Abstract
Poloxamers (known by the trade name Pluronic®) are triblock copolymer surfactants that contain two polyethylene glycol blocks and one polypropylene glycol block of various sizes. Poloxamers are widely used as nanoparticle dispersants for nanotoxicity studies wherein nanoparticles are sonicated with a dispersant to prepare suspensions. It is known that poloxamers can be degraded during sonication and that reactive oxygen species contribute to the degradation process. However, the possibility that poloxamer degradation products are toxic to mammalian cells has not been well studied. We report here that aqueous solutions of poloxamer 188 (Pluronic® F-68) and poloxamer 407 (Pluronic® F-127) sonicated in the presence or absence of multi-walled carbon nanotubes (MWNTs) can became highly toxic to cultured cells. Moreover, toxicity correlated with the sonolytic degradation of the polymers. These findings suggest that caution should be used in interpreting the results of nanotoxicity studies where the potential sonolytic degradation of dispersants was not controlled.
Keywords: nanotoxicology, nanoparticles, poloxamer, carbon nanotubes, ultrasound
Introduction
Non-ionic polyether surfactants, such as poloxamer triblock copolymers (known also by the trade name Pluronic®), are frequently used as dispersants to prepare various nanoparticle suspensions, especially with hydrophobic nanoparticles like carbon nanotubes and related material. Poloxamers contain two polyethylene glycol (PEG) blocks and one polypropylene glycol (PPG) block of various sizes and are popular for in vitro and in vivo nanotoxicity studies because they are relatively non-toxic dispersants. Typically, nanoparticles are sonicated with the desired poloxamer to produce a suspension using a variety of probe or bath sonicators with varying frequencies, power levels, and pulse regimes, and with sonication times from a few minutes up to 6 h or more. However, the application of ultrasound by sonication is well-known to degrade a variety of polymers by inducing cavitation bubbles that collapse and generate extreme local conditions, including high heat, pressure and shear forces (Neppiras 1984; Suslick 1990; Suslick & Flannigan 2008). Further, in aqueous media, sonication splits water into hydrogen atoms and hydroxyl radicals that combine to produce H2O2 and initiate free radical pathways of polymer degradation. For example, sonication in aqueous media is known to degrade poloxamers (Koda et al. 1994; Watanabe et al. 2010), PEG itself (Vijayalakshmi & Madras 2004; Vijayalakshmi & Madras 2005; Kawasaki et al. 2007), the PEG-containing surfactant C12E8 (Singla et al. 2009), and a lipid/PEG conjugate (Zeineldin et al. 2009). In most of these examples, free radical attacks on the polymers are proposed to contribute to their degradation, which also generates a variety of organic acids, alcohols, and aldehydes en route to oxidative degradation. Thus, sonication of polyether compounds has the potential to generate free radicals, reactive oxygen species (ROS), and organic compounds that are toxic. It is therefore important to assess whether sonication of polyether dispersants themselves may contribute to the toxicity of sonicated nanoparticle suspensions.
We studied here suspensions of multi-walled carbon nanotubes (MWNTs) prepared by sonication with the protein bovine serum albumin (BSA) or with two different poloxamers, Pluronic® F-68 (poloxamer 188) and Pluronic® F-127 (poloxamer 407). F-68 and F-127 were chosen for study because they are two representatives of the Pluronic® surfactant series that are widely used in dispersing carbon nanoparticles. MWNT suspensions prepared with F-68 or F-127, but not BSA, became highly toxic to cultured cells after probe or bath sonication, depending on sonication time, power, and frequency. Moreover, solutions of F-68 and F-127 became toxic when sonicated in the absence of MWNTs. For example, under very common conditions of probe sonication, material toxic to cultured mammalian cells was detected in F-68 and F-127 solutions within 5 min of sonication. The toxicity of the poloxamers correlated with their degradation during sonication. These results emphasize the importance of assessing sonication effects on dispersants used in nanotoxicity studies.
Methods
Tissue culture and other materials
Dulbecco’s modified Eagle medium (DMEM) and trypsin were purchased from Gibco (Grand Island, NY). Foetal bovine serum (FBS) was purchased from HyClone (Logan, UT). MWNT powder was purchased from Nanostructured & Amorphous Materials, Inc. (Houston, TX). The MWNT powder was produced by a catalytic chemical vapour deposition process, was >95% in purity, and comprised nanotubes with outer diameters ranging from 10 to 20 nm, inner diameters of 5–10 nm, and lengths of 0.5–2 μm, as described by the manufacturer. Caution: a fine particulates respirator should be worn when handling dry MWNT powders. BSA, Pluronic® F-68, Pluronic® F-127, and PEG were purchased from Sigma Aldrich (St. Louis, MO). The average molecular weights reported by the supplier were 66,430, 8,350, 12,600, and 8,000, respectively. All other chemicals were purchased from Sigma Aldrich and were used as received.
Sonication
Sonication was done with either a bath or a probe sonicator. Probe sonication used a 3-mm diameter probe attached to a Branson 250 Sonifier. The probe was centred 5 mm from the bottom of a 1.5 mL microcentrifuge tube that contained 1 mL of sample. The microcentrifuge tube was placed in ice-water and the sonicator was operated in a continuous mode at 10–21 W for 2–30 min. For bath sonication, a glass vial containing 10 mL of sample solution was secured in a floating rack and immersed in the centre of an ultrasonic bath unit (Elma P30H) that was operated at 60–120 W (50–100% power level) and 37 or 80 kHz frequencies. The temperature of the bath was kept below 18°C by using a cooling coil connected to a refrigerated water bath circulator (Isotemp 1006S) and by refilling the bath with ice-cold water at 30-min intervals. The position of a vial during sonication is a key variable in bath sonication (Nascentes et al. 2001; Taurozzi et al. 2011). Therefore, the bath was marked with a grid and each vial was sonicated sequentially in a central grid location. The temperature dependence of the critical micellar concentration of poloxamers is also a key variable. The F-127 and F-68 solutions at concentrations of 0.2 mM are below their critical micellar concentrations under the conditions of sonication used here (Alexandridis et al. 1994).
