Abstract
There has been intensified interest in the neuropeptides oxytocin (OT) and arginine vasopressin (AVP) in autism spectrum disorders (ASD) given their role in affiliative and social behavior in animals, positive results of treatment studies using OT, and findings that genetic polymorphisms in the AVP-OT pathway are present in individuals with ASD. Nearly all such studies in humans have focused only on males. With this preliminary study, we provide basic and novel information on the involvement of OT and AVP in autism with an investigation of blood plasma levels of these neuropeptides in 75 preadolescent and adolescent girls and boys ages 8–18: 40 with high-functioning ASD (19 girls, 21 boys) and 35 typically developing children (16 girls, 19 boys). We related neuropeptide levels to social, language, repetitive behavior, and internalizing symptom measures in these individuals. There were significant gender effects: Girls showed higher levels of OT while boys had significantly higher levels of AVP. There were no significant effects of diagnosis on OT or AVP. Higher OT values were associated with greater anxiety in all girls and with better pragmatic language in all boys and girls. AVP levels were positively associated with restricted and repetitive behaviors in girls with ASD but negatively (non-significantly) associated with these behaviors in boys with ASD. Our results challenge the prevailing view that plasma OT levels are lower in individuals with ASD and suggest there are distinct and sexually dimorphic mechanisms of action for OT and AVP underlying anxiety and repetitive behaviors.
Lay Abstract
Oxytocin (OT) and arginine vasopressin (AVP) are neuropeptides that are involved in affiliative and social behavior. Previous studies have shown that boys with autism spectrum disorders (ASD) have lower levels of OT than boys without ASD, and treatment studies have found that intranasal infusions of OT increase social behaviors in mostly males with ASD. With this study, we provide basic and novel information on the involvement of OT and AVP in ASD with an investigation of blood plasma levels of these neuropeptides in 75 preadolescent and adolescent girls and boys ages 8–18: 40 with high-functioning ASD and 35 typically developing children. We related OT and AVP levels to social, language, repetitive behavior, and internalizing symptom measures in these individuals. Girls had higher levels of OT while boys had higher levels of AVP. There were no differences in OT or AVP levels between the ASD and typically developing groups. Higher OT values were associated with greater anxiety in all girls and with less impaired social language in all boys and girls. Higher levels of AVP were associated with greater restricted and repetitive behaviors in girls with ASD whereas lower levels of AVP levels were associated with lower levels of these behaviors in boys with ASD. Results challenge the prevailing view that OT levels are lower in individuals with ASD, and suggest there are distinct mechanisms of action for OT and AVP underlying anxiety and repetitive behavior symptoms for boys versus girls.
Keywords: Neuropeptides, oxytocin, vasopressin, autism, sex differences, repetitive behaviors, anxiety
Autism spectrum disorders (ASD) are common, debilitating neurodevelopmental disorders involving impairments in social and language functioning, and the presence of restricted interests and repetitive behaviors. A growing body of research suggests that the neuropeptides oxytocin (OT) and arginine vasopressin (AVP) promote affiliative and social behavior in rodents and other species including humans (Donaldson & Young, 2008; Insel, 2010); that intranasal administration of OT enhances social cognition, responsive care taking, and trust (Bartz et al., 2010; Domes et al., 2007; Naber, van Ijzendoorn, Deschamps, van Engeland, & Bakermans-Kranenburg, 2010; Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005); and that certain alleles in genes in the AVP-OT pathway are found more frequently in persons with ASD (Ebstein et al., 2009). These findings have led to intensified efforts to clarify the roles of OT and AVP in the pathophysiology of ASD and to translate resulting knowledge to treatment development (Carter, 2007; Hammock & Young, 2006; Insel, O’Brien, & Leckman, 1999).
In mammals, OT and AVP are synthesized primarily in the brain’s paraventricular and supraoptic nuclei, with AVP also synthesized in the suprachiasmatic nucleus. They are transported by neurosecretory axons to the posterior hypothalamus before being sent to the pituitary for peripheral release, or projected to various regions of the brain and central nervous system involved in reproductive, social, and aggressive behaviors (Donaldson, Yang, Chan, & Young, 2009). The effects of OT and AVP frequently interact with one another (Carter, 2007), are species specific, are sexually dimorphic (deVries & Panzica, 2006), and interact with gonadal steroids (Choleris et al., 2003). Furthermore, there are profound differences in the forebrain distribution of receptors for OT and AVP, likely contributing to the differences in the function of these neuropeptides across species (Insel, 2010).
