The scaling of behavioral performance and neuronal responses with visual luminance contrast is one of the most basic and well-accepted aspects of visual processing [1, 2]. This relationship between contrast and visual perception can be experienced informally (e.g., by driving in fog), and numerous studies confirm that many visual skills are impaired under low-contrast conditions. However, a number of studies show a more complex interaction between contrast and visual processing. For single neurons the spatiotemporal structure of visual receptive fields is different under conditions of low and high contrast [3–9], and psychophysical experiments reveal counterintuitive cases where low-contrast conditions change the content [10] and even improve the accuracy [11] of visual perception. Here, we evaluated subjects’ ability to estimate the direction of moving dot fields presented at high or low contrast and compared these results to neural responses recorded from MT of alert macaque monkeys. We find that motion stimuli involving small spatial displacements yield better visual performance at lower contrast, and that this improvement is mirrored in the activity of MT neurons. These data link contrast-adaptive responses in area MT with behavioral performance and demonstrate that brighter is not always better for motion processing.
We first tested subjects (n=8) on their ability to estimate the motion direction of random dot fields presented at high (119 cd/m2) or low (12.7 cd/m2) contrast on a dark background (4.5 cd/m2), while varying the amount of displacement undergone by the stimulus dots on each monitor refresh. In this task, subjects viewed a dot field for 400 ms and reported the direction by rotating the orientation of a response bar. Stimuli were shown at variable directions and temporal displacements (see supplemental methods), and we manipulated motion coherence to maintain an appropriate level of task difficulty (see 100% coherence control below). We found (Figure 1a) that motion perception depended both on stimulus contrast and displacement. Surprisingly, subjects were better at determining the motion direction of stimuli with small displacements at low contrast (blue) than at high contrast (red). For larger displacements this effect reversed. The interaction between contrast and displacement was highly significant (p<.0001; repeated-measures ANOVA) and largely independent of temporal displacement (see supplemental data: Table S2 and Figure S5).
Figure 1.
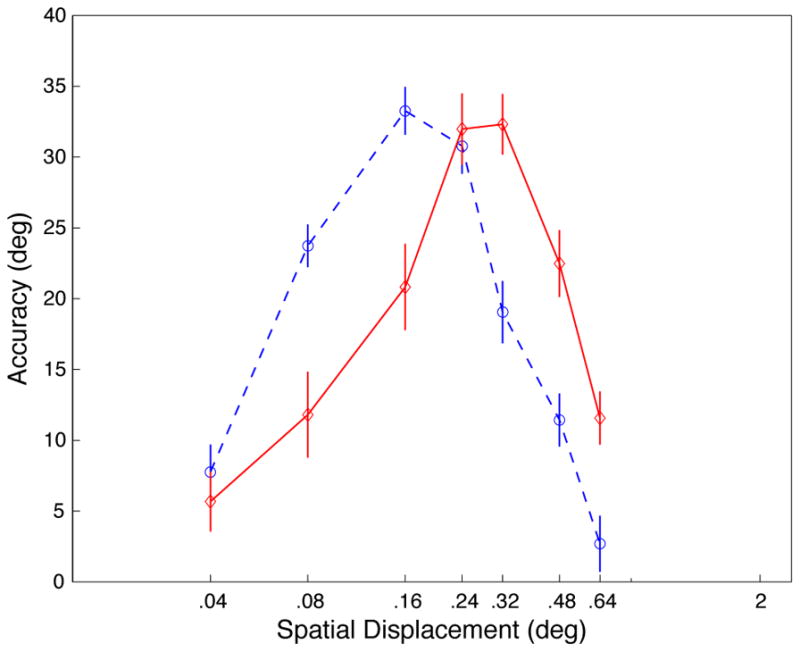
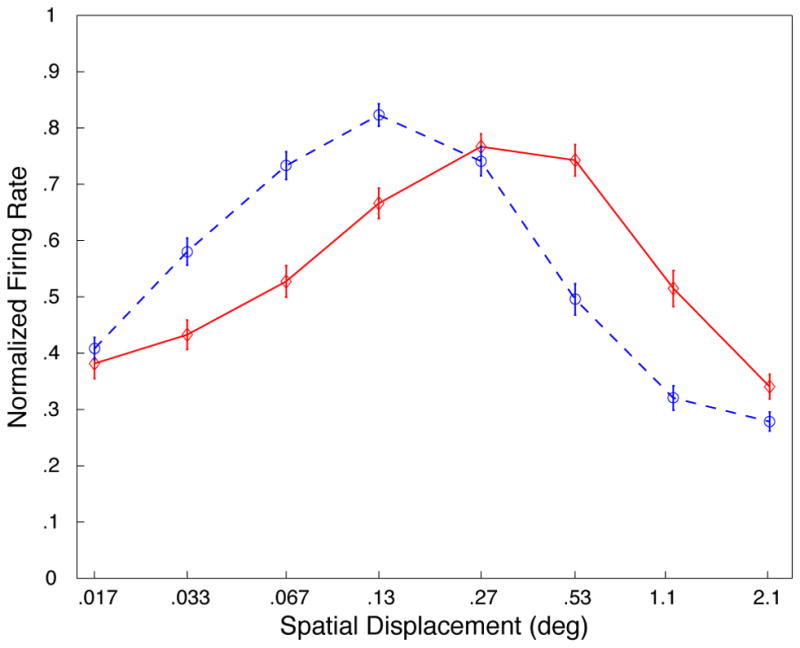