Preparation of MWNT suspensions
The bath sonication/centrifugation procedure described in our previous work (Wang et al. 2011) was used with slight modifications to disperse MWNT powder in either a 10 mg/mL BSA solution buffered with HEPES at pH 7.4 (HB), a 0.2 mM F-127 solution, or a 0.2 mM F-68 solution. In general, 10 mL of HB, F-127, or F-68 solution was added to a glass vial containing 10.0 mg as-received MWNT powder and sonicated. After sonication, 1 mL of the MWNT suspension was diluted to various concentrations and the absorbance at 500 nm was measured using a microplate reader (BioTek SynergyMx) to construct a calibration curve. The remaining suspension was centrifuged at 20,000 g for 5 min to remove MWNT bundles, metal catalysts, and other heavier impurities. The supernatant collected was marked as the stock MWNT suspension; MWNT-BSA suspensions were stored at 4°C and MWNT-F-68 and MWNT-F-127 suspensions were stored at room temperature. The concentration of MWNTs and related carbonaceous impurities in the resulting stock MWNT suspension was estimated from the absorbance at 500 nm based on the extinction coefficient obtained from the calibration curve.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE is a commonly used technique for separating proteins according to their electrophoretic mobility (Laemmli 1970). A slightly modified SDS-PAGE procedure was used to monitor the degradation of F-127 and F-68 based on changes in the electrophoretic migration of surfactant polymers in the presence of SDS (Kurfürst 1992). Briefly, a 4% stacking gel on top of a 15% resolving gel was prepared using a Hoefer Mini Vertical Gel Caster for 10 × 8 cm plates with 1.5-mm thick spacers and 10-well combs. Aliquots of dispersants or MWNT suspensions were mixed with 2X SDS sample loading buffer to a final concentration of 2% SDS and 5% 2-mercaptoethanol, and boiled for 3 min. Samples were subsequently loaded into the wells of the gel and an electric current was applied at a constant 100V for 2 h. Following electrophoresis, the gels were stained for polymers that contain PEG according to a modified procedure described by Kurfürst (1992). Briefly, gels were fixed in 50% methanol and 10% acetic acid for 10 min, rinsed with distilled water, and placed in a 5% barium chloride solution for 10 min. After rinsing with distilled water to remove excess barium chloride, gels were placed in 5% iodine solution for 5 min and rinsed with distilled water repeatedly until the yellow background was removed.
Dynamic light scattering (DLS)
The particle size distributions of dispersant solutions were analysed by DLS using a 633 nm laser source at a fixed angle of 173° (Zetasizer Nano-ZS 3600, Malvern Instrument, Worcestershire, UK). 0.2 mM F-68 and F-127 solutions were bath sonicated at 37 kHz and 120 W, or at 80 kHz and 100 W, for 10 – 240 min. One millilitre aliquots of non-sonicated and sonicated samples were placed in a disposable polystyrene cuvette and five consecutive measurements of twelve-10 s runs per measurement, were taken at 25°C.
Cell culture
Normal rat kidney (NRK) cells were obtained from the American Type Culture Collection (Manassas, VA) and cultured in DMEM supplemented with 3.7 mg/mL sodium bicarbonate and 10% (v/v) FBS in a 37 °C incubator with 90% air and 10% CO2. NRK cells were chosen for this study because they are a robust cell line and we have considerable data on how these cells respond to a variety of SWNTs (Wang et al. 2011) that would allow us to compare their responses to MWNTs. To determine the number of cells in a given sample, cells grown in tissue culture dishes were first detached using 0.05% (w/v) trypsin. Aliquots were diluted in an isotonic solution (Isoton II) and the number of cells was measured using a Beckman Coulter Particle Counter (Miami, FL).
Cytotoxicity assays
A standardized cytotoxicity assay described in our previous SWNT toxicity work was used with minor modifications (Wang et al. 2011). Briefly, the MWNT suspensions for cytotoxicity testing were prepared by diluting the stock MWNT suspensions with non-sonicated dispersant solutions to a MWNT concentration of 200 μg/mL. A MWNT concentration of 200 μg/mL was used because it is about the limit achievable with BSA as the dispersant after 1 h of sonication. MWNT suspensions were diluted 1:1 with 2X concentrated DMEM that contained 20% FBS, 20 mM HEPES, and the antibiotics Penicillin (200 U/mL) and Streptomycin (0.2 mg/mL) to give a final concentration of MWNTs at 100 μg/mL. The 0.2 mM poloxamer solutions sonicated in the absence of MWNTs were also diluted 1:1 with 2X concentrated media and the concentration was reduced to 0.1 mM in the testing media. Sonication of poloxamer solutions reduced the pH in the absence of buffers, but the pH was buffered to neutrality upon dilution with the HEPES-buffered testing media.
2 × 105 NRK cells/well were plated in 48-well plates in DMEM medium with 5% FBS and incubated at 37°C for 24 h. The regular cell culture media was replaced with freshly prepared testing media that contained either a MWNT suspension, dispersant, or control medium that contained no MWNT suspension or dispersant. Cells were incubated at 37°C for 24 h and washed three times with phosphate buffered saline. To determine the total amount of nucleic acid content in a well, 200 μL of 0.1% crystal violet in 10% ethanol (w/v) was added to each well and the plate was rocked for 20 min. Excess crystal violet was washed away with water and cell-associated crystal violet was extracted for 20 min with 200 μL of 10% acetic acid; 100 μL of each extract was transferred to a corresponding well in a 96-well plate. The absorbance of each extract was read at 590 and 700 nm with a microplate reader. The background absorbance at 700 nm was subtracted from that at 590 nm. All cytotoxicity assays were an average of at least four samples and were expressed as a per cent of the control, which was set at 100%.
Phase contrast light microscopy
NRK cells grown in four-well plates were incubated for up to 24 h at 37°C in testing media that contained 0.1 mM sonicated or non-sonicated poloxamer solutions. Bright field images of cells were acquired using an inverted light microscope (Nikon Eclipse TS100) equipped with a digital camera (Nikon DS-5M).
Dialysis
Cellulose ester dialysis tubing supplied in ready-to-use devices (Float-A-Lyzer® G2) were purchased from Spectrum Labs (Rancho Dominguez, CA). The approximate molecular weight cut off of the tubing was 300,000 Daltons. The devices were pre-washed with water according to the instructions provided by the manufacturer. Each dialysis device was loaded with 5 mL of MWNT suspension and placed in a beaker filled with 2 L of 0.2 mM non-sonicated F-68 or F-127 solutions. The samples were dialysed at room temperature with four solution changes within the first 24 h. One millilitre of sample was then removed and set aside for analysis. The remaining sample was dialysed for another 24 h with four more solution changes and a second sample was removed for analysis. The concentration of MWNTs and related carbonaceous impurities in the dialysed samples were estimated from the absorbance at 500 nm. Both dialysed and non-dialysed samples were analysed for toxicity by the crystal violet assay and for the presence of degradation products by SDS-PAGE as described in previous sections.