It is, as of yet, unclear what peripherally measured levels of OT and AVP (in plasma, saliva, or urine) represent, versus central levels. Work in primates suggests that plasma and CSF levels of OT are dissociated (Amico et al. 1990). However, peripheral levels of OT and AVP are clearly responsive to social stimuli (Kenkel et al., 2012; Schneiderman, Zagoory-Sharon, Leckman, & Feldman, 2012; Seltzer, Ziegler, & Pollak, 2010; Wismer, Ziegler, Kurian, Jacoris, & Pollak, 2005). Thus, it is possible that previous studies found asynchrony between plasma and CSF either because (1) there were issues of timing for the sampling, or (2) CSF was not the appropriate central measure (Landgraf & Neumann, 2004). Likewise, it is unclear if intranasally administered OT actually enters the brain at levels any higher than intravenously administered OT. The commonly cited source for the permeability of the blood-brain barrier to peptides (Born et al., 2002) did not actually test OT but instead focused on intranasal AVP. It is possible that intranasal OT also operates via peripheral feedback to the central nervous system (see Norman et al. 2011).
Research on OT and AVP in humans has focused on both peripheral measurements of peptides and central administration, mainly of OT. Broadly, both OT and AVP appear to be involved in social and emotional behavior, particularly anxiety. OT has generally been associated with positive social behaviors such as social recognition, social contact, pair bonding, and parenting (Hammock & Young, 2005; Insel & Fernald, 2004); anxiolysis (Gimpl & Fahrenholz, 2001; McCarthy, McDonald, Brooks, & Goldman, 1996); and reduced activation of the HPA axis (Neumann, Kromer, Toschi, & Ebner, 2000). Plasma levels of OT during pregnancy correlate with bonding-related thoughts, maternal-fetal bonding post-partum, and affectionate touch by mothers (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010; Feldman, Weller, Zagoory-Sharon, & Levine, 2007; Levine, Zagoory-Sharon, Feldman, & Weller, 2007). It has been suggested that OT may serve as a protective factor against the development of affective disorders, given its anxiolytic and antidepressant properties (Gimpl & Fahrenholz, 2001; Grippo, Trahanas, Zimmerman, Porges, & Carter, 2008). In men, intranasal administration of OT has been shown to increase stimulatory contact and reduce hostility in fathers of infants (Naber et al., 2010), improve theory of mind (Domes et al., 2007), enhance trust (Kosfeld et al., 2005), increase social memory (Guastella, Mitchell, & Mathews, 2008; Savaskan et al., 2008), and improve empathic accuracy (Bartz et al., 2010). AVP has also been associated with positive social behaviors including pair bonding in male prairie voles (Wang, Ferris, & deVries, 1994).
While both OT and AVP are involved in positive social behaviors, it should be noted that their effects are not always positive, and central administration can lead to excessive self-grooming (Argiolas & Gessa, 1991; Gimpl & Fahrenholz, 2001). AVP has been shown to be involved in aggression and can be anxiogenic at some dosages (Keverne & Curley, 2004). High plasma levels of OT and AVP and elevated levels of OT- or AVP-expressing neurons have been associated with both depression (Bao, Meynen, & Swaab, 2008; Meynen et al., 2006; Purba, Hoogendijk, Hofman, & Swaab, 1996; van Londen et al., 1997, 1998) and anxiety (de Kloet et al., 2008). Additionally, dysregulation in OT and AVP has been related to obsessive-compulsive symptoms (Leckman et al., 1994; Swedo et al., 1992).
Because of the role of OT and AVP in social behaviors, recent research has aimed to determine whether and how they are involved in the pathogenesis of ASD. Neuropeptide research in individuals with ASD has examined plasma levels of OT and AVP, treatments involving central OT administration, and genetics of the AVP-OT pathway. Modahl et al. (1998) found lower levels of plasma OT in prepubertal boys with autism relative to typically developing (TYP) children. They also found positive associations between OT and measures of social and adaptive behavior in TYP children, but not those with autism; in contrast, children with autism, especially those considered aloof, showed the opposite relationship between OT and functioning (Modahl et al., 1998). Al-Ayadhi (2005) reported lower levels of OT and AVP in plasma of adult males with ASD. In a small sample, Andari et al. (2010) also found lower plasma OT levels in high functioning young adult males with ASD. Two studies of AVP in individuals with ASD have shown that levels are lower in low functioning children (Leboyer et al., 1992) and adults (Boso et al., 2007).