A, Results of behavioral experiments. Displacement tuning curves for low contrast (blue; dashed) and high contrast (red; solid). Data are averaged across direction, coherence, and temporal displacement (data for different coherences and TDs shown in supplemental Figures S3, S4, S5). Accuracy is computed as the average absolute error in direction judgment subtracted from 90°; a value of 90 represents perfect performance, and 0 is chance performance (distributions of errors show in supplemental Figures S7 and S10). B, Neurophysiological data from 94 MT neurons (previously described in [4]). Displacement tuning curves for low contrast (blue; dashed) and high contrast (red; solid). Error bars represent standard error.
To study the potential neuronal substrate of these perceptual findings, we reanalyzed previously-published data from 94 cells recorded from the middle temporal (MT) area in two alert macaque monkeys [4]. As in the behavioral experiments, the motion stimuli were presented at low (2.2 cd/m2) or high (139.5 cd/m2) contrast on a dark background (0.025 cd/m2), and with different spatial displacements on different trials (see supplemental methods). The resulting displacement tuning functions for the neuronal population (Figure 1b) show a striking resemblance to our human behavioral data, with the interaction between displacement and contrast again being highly significant (p<.0001). A similar effect was observed in a smaller sample of neurons (N=40) for which we examined the difference in responses to stimuli moving in the preferred and anti-preferred directions (see supplemental Figure S2).
Our results demonstrate a strong similarity between the behavior of MT cells and human perception. However, the stimuli used in the two experiments were not identical, mainly because we chose to use low motion coherence in the psychophysical experiments in order to avoid ceiling effects exhibited by some observers in the 100% coherence condition. Nevertheless, to evaluate whether similar behavior would emerge with stimuli that more precisely matched those in the neurophysiology experiments, we ran 16 additional subjects with 100% coherent motion (and other matched stimulus parameters; see supplemental methods). These results, shown in Figure 2a, replicate the original interaction between displacement and contrast (p<.01). As expected, many subjects were at or near ceiling performance with 100% coherent motion, which caused the profiles in Figure 1b to be blurred relative to the profiles shown in Figure 1a.
Figure 2.