Results
F-68 and F-127 are better than BSA at dispersing MWNTs
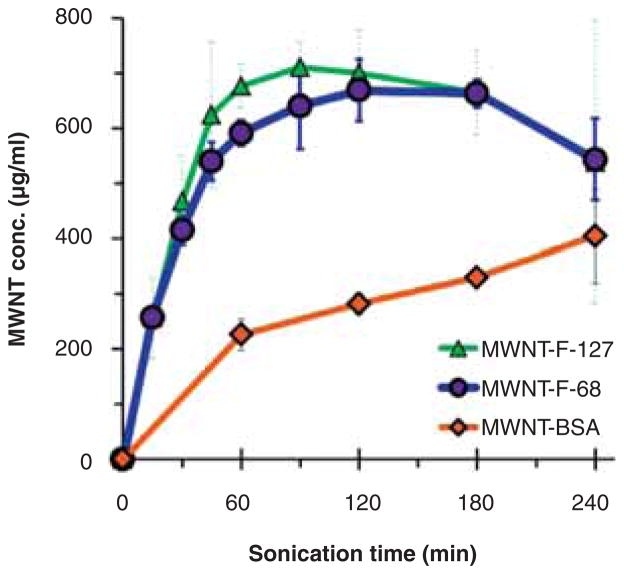
To compare the ability of BSA, F-68, and F-127 to disperse MWNTs,10 mg of dry MWNT powder was placed in a glass vial, 10 mL of each dispersant solution was added, and samples were bath sonicated at 37 kHz and 120 W for the desired times. The concentrations of MWNTs in the MWNT-F-68 and MWNT-F-127 suspensions increased rapidly within the first hour of sonication, reaching a maximum of ~650 μg/mL at 120 min, while the concentrations of MWNTs in MWNT-BSA suspensions increased slowly as sonication time increased, reaching a maximum of ~400 μg/mL after 240 min (Figure 1). These results indicated that the non-ionic triblock polymers F-68 and F-127 outperformed the protein BSA in dispersing MWNTs. All MWNT suspensions were stable in media of physiological salt and pH conditions, regardless of the sonication time and dispersants used in the sonication process.
Figure 1.
Effectiveness of F-68, F-127 or BSA in dispersing MWNTs using bath sonication at 37 kHz and 120 W. The MWNT suspensions were prepared by sonicating 10.0 mg MWNT powder with 10.0 mL of 0.2 mM F-68, F-127 or 10.0 mg/mL HEPES buffered BSA (pH 7.4) for the indicated length of time. Aliquots of the resulting suspension were centrifuged at 20,000 g for 5 min and 90% of the supernatant was collected from the top. The concentration of the MWNTs in the supernatant was determined by the absorbance at 500 nm, compared to that of the non-centrifuged sample. Each data point is the mean of three independent experiments ± SD.
Surprisingly, the concentrations of the MWNT-F-68 and MWNT-F-127 suspensions actually decreased after the third hour of sonication. To see if this trend continued to longer times, sonication was extended to 15 h for 0.2 mM F-68 at which time the concentration of MWNTs in the suspension dropped to nearly zero (data not shown). This suggested that the structure of the poloxamer polymers might be altered during sonication thereby reducing their effectiveness as dispersants.
F-68 and F-127 solutions become cytotoxic after sonication in the presence or absence of MWNTs
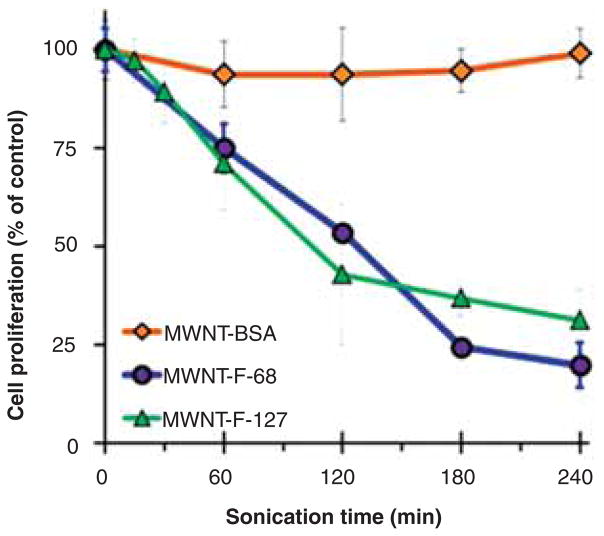
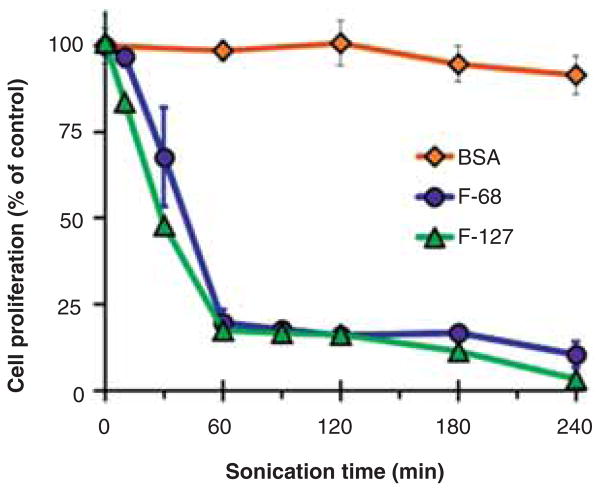
The cytotoxicity of MWNT suspensions prepared at different times after bath sonication at 37 kHz and 120 W was assessed in a proliferation assay with cultured NRK cells where the concentration of MWNTs was a constant 100 μg/mL. Suspensions prepared with F-68 or F-127 became highly cytotoxic with sonication time, whereas those prepared with BSA did not (Figure 2). Since the MWNT concentration was the same regardless of the dispersant, this suggested that the toxic material in the suspensions made with F-68 and F-127 might have been generated during sonication from the poloxamers themselves. When F-68 and F-127 were bath sonicated in the absence of MWNTs, they quickly became toxic, whereas sonicated BSA was not toxic (Figure 3).
Figure 2.
Cytotoxicity of MWNTs suspended in F-68, F-127 or BSA as a function of bath sonication time at 37 kHz and 120 W. After sonication, MWNT suspensions were diluted with non-sonicated F-68, F-127 or BSA solution to an equal MWNT concentration of 200 μg/mL. After dilution with an equal volume of 2X concentrated cell culture media, NRK cells were incubated for 24 h with the various MWNT suspensions at a final MWNT concentration of 100 μg/mL or in control media that contained no MWNTs. Cytotoxicity was assessed by cell proliferation, quantified by the crystal violet assay. Each data point is the mean of three independent experiments ± SD.
Figure 3.
Cytotoxicity of F-68, F-127 and BSA as a function of bath sonication time in the absence of MWNTs. F-68 and F-127 solutions at a concentration of 0.2 mM and HEPES buffered BSA (pH 7.4) at 10.0 mg/mL were sonicated in the absence of MWNTs for various lengths of time using an ultrasonic bath at 37 kHz and 120 W. NRK cells were incubated for 24 h in media containing various sonicated F-68, F-127, BSA solutions, or non-sonicated solutions as controls. The final concentration of F-68 or F-127 in media was 0.1 mM and that of BSA was 5 mg/mL. Cytotoxicity was assessed by cell proliferation, quantified by the crystal violet assay. Each data point is the mean of three independent experiments ± SD.
