1177/10454411920030030401. [DOI] [PubMed] [Google Scholar]
- Spencer RM, Ivry RB, Zelaznik HN. Role of the cerebellum in movements: Control of timing or movement transitions? Experimental Brain Research. 2005;161:383–396. doi: 10.1007/s00221-004-2088-6. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Stark RE, Blackwell PB. Oral volitional movements in children with language impairments. Child Neuropsychology. 1997;3:81–97. [Google Scholar]
- Stark RE, Tallal P. Perceptual and motor deficits in language impaired children. In: Keith RW, editor. Central Auditory and Language Disorders in Children. San Diego: College-Hill Press; 1981. pp. 121–144. [Google Scholar]
- Tallal P, Miller S, Bedi G, Byma G, Wang X, Nagarajam S, Schreiner C, Jenkins W, Merzenich M. Language comprehension in language impaired children improved with acoustically modified speech. Science. 1996;271:81–84. doi: 10.1126/science.271.5245.81. [DOI] [PubMed] [Google Scholar]
- Tallal P, Stark RE. Speech acoustic-cue discrimination abilities of normally developing and language-impaired children. Journal of the Acoustical Society of America. 1980;69:568–574. doi: 10.1121/1.385431. [DOI] [PubMed] [Google Scholar]
- Tan S. Concurrent validity of motor tests used to identify children with motor impairment. Adapted physical activity quarterly. 2001;18:168–182. [Google Scholar]
- Thomson JM, Goswami U. Rhythmic processing in children with developmental dyslexia: Auditory and motor rhythms link to reading and spelling. Journal of Physiology-Paris. doi: 10.1016/j.jphysparis.2008.03.007. (in press) [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Maniela-Arnold E, Zhang X. Procedural learning in adolescents with specific language impairment. Language, Learning, and Development. 2007;3:269–293. [Google Scholar]
- Ullman MT, Pierpont EI. Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex. 2005;41:399–433. doi: 10.1016/s0010-9452(08)70276-4. [DOI] [PubMed] [Google Scholar]
- Van der Lely H. SLI in children: Movement, economy, and deficits in the computational-syntactic system. Language Acquisition. 1998;7:161–192. [Google Scholar]
- Wiart L, Darrah J. Review of four tests of gross motor development. Developmental Medicine & Child Neurology. 2001;43:279–285. doi: 10.1017/s0012162201000536. [DOI] [PubMed] [Google Scholar]
- Williams HG, Woollacott MH, Ivry RB. Timing and motor control in clumsy children. Journal of Motor Behavior. 1992;24:165–172. doi: 10.1080/00222895.1992.9941612. [DOI] [PubMed] [Google Scholar]
- Zelaznik HN, Spencer RMC, Ivry RB. Behavioral analysis of human movement timing. In: Grondin S, editor. Psychology of time. Bingley, United Kingdom: Emerald Group; 2008. pp. 233–260. [Google Scholar]
- Zelaznik HN, Smith A, Franz EA. Motor performance of stutterers and nonstutterers on timing and force control tasks. Journal of Motor Behavior. 1994;26:340–347. doi: 10.1080/00222895.1994.9941690. [DOI] [PubMed] [Google Scholar]
- Zelaznik HN, Smith A, Franz EA, Ho M. Differences in bimanual coordination associated with stuttering. Acta Psychologica. 1997;96:229–243. doi: 10.1016/s0001-6918(97)00014-0. [DOI] [PubMed] [Google Scholar]