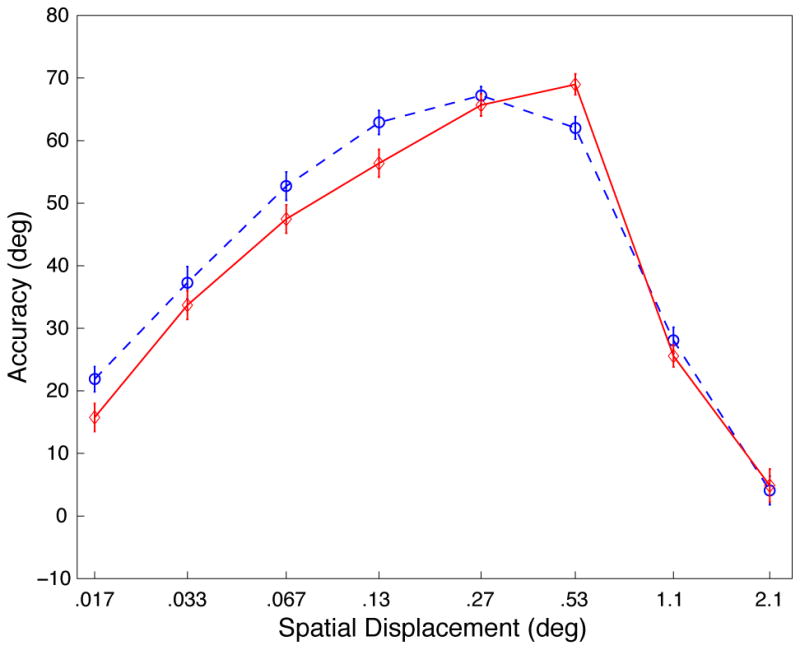
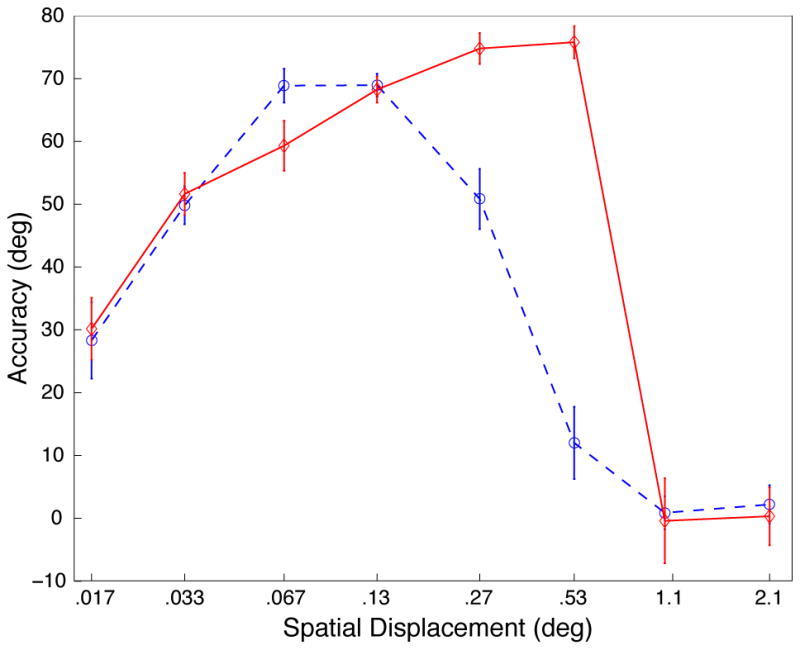
Results of behavioral control experiments (see Figure 1a for plot descriptions). A, Experiment 2, displacement tuning curves for 100% coherent dot fields matching the stimulus parameters of the physiological experiment (see supplemental methods for details; data for each TD shown in supplemental Figure S6). B, Experiment 3, displacement tuning curves for contrast control experiment (see supplemental methods for details). Distributions of errors show in supplemental Figures S8, S9 and S10).