In comparing the results in Figures 2 and 3, sonicating the MWNT-F-68 suspension for 1 h only reduced cell proliferation to 75% (Figure 2), whereas F-68 sonicated alone for 1 h reduced cell proliferation to about 25% (Figure 3). This difference is explained by the fact that the sonicated F-68 sample was used without further dilution prior to mixing with 2X complete media such that the final concentration of sonicated poloxamer in the testing media was 0.1 mM. The MWNT suspensions, on the other hand, were diluted in fresh non-sonicated poloxamer to a MWNT concentration of 200 μg/mL prior to another twofold dilution in 2X complete media to achieve a final concentration of 100 μg/mL. Thus, the concentration of sonicated poloxamer in media containing MWNT suspensions was lower than 0.1 mM. IC50 values were determined from dose–response curves after bath sonication of 0.2 mM F-68 and F-127 at 37 kHz and 120 W for either 1 or 4 h. After 1 h of sonication, the IC50 values were 53.9 ± 6.5 and 18.6 ± 0.4 μM for F-68 and F-127, respectively, and after 4 h of sonication the values were 14.6 ± 2.5 and 7.2 ± 0.3 for F-68 and F-127, respectively. Thus, the longer a poloxamer was sonicated, the more toxic it became, and F-127 became more toxic than F-68 under similar sonication times.
The results in Figures 2 and 3 also raise the question of whether there is any interaction between the poloxamers or their degradation products and the MWNTs during sonication that alters the toxicity compared to sonication of poloxamers alone. This question can be addressed using the data in Figures 1 and 2 plus the known dose–response curves for the poloxamers that were used to determine the IC50 values for poloxamers sonicated alone. In Figure 1 after 1 h of sonication with 0.2 mM F-68, the concentration of the MWNTs was 591 μg/mL. When the dispersion was diluted to give 100 μg/mL MWNTs, used in the proliferation assay of Figure 2 at 1 h of sonication, the concentration of F-68 is calculated to be 0.034 mM. From Figure 2, cell proliferation was reduced to 75% of control values for the dispersion that contained 100 μg/mL MWNTs and 0.034 mM of F-68. From the dose–response curve of F-68 sonicated for 1 h alone, the prediction is that 0.034 mM F-68 would reduce cell proliferation to 69% of control values, suggesting a slight protective effect of MWNTs in decreasing the toxicity of F-68. A similar calculation for F-127 shows that 100 μg/mL MWNTs contained 0.030 mM F-127 and reduced cell proliferation to 71% of control values after 1 h of sonication (from Figure 2). The predicted decrease in cell proliferation of 0.030 mM F-127, sonicated for 1 h in the absence of MWNTs, is 31% of control values based on the dose–response curve, suggesting an even greater effect of MWNTs on decreasing the toxicity of F-127. One potential explanation for this difference is that MWNTs bind and sequester toxic hydrophobic poloxamer degradation products and that F-127 generates more of these toxic products because it has a larger hydrophobic block than F-68; however, further work would be necessary to better understand the mechanism underlying this effect.
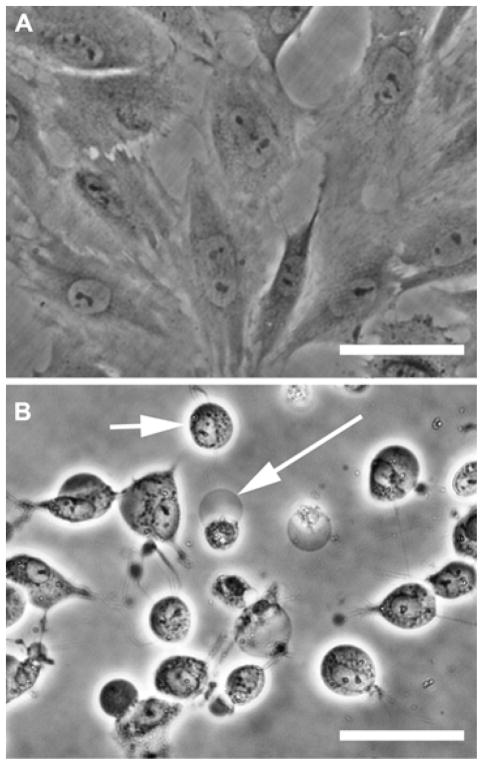
Whether sonicated poloxamers are cytostatic or cytotoxic is not distinguished by the cell proliferation assay. However, phase contrast images of cells treated with F-68 or F-127 sonicates showed dead and dying cells. For example, exposing NRK cells for 12 h to F-68 that had been sonicated for 4 h revealed rounded cells and plasma membrane blebs characteristic of dead and dying cells, whereas untreated cells had typical fibroblast morphology (Figure 4). Nascent membrane blebs and rounded cells were also evident in cell cultures within an hour after treatment with sonicated polymers (image not shown). These data are evidence that the failure of NRK cells to proliferate after treatment with sonicated poloxamers is the result of a cytotoxic mechanism rather than a cytostatic effect.
Figure 4.
Phase contrast images of NRK cells exposed to sonicated F-68. F-68 (0.2 mM) was either bath sonicated in the absence of MWNTs for 4 h at 37 kHz and 120 W, or not sonicated. The samples were diluted with an equal volume of medium and incubated with NRK cells for 12 h. (A) Control cells exposed to F-68 that was not sonicated. (B) Cells exposed to F-68 that had been sonicated for 4 h. The long arrow points to a membrane bleb and the short arrow points to a rounded cell. Scale bar is 50 μm.
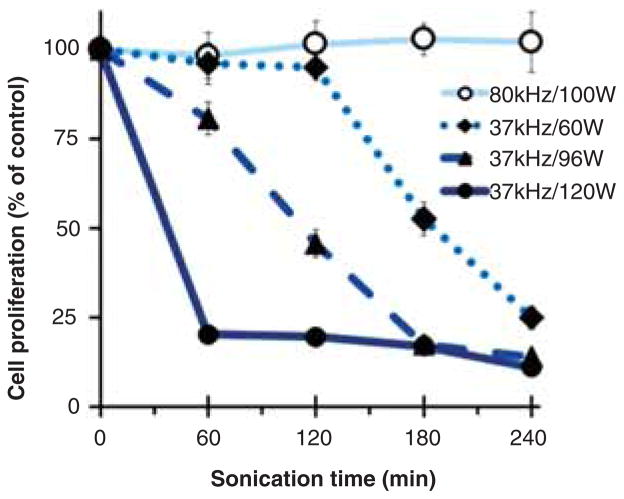
It is well-known that the degradation of polymers is affected by ultrasound frequency and power (Suslick 1990; Suslick & Price 1999). We therefore investigated the effect of bath sonication power and frequency on the generation of toxic materials in F-68 solutions. F-68 (0.2 mM) was bath sonicated for 1–4 h at a frequency of 37 kHz and at a power level of 120, 96, or 60 W, or at a higher frequency of 80 kHz with a power level of 100 W. Toxicities of the non-sonicated and sonicated F-68 samples were determined after 24 h exposure with NRK cells. As shown in Figure 5, toxic products were generated most rapidly by sonication at 37 kHz and 120 W, and reducing the power at 37 kHz to either 96 W or 60 W reduced, but did not eliminate, the production of toxic material. However, increasing the frequency to 80 kHz at 100 W failed to produce toxic material even after 240 min of sonication.
Figure 5.