While these previous studies have focused on measuring peripheral levels of OT and AVP, several treatment studies have emerged in which OT was intranasally administered to individuals with ASD in order to introduce the neuropeptide directly into the brain. In the first such study, Hollander et al. (2003) reported that intravenous OT infusion reduced restricted and repetitive behaviors in adults (mostly men) with high functioning ASD. Later studies showed that intravenous OT administration improved comprehension of affective speech in this same sample (Hollander et al., 2007). More recently, Andari et al. (2010) demonstrated that OT inhalation increased cooperation and feelings of trust and time spent gazing at the eye regions of faces in young adults with high functioning ASD. In the first study of adolescents, Guastella et al. (2010) found that intranasal OT administration improved emotion recognition from eye regions in high functioning boys.
Additional support for the contention that OT and AVP play a role in the pathophysiology of ASD comes from studies suggesting that genetic polymorphisms in the AVP-OT pathway contribute to deficits in socialization in individuals with ASD (Jacob et al., 2007; Kim et al., 2002; Liu et al., 2010; Wassink et al., 2004; Wermter et al., 2010; Wu et al., 2005; Yirmiya et al., 2006; Ylisaukko-oja et al., 2006), and that OT receptor knockout mice show social and communication impairments (Pobbe et al., 2012).
Despite the burgeoning OT and AVP literatures, there is still much unknown about plasma levels of OT and AVP in individuals with ASD, and none in both high functioning boys and girls during childhood and adolescence. Peripherally, work in animals has found higher levels of plasma OT in females than males (Kramer, Cushing, Carter, Wu, & Ottinger, 2004), and human studies have found higher levels of plasma AVP in males than females (Asplund & Aberg, 1991). The effects of OT and AVP are also known to be sexually dimorphic (Carter, 2007; deVries & Panzica, 2006). Thus, including females in studies of neuropeptides in ASD is critical given potential sex differences in these systems. Such studies are a clinically significant first step towards understanding the relationship of OT and AVP to pathophysiology, and may provide useful information about therapeutic levels of these neuropeptides for treatment trials.
The primary aims of the current study were to (1) determine plasma levels of OT and AVP in TYP girls and boys and those with ASD, and (2) to relate these levels to measures of social, language, repetitive behavior, and internalizing symptoms; the ultimate goal is to identify novel associations to be rigorously explored in future research. Based on the animal literature and on the extant literature about neuropeptide plasma levels in ASD (focused mostly on males), we hypothesized that (1) girls would have higher plasma OT levels than boys (Kramer et al., 2004) while boys would have higher plasma AVP levels than girls (Asplund & Aberg, 1991); (2) TYP children would have higher levels of OT than those with ASD; and (3) low OT levels would be associated with higher levels of social language, repetitive behavior, and internalizing symptoms in all participants. We note at the outset the exploratory nature of this study given the extremely limited literature investigating these issues.
Method
Participants
A total of 79 children and adolescents (ages 8–18) participated in this study. Participants with ASD were recruited from local physicians, psychologists, speech-language pathologists, occupational therapists, advocacy groups, state centers for the developmentally disabled, ASD support groups, and the MIND Institute’s Subject Tracking System, which includes children with developmental disorders as well as TYP children. Participants with ASD were required to meet criteria for Autistic Disorder, Asperger’s Syndrome, or PDD-NOS according to DSM-IV-TR criteria (American Psychiatric Association, 2000). Exclusion was based on parent-reported diagnosed depression, anxiety, ADHD, Fragile X, Tourette’s, or seizures. The decision to include individuals with high-functioning autism, Asperger’s, and PDD-NOS was made based on studies showing that it is difficult to reliably distinguish between them (Macintosh & Dissanayake, 2004; Ozonoff & Griffith, 2000).
Neuropeptide data were initially inspected for outliers using Grubb’s test for outliers and four participants were removed due to high levels of AVP (one TYP girl, one boy with ASD) or OT (one TYP girl, one TYP boy). Thus, the final sample contained a total of 75 participants and four age-matched groups were formed: 19 girls with ASD, 21 boys with ASD, 16 TYP girls, and 19 TYP boys. Twelve individuals in the ASD group (6 girls, 6 boys) were taking psychotropic medications (3 on atypical antipsychotics, 8 on SSRIs, and 5 on stimulants). The ASD groups were matched on FSIQ as were the TYP groups; however, the ASD groups had significantly lower FSIQ scores than their TYP counterparts, t(32) = −3.30, p = .002 for girls and t(38) = −3.24, p = .002 for boys. Participant characteristics are displayed in Table 1. All procedures were approved by the UC Davis Medical Center Institutional Review Board.