We also examined the contribution of overall stimulus luminance to psychophysical performance. This was motivated by the fact that, in the previous experiment, changing the dot contrast caused a slight change in the overall stimulus luminance. To evaluate whether our results were due to changes in contrast or luminance, we tested 12 subjects with a stimulus consisting of light and dark dots on a gray background. This manipulation rendered the mean luminance constant across all conditions, but the pattern of results (Figure 2b) was largely unchanged. Again there was a significant interaction between contrast and displacement (p<.0001), suggesting an effect related to stimulus contrast, rather than luminance.
The results presented here are consistent with information-theoretic hypotheses about the influence of contrast on visual processing. To maximize information transmission, the system at high contrast suppresses redundant information [12], which, given the typical pattern of velocities on the retina during self-motion, leads to suppression of large, slow stimuli [4]. On the other hand, at low contrast, the sole basis for distinguishing visual signal from random noise is the signal’s regularity across space and time. In this case, preserving redundancy becomes critical, and spatial pooling and temporal summation are desired.
Supplementary Material
Acknowledgments
ARS was supported by NSF (BCS-0549036) and NIH (R21 EY017737), PKP was supported by NIH (R01-DC02852), NSF (IIS-0205271 and SBE-0354378) and ONR (N00014-01-1-0624). CCP was supported by CIHR (MOP-79352).
Bibliography
- 1.Sclar G, Maunsell JH, Lennie P. Coding of image contrast in central visual pathways of the macaque monkey. Vision Res. 1990;30:1–10. doi: 10.1016/0042-6989(90)90123-3. [DOI] [PubMed] [Google Scholar]
- 2.Shapley R. Visual sensitivity and parallel retinocortical channels. Annu Rev Psychol. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- 3.Shapley RM, Victor JD. The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol. 1978;285:275–298. doi: 10.1113/jphysiol.1978.sp012571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pack CC, Hunter JN, Born RT. Contrast dependence of suppressive influences in cortical area MT of alert macaque. J Neurophysiol. 2005;93:1809–1815. doi: 10.1152/jn.00629.2004. [DOI] [PubMed] [Google Scholar]
- 5.Livingstone MS, Conway BR. Contrast affects speed tuning, space-time slant, and receptive-field organization of simple cells in macaque V1. J Neurophysiol. 2007;97:849–857. doi: 10.1152/jn.00762.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peterson MR, Li B, Freeman RD. Direction selectivity of neurons in the striate cortex increases as stimulus contrast is decreased. J Neurophysiol. 2006;95:2705–2712. doi: 10.1152/jn.00885.2005. [DOI] [PubMed] [Google Scholar]
- 7.Polat U, Mizobe K, Pettet MW, Kasamatsu T, Norcia AM. Collinear stimuli regulate visual responses depending on cell’s contrast threshold. Nature. 1998;391:580–584. doi: 10.1038/35372. [DOI] [PubMed] [Google Scholar]
- 8.Sceniak MP, Ringach DL, Hawken MJ, Shapley R. Contrast’s effect on spatial summation by macaque V1 neurons. Nat Neurosci. 1999;2:733–739. doi: 10.1038/11197. [DOI] [PubMed] [Google Scholar]
- 9.Krekelberg B, van Wezel RJ, Albright TD. Adaptation in macaque MT reduces perceived speed and improves speed discrimination. J Neurophysiol. 2006;95:255–270. doi: 10.1152/jn.00750.2005. [DOI] [PubMed] [Google Scholar]
- 10.Stone LS, Thompson P. Human speed perception is contrast dependent. Vision Res. 1992;32:1535–1549. doi: 10.1016/0042-6989(92)90209-2. [DOI] [PubMed] [Google Scholar]
- 11.Tadin D, Lappin JS, Gilroy LA, Blake R. Perceptual consequences of centre-surround antagonism in visual motion processing. Nature. 2003;424:312–315. doi: 10.1038/nature01800. [DOI] [PubMed] [Google Scholar]
- 12.van Hateren JH. Real and optimal neural images in early vision. Nature. 1992;360:68–70. doi: 10.1038/360068a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