Effects of bath sonication time, power and frequency on the cytotoxicity of F-68. 10 mL of 0.2 mM F-68 was sonicated in the absence of MWNTs for the indicated length of time using an ultrasonic bath operated either at 37 kHz with a power level of 60, 96 and 120 W or at 80 kHz with a power level of 100 W. NRK cells were incubated for 24 h in media containing various sonicated F-68 samples or non-sonicated solutions as control. The final concentration of F-68 in media was 0.1 mM. Cytotoxicity was assessed by cell proliferation and quantified by the crystal violet assay. Each data point is the mean of three independent experiments ± SD.
The generation of toxic material after probe sonication at 20 kHz and 18 W was also tested where the cavitation power is concentrated at the tip of the probe, is delivered directly to the liquid, and is not dissipated through the walls of the glass vial holding the sample. Figure S1 shows that exposing cells to F-68 and F-127 that had been probe sonicated for as little as 5 min resulted in reduced cell proliferation. The toxicity of F-68 and F-127 subjected to a 20-min probe sonication at 20 kHz with power levels of 10, 14, 18, or 21 W was also tested. Similar to the results obtained using bath sonication, both F-68 and F-127 became more toxic to NRK cells after probe sonication at higher power levels (Figure S2A).
A structural feature of both F-68 and F-127 is PEG. We therefore asked whether sonicating PEG alone would generate toxic material. Indeed, a solution of 0.2 mM PEG (average molecular weight of 8000) alone also became highly toxic after bath sonication in less than 15 min (Figure S3A). Altogether, these data suggest that bath sonicating or probe sonicating F-68 and F-127 in the presence or absence of MWNTs generates toxic material that is a function of sonication time, frequency and power. The toxic material most likely originates from the polyether backbone of the poloxamer since sonicating BSA did not generate toxic products, whereas sonicating PEG alone did.
Detecting degradation of F-68, F-127 and PEG by SDS-PAGE and DLS
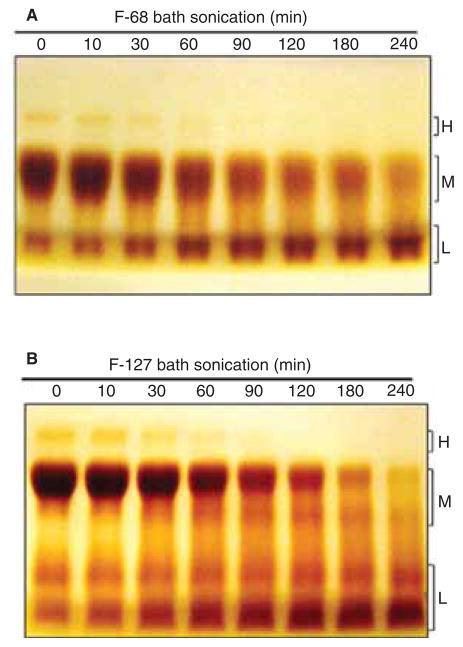
The collapse of cavitation bubbles generated during sonication can create sufficient heat, pressures, and shear forces to degrade polymers containing PEG, PPG or both (Koda et al. 1994; Suslick & Price 1999; Vijayalakshmi & Madras 2004; Vijayalakshmi & Madras 2005; Kawasaki et al. 2007; Singla et al. 2009; Zeineldin et al. 2009; Watanabe et al. 2010). To determine whether F-68 and F-127 were degraded during sonication, we analysed changes in their size as a function of bath sonication time by SDS-PAGE, detecting the polymers in the gel by staining with BaI2 (Kurfürst 1992). In the absence of sonication, gels loaded with F-68 and F-127 showed bands in mainly three regions of the gel, designated high molecular mass (H), medium molecular mass (M), and low molecular mass (L), with the majority of the material in the M region (Figure 6A and B). As bath sonication proceeded, the intensity of bands in the H and M regions declined and bands in the L region increased, suggesting that larger poloxamer polymers were degraded during sonication to smaller material. F-68 and F-127 were also degraded when bath sonicated at 37 kHz and 120 W in the presence of MWNTs (data not shown, but see Figure 8). The effect of probe sonication on F-68 and F-127 as a function of power is shown in Figure S2B. As the power increased from 0 to 21 W, more degradation was observed and the amount of degradation correlated with toxicity (Figure S2A). PEG alone also showed time-dependent degradation in bath sonication (Figure S3B) that correlated with toxicity (Figure S3A). The degradation of F-68 (at 0.2 mM, which is below the critical micellar concentration at 25°C) by bath sonication at 37 kHz and 120 W was also confirmed by DLS. The hydrodynamic diameter of non-sonicated F-68 monomer was 5.7 ± 1.2 nm, which is similar to results reported in the literature (Bahadur et al. 1992; Zhou & Chu 1988). Within 15 min of sonication, the percent intensity of the F-68 peak area had declined by ~50% and after 180 min of sonication no particles in the 4–6 nm region were detected, verifying that sonication degraded poloxamers.
Figure 6.
Degradation of F-68 and F-127 as a function of bath sonication time at 37 kHz and 120 W. Representative gels were loaded with (A) F-68 or (B) F-127 samples following bath sonication for various lengths of time. Intact polymer and degradation products in the sonicated and non-sonicated samples were detected by SDS-PAGE followed by BaI2 staining. BaI2 stained bands were denoted H, M and L corresponding to the high, medium and low molecular mass, respectively.
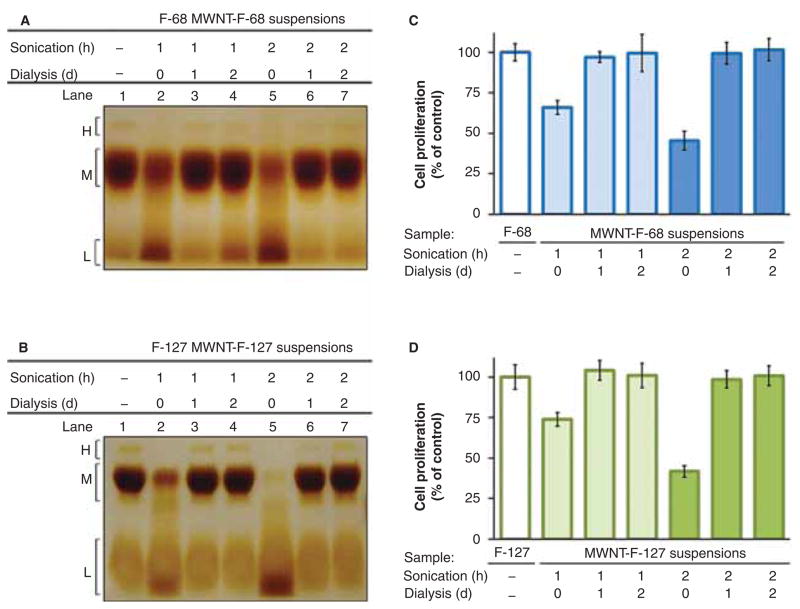
Figure 8.
Effects of dialysis on the removal of degradation products and toxicity in MWNT-F-68 and MWNT-F-127 suspensions after bath sonication. MWNT suspensions were prepared by bath sonicating in 0.2 mM F-68 or F-127 at 37 kHz and 120 W for 1 or 2 h followed by centrifugation at 20,000 g for 5 min. The suspensions were dialysed against non-sonicated 0.2 mM F-68 or F-127 for 1 or 2 days using a dialysis membrane with a 300 kDa molecular weight cut-off. Representative gels were loaded with (A) MWNT-F-68 or (B) MWNT-F-127 suspensions before and after dialysis. Degradation products from the samples in the gels were detected by SDS-PAGE followed by BaI2 staining. BaI2 stained bands were denoted H, M and L corresponding to the high, medium and low molecular mass, respectively. The cytotoxicity of (C) MWNT-F-68 or (D) MWNT-F-127 suspensions and their corresponding dialysed samples was assessed by 24-h incubation of NRK cells in media containing various MWNT suspensions at a final concentration of 100 μg/mL or in the absence of MWNTs as control, quantified by crystal violet assay. Each data point is the mean of three independent experiments ± SD.
In the previous section, we showed that sonicating F-68 at 80 kHz and 100 W did not generate toxic materials (Figure 5). If there is a correlation between degradation and toxicity, sonicating MWNTs with F-68 or F-127 at 80 kHz and 100 W should not degrade the polymers. When this prediction was tested, there was no apparent degradation of either F-68 or F-127 even after 12 h of sonication in the presence of MWNTs (Figure S4). Together, the results in this section demonstrate a correlation between F-68 or F-127 degradation and production of toxic material upon sonication at 37 kHz (bath) or 20 kHz (probe) and that both toxicity and degradation are functions of sonication power.
Glutathione partially protects cells from sonicated F-68
The sonolytic degradation of poloxamer triblock copolymers and of PEG is believed to occur through ROS and free radical mechanisms (Kawasaki et al. 2007; Watanabe et al. 2010; Singla et al. 2009). Many ROS are short-lived and to see whether toxicity in sonicated samples persisted, we measured the loss in toxicity of sonicated F-68 over a period of 8 weeks after sonication. There was no significant loss in the toxicity to NRK cells, suggesting that the toxic species was not exclusively a short-lived ROS (data not shown). H2O2 is a relatively long-lived ROS and to determine whether toxic levels of H2O2 were generated during bath sonication of F-68 at 37 kHz and 120 W, catalase was added immediately after sonication to enzymatically break down H2O2 prior to toxicity testing. Catalase protected cells from a non-sonicated control solution of F-68 that was spiked with lethal levels of H2O2, but did not protect cells from sonicated F-68 (data not shown). Thus, there did not appear to be toxic levels of H2O2 in the sonicated material.
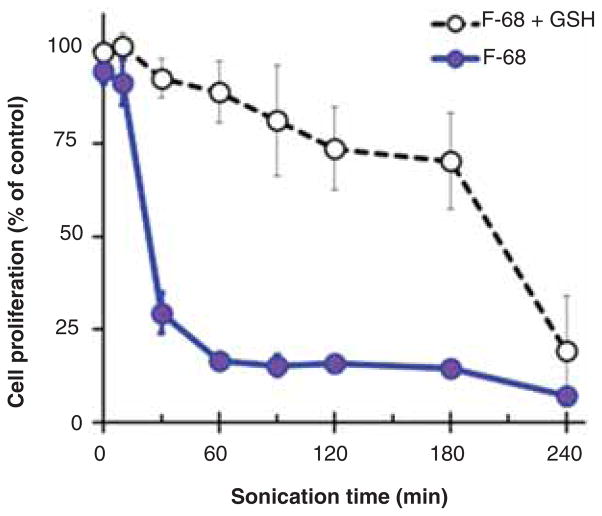
We next determined whether the antioxidant glutathione, well-known to neutralize free radicals and ROS, protected cells from the toxic material in sonicated F-68. F-68 was bath sonicated for different times and freshly prepared glutathione (10 mM) was added prior to mixing with cell culture media. Cells were then exposed to the F-68 samples for 24 h. As shown in Figure 7, the toxicity of sonicated F-68 samples was partially reduced in the presence of glutathione, but after 240 min of sonication, glutathione had little effect. This suggests that glutathione may quench damaging free radicals and ROS present during early times of sonication; however, as time goes on, stable toxic degradation products accumulate that are not affected by glutathione.
Figure 7.
Effect of glutathione on the cytotoxicity of F-68 after bath sonication. F-68 at a concentration of 0.2 mM was bath sonicated at 37 kHz and 120 W. NRK cells were incubated for 24 h in media containing either sonicated or non-sonicated F-68. Freshly prepared glutathione was added to the media at the beginning of the incubation. The final concentrations of F-68 and glutathione in media were 0.1 and 10.0 mM, respectively. Control cells were incubated in medium that contained no F-68 or glutathione. Cytotoxicity of sonicated F-68 in the presence or absence of glutathione was quantified using the crystal violet assay. Each data point is the mean of three independent experiments ± SD.
Detoxification of MWNT suspensions and the removal of poloxamer fragments by dialysis
Since F-68 and F-127 are such effective dispersants and have been so widely used in the nanomaterials field, an important question is whether conditions can be developed that preserve their ability to disperse materials without the problems of toxic products generated by sonication. One possibility is to disperse material by bath sonication at 80 kHz and 100 W because toxic degradation products are not generated under these conditions (see Figure 5 and Figure S4). However, bath sonicating MWNTs with either F-68 or F-127 at 80 kHz and 100 W for 2 h resulted in suspensions containing MWNT concentrations of only ~50 μg/mL, compared to over 600 μg/mL after sonication at 37 kHz and 120 W. Thus, sonicating under mild conditions avoids toxic degradation products, but at the expense of a tenfold reduction in the amount of dispersed MWNTs.
An alternative approach is to dialyse suspensions sonicated at 37 kHz and 120 W against an external solution containing non-sonicated F-68 or F-127. This should remove toxic poloxamer degradation products and replace them with intact polymer to prevent re-aggregation of MWNTs. To test this idea, MWNTs were bath sonicated at 37 kHz and 120 W for 1 or 2 h with 0.2 mM F-68 or F-127, centrifuged, and dialysed for 1 or 2 days against buffer containing 0.2 mM F-68 or F-127 using a membrane with an average molecular weight cut-off of 300 kDa. Samples were tested for toxicity and analysed by SDS-PAGE to monitor the presence of polymer degradation products. Representative gels loaded with MWNT-F-68 and MWNT-F-127 suspensions before and after dialysis are shown in Figures 8A and 8B. Non-sonicated F-68 and F-127 aliquots were loaded as references in lane 1. Without subsequent dialysis, sonication for 1 or 2 h showed a decrease in the intensity of bands in H and M regions with the expected increase in the intensity of lower molecular weight bands in the L region. After dialysis the intensities of bands in the H, M, and L regions were restored to pre-sonication levels, indicating that degraded polymer had been removed and replaced by intact polymer. Figures 8C and 8D show the toxicity of the corresponding MWNT-F-68 and MWNT-F-127 suspensions before and after dialysis compared to those of the non-sonicated F-68 and F-127 solutions. Before dialysis, both MWNT-F-68 and MWNT-127 suspensions were toxic to NRK cells after 24 h incubation. As expected, suspensions sonicated for 2 h were more toxic than those sonicated for 1 h. After dialysis, however, none of the suspensions were toxic to the cells. The MWNT concentrations remained constant before and after dialysis suggesting that the MWNTs were stably dispersed throughout the dialysis process. Altogether these results demonstrate that a well-dispersed non-toxic MWNT suspension with a high MWNT concentration can be prepared with F-68 or F-127 using a sonication and dialysis approach.
Discussion
When the ability of BSA and two poloxamers (Pluronic® F-68 and F-127) to suspend MWNTs upon sonication was compared, the poloxamers were much more efficient than BSA. However, we also noticed that the concentration of suspended MWNTs first increased and then declined as a function of sonication time in both F-68 and F-127. This suggested that the structure of the poloxamers might be altered by sonication to a form that was no longer an effective dispersant, leading to re-aggregation of the MWNTs. Indeed, the sonolytic degradation of PEG/PPG block copolymers and related polyethers in aqueous solution is well documented in the literature (Koda et al. 1994; Vijayalakshmi & Madras 2004; Vijayalakshmi & Madras 2005; Kawasaki et al. 2007; Singla et al. 2009; Zeineldin et al. 2009; Watanabe et al. 2010). However, the highly toxic nature of the degradation products to mammalian cells has not been previously reported to our knowledge.
When the toxicity of MWNTs sonicated with either BSA or poloxamers was compared, it was evident that MWNTs suspended in poloxamers became highly toxic during sonication whereas MWNTs in BSA did not. Several lines of evidence support the conclusion that the toxic material in MWNT/poloxamer suspensions is derived from the poloxamers, and not associated with the MWNTs. First, MWNTs sonicated with BSA did not become toxic, while MWNTs sonicated with the poloxamers did. Second, sonicating BSA alone did not generate toxic material, while sonicating the poloxamers alone produced highly toxic solutions. Poloxamer solutions became toxic after either bath or probe sonication and toxicity was a function not only of sonication time, frequency, and power, but also of poloxamer concentration. Third, bath sonicating (37 kHz, 120 W) PEG alone, a structural block shared by F-68 and F-127, produced a highly toxic solution.
Additional evidence correlated the toxicity of sonicated F-68 and F-127 with poloxamer degradation. SDS-PAGE of F-68 and F-127, coupled with chemical detection of polymer bands stained with BaI2, showed a decline in the intensities of high and medium molecular weight bands and an increase in the intensities of low molecular weight bands as a function of sonication time with either bath or probe sonicators. Generation of the low molecular weight bands was a function of sonication time, frequency, and power that correlated with the toxicity of the solutions after sonication. For example, bath sonicating F-68 and F-127 at 37 kHz and 120 W, or probe sonicating at 20 kHz and 21 W, both degraded the polymers and produced toxic solutions, whereas bath sonicating at 80 kHz and 100 W did not degrade the polymers, nor did it produce toxic solutions. DLS analyses performed below the critical micellar concentration of F-68 also showed loss of intact polymer peaks upon bath sonication at 37 kHz and 120 W. This is consistent with theory and experiments that there is an inverse relationship between frequency and the size of cavitation bubbles and that larger bubbles generated at lower frequencies generally create more extreme heat and pressure when they implode (Suslick 1990; Sostaric & Riesz 2002; Suslick & Flannigan 2008).
The implosion of cavitation bubbles in aqueous solution generates temperatures in excess of 5,000 °C and cleaves water to hydrogen atoms and hydroxyl radicals, which then recombine to generate H2O2 (Suslick 1990; Suslick & Flannigan 2008). Mass spectrometry studies on the sonolytic degradation of PEG, PEG/PPG copolymers, and a PEG-containing surfactant (C12E8), all suggest a free radical mechanism of degradation (Kawasaki et al. 2007; Singla et al. 2009; Watanabe et al. 2010). Thus, one candidate for a toxic component present in sonicated poloxamer solutions was H2O2, but treating poloxamer sonicates with catalase to degrade H2O2 did not reduce toxicity. It is possible, however, that H2O2 produced by sonolysis of water was rapidly consumed in oxidation of the poloxamers and did not accumulate to a toxic steady state level. The free radical scavenger glutathione partially detoxified poloxamer sonicates, but the effect declined with sonication time and was almost absent after 2 h of sonication. This suggests that there may be at least two types of toxic substances in poloxamer sonicates that change as a function of time: Free radical intermediates associated with the polymer backbone present during early stages of degradation, but which decline as oxidation of the polymer proceeds towards completion, and toxic organic degradation products, perhaps small molecular weight aldehydes and carboxylic acids that have been previously detected in sonicated poloxamer solutions (Watanabe et al. 2010). Further studies are necessary to better identify the toxic species generated by sonolysis of poloxamers.
Poloxamers and related non-ionic surfactants are efficient and popular surfactants for suspending carbon nanotubes of various types by sonication (Ciofani et al. 2008; Mejia et al. 2011), and it would be useful to develop conditions where they may still be used without the disadvantage of producing toxic degradation products. One approach is to sonicate at 80 kHz and 100 W where no polymer degradation was observed; however, exfoliation and suspension MWNTs under these conditions was very inefficient. An alternative was to sonicate with conditions that give optimum MWNT suspension, and then replace degraded surfactant with intact surfactant by dialysis. We found that dialysing MWNT suspensions prepared with either F-68 or F-127 against non-sonicated surfactant completely replaced the degraded polymers and detoxified the MWNT suspensions. The duration of dialysis to reduce the concentration of toxic poloxamer products may vary for different cell types whose sensitivity to the toxic products may be different than NRK cells. The dialysis method also has the advantage that the structure of the poloxamer coating the MWNTs is defined after intact surfactant replaces degraded surfactant. This is an important point because even if a sonicated suspension is diluted to reduce toxicity, alterations in the structure of the surfactant that coats the nanoparticles may introduce variables in cell experiments. There is an example of this in the literature where single-walled carbon nanotubes (SWNTs) suspended by sonication with a lipid/PEG construct had structurally altered PEG associated with the SWNTs that affected the interaction of the construct with cells (Zeineldin et al. 2009).
The nanotoxicity literature is complex and often contains conflicting results and conclusions. Difficulties in reproducibly preparing nanoparticle suspensions by sonication across a spectrum of instruments and in different laboratories can be a major source of inconsistencies, as discussed recently by Taurozzi et al. (2011). The actual power of the acoustic field delivered to a sample cannot be assumed to be the same as the power setting and can be difficult to control. For bath sonication, position in the bath is one of the crucial factors (Nascentes et al. 2001; Taurozzi et al. 2011). For probe sonication, the condition of the probe tip and the volume of liquid are among the important variables. Taurozzi et al. (2011) also warn that sonolysis of polymers during dispersion can be a problem. The results presented here provide an example of the latter for PEG and the widely used poloxamer surfactant series related to PEG. It would be prudent in nanotoxicity studies to test the toxicity of a polymer sonicated alone to determine whether polymer sonolysis may contribute to toxicity or other cell responses.
Conclusions
We conclude that suspensions of MWNTs prepared with Pluronic® F-68 (poloxamer 188) or Pluronic® F-127 (poloxamer 407), but not BSA, may become highly toxic to cultured cells after sonication with either bath or probe sonicators, depending on sonication time, frequency, and power. A search on Google Scholar using all the four terms “carbon”, “nanotubes”, “toxicity” and “pluronic” (as of July, 2012, patents and legal documents excluded) returned over 1400 articles, an estimate of how widely used these polymers are in nanotoxicity studies with carbon nanotubes. Whereas some reports in the literature did not use conditions likely to degrade poloxamers, a sampling of the articles revealed many where nanoparticles were sonicated under conditions that are likely to have produced suspensions containing toxic poloxamer degradation products. Thus, caution should be used in interpreting the results of nanotoxicity studies in the literature where the sonolytic degradation of dispersants has not been eliminated as a potential source of toxic material.
Supplementary Material
Acknowledgments
We are grateful to Dr. Carole Mikoryak for valuable discussions and for reading the manuscript, to Dr. Jie Zheng for assistance with initial DLS data, and to Prashant Raghavendran for early work on MWNTs that was not included in the manuscript.
Footnotes
Declaration of interest
The authors thank the University of Arizona SEMATECH/Semiconductor Research Corporation Engineering Research Center for Environmentally Benign Semiconductor Manufacturing (Grants ERC425-027 and ERC425-042) and the National Cancer Institute (Grant R15-CA152917) for partially supporting this work. The authors report no conflict of interest. The authors alone are responsible for the content and writing the manuscript.
References
- Alexandridis P, Holzwarth JF, Hatton TA. Micellization of poly (ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) tri-block copolymers in aqueous solutions: Thermodynamics of copolymer association. Macromolecules. 1994;27:2414–2425. [Google Scholar]
- Bahadur P, Li P, Almgren M, Brown W. Effect of potassium fluoride on the micellar behavior of Pluronic F-68 in aqueous solution. Langmuir. 1992;8:1903–1907. [Google Scholar]
- Ciofani G, Raffa V, Pensabene V, Menciassi A, Dario P. Dispersion of multi-walled carbon nanotubes in aqueous Pluronic F127 solutions for biological applications. Fullerenes Nanotubes Carbon Nanostructures. 2008;17:11–25. [Google Scholar]
- Kawasaki H, Takeda Y, Arakawa R. Mass spectrometric analysis for high molecular weight synthetic polymers using ultrasonic degradation and the mechanism of degradation. Anal Chem. 2007;79:4182–4187. doi: 10.1021/ac062304v. [DOI] [PubMed] [Google Scholar]
- Koda S, Mori H, Matsumoto K, Nomura H. Ultrasonic degradation of water-soluble polymers. Polymer (Guildf) 1994;35:30–33. [Google Scholar]
- Kurfürst MM. Detection and molecular weight determination of polyethylene glycol-modified hirudin by staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal Biochem. 1992;200:244–248. doi: 10.1016/0003-2697(92)90460-o. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mejia J, Tichelaar F, Saout C, Toussaint O, Masereel B, Mekhalif Z, et al. Effects of the dispersion methods in Pluronic F108 on the size and the surface composition of MWCNTs and their implications in toxicology assessment. J Nanopart Res. 2011;13:655–667. [Google Scholar]
- Nascentes CC, Korn M, Sousa CS, Arruda MAZ. Use of ultrasonic baths for analytical applications: a new approach for optimisation conditions. J Braz Chem Soc. 2001;12:57–63. [Google Scholar]
- Neppiras EA. Acoustic cavitation series: part one: Acoustic cavitation: an introduction. Ultrasonics. 1984;22:25–28. [Google Scholar]
- Singla R, Grieser F, Ashokkumar M. Kinetics and mechanism for the sonochemical degradation of a nonionic surfactant. J Phys Chem A. 2009;113:2865–2872. doi: 10.1021/jp808968e. [DOI] [PubMed] [Google Scholar]
- Sostaric JZ, Riesz P. Adsorption of surfactants at the gas/solution Interface of cavitation bubbles: an ultrasound intensity-independent frequency effect in sonochemistry. J Phys Chem B. 2002;106:12537–12548. [Google Scholar]
- Suslick KS. Sonochemistry. Science. 1990;247:1439–1445. doi: 10.1126/science.247.4949.1439. [DOI] [PubMed] [Google Scholar]
- Suslick KS, Flannigan DJ. Inside a collapsing bubble: Sonoluminescence and the conditions during cavitation. Annu Rev Phys Chem. 2008;59:659–683. doi: 10.1146/annurev.physchem.59.032607.093739. [DOI] [PubMed] [Google Scholar]
- Suslick KS, Price GJ. Applications of ultrasound to materials chemistry. Annu Rev Mater Sci. 1999;29:295–326. [Google Scholar]
- Taurozzi JS, Hackley VA, Wiesner MR. Ultrasonic dispersion of nanoparticles for environmental, health and safety assessment –issues and recommendations. Nanotoxicology. 2011;5:711–729. doi: 10.3109/17435390.2010.528846. [DOI] [PubMed] [Google Scholar]
- Vijayalakshmi SP, Madras G. Effect of temperature on the ultrasonic degradation of polyacrylamide and poly(ethylene oxide) Polym Degradat Stab. 2004;84:341–344. [Google Scholar]
- Vijayalakshmi SP, Madras G. Effect of initial molecular weight and solvents on the ultrasonic degradation of poly(ethylene oxide) Polym Degradat Stab. 2005;90:116–122. [Google Scholar]
- Wang R, Mikoryak C, Li S, Bushdiecker D, Musselman IH, Pantano P, et al. Cytotoxicity screening of single-walled carbon nanotubes: detection and removal of cytotoxic contaminants from carboxylated carbon nanotubes. Mol Pharm. 2011;8:1351–1361. doi: 10.1021/mp2001439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Okabayashi M, Kurokawa D, Nishimoto Y, Ozawa T, Kawasaki H, et al. Determination of primary bond scissions by mass spectrometric analysis of ultrasonic degradation products of poly(ethylene oxide-block-propylene oxide) copolymers. J Mass Spectrom. 2010;45:799–805. doi: 10.1002/jms.1771. [DOI] [PubMed] [Google Scholar]
- Zeineldin R, Al-Haik M, Hudson LG. Role of polyethylene glycol integrity in specific receptor targeting of carbon nanotubes to cancer cells. Nano Lett. 2009;9:751–757. doi: 10.1021/nl8033174. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Chu B. Light-scattering study on the association behavior of triblock polymers of ethylene oxide and propylene oxide in aqueous solution. J Colloid Interface Sci. 1988;126:171–180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.