Table 1.
Participant characteristics.
| Girls with ASD (n = 19) | Boys with ASD (n = 21) | TYP girls (n = 16) | TYP boys (n = 19) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M (range) | SD | M (range) | SD | M (range) | SD | M (range) | SD | |
| Age (Years) | 11.79 (8–18) | 3.43 | 12.24 (8–18) | 3.56 | 12.94 (8–18) | 3.19 | 11.74 (8–17) | 2.49 |
| OT (pg/ml) | 525.23 (169–1378) | 325.75 | 357.12 (92–793) | 184.05 | 434.33 (191–1468) | 332.27 | 361.52 (87–1318) | 315.26 |
| AVP (pg/ml) | 85.72 (27–206) | 41.62 | 101.14 (23–214) | 48.56 | 70.35 (34–114) | 25.51 | 114.58 (37–293) | 82.68 |
| FSIQ | 101.39 (75–129) | 15.63 | 103.10 (76–145) | 17.76 | 116.94 (100–135) | 11.11 | 119.58 (88–139) | 13.90 |
| VIQ | 108.94 (85–128) | 12.87 | 102.67 (69–132) | 17.32 | 118.38 (91–138) | 13.65 | 120.26 (95–141) | 12.99 |
| PIQ | 93.50 (63–125) | 18.04 | 105.48 (80–149) | 17.26 | 111.50 (88–131) | 11.52 | 114.79 (86–133) | 14.20 |
| ADOS total | 10.50 (7–18) | 2.87 | 10.90 (7–22) | 4.06 | -- | -- | -- | -- |
| ADOS communication | 2.89 (1–5) | 0.96 | 3.76 (2–8) | 1.89 | -- | -- | -- | -- |
| ADOS social | 7.61 (4–13) | 2.45 | 7.14 (5–14) | 2.46 | -- | -- | -- | -- |
| SCQ total | 20.95 (12–31) | 5.41 | 22.90 (11–34) | 7.27 | 1.75 (0–8) | 2.41 | 1.84 (0–8) | 2.17 |
| Time of blood draw | 14:20 (10:00–17:00) | 02:05 | 14:03 (9:45–17:00) | 02:26 | 14:49 (10:30–17:00) | 01:56 | 14:15 (9:45–17:00) | 02:30 |
Note. ASD = Autism Spectrum Disorder; TYP = Typically Developing; OT = Oxytocin; AVP = Arginine Vasopressin; FSIQ = Full Scale IQ; VIQ = Verbal IQ; PIQ = Performance IQ; ADOS = Autism Diagnostic Observation Schedule; SCQ = Social Communication Questionnaire.
Qualification Measures
Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999)
The four-subtest (Vocabulary, Block Design, Similarities, Matrix Reasoning) version of the WASI was used. The WASI produces Verbal (VIQ), Performance (PIQ), and Full Scale IQ (FSIQ) Standard Scores with means of 100 and SDs of 15. Test-retest reliability for IQ scales ranges from .88–.93. Participants were required to have FSIQ scores of at least 75.
Autism Diagnostic Observation Schedule (ADOS-G; Lord et al., 2000)
To confirm diagnosis, participants with ASD were administered module 3 or 4 of the ADOS-G by a trained clinician. The ADOS-G is a semi-structured protocol that offers standardized observation of social-communication behavior. Lord et al. (2000) showed that mean inter-rater agreement was 88% across all items for modules 3 and 4; internal consistency ranged from .91–.94. Inter-rater agreement in diagnostic classification based on the ADOS-G algorithm for all modules exceeds 90%.
Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003)
Participants’ parents completed the SCQ, a 40-item questionnaire to evaluate communication and social skills. It contains parallel questions to those on the Autism Diagnostic Interview-Revised (Lord, Rutter, & Le Couteur, 1994), the gold standard parent-report diagnostic measure, in a briefer format. A cutoff of 15 gives sensitivity of .96 and specificity of .80 for autism versus other diagnoses (Berument, Rutter, Lord, Pickles, & Bailey, 1999). Children with ASDs were required to score ≥ 11 while TYP groups were required to score <10.
Autism Symptom Measures
Social Responsiveness Scale (SRS; Constantino, 2002)
The SRS is a 65-item parent report used to assess autism symptom severity. Subscales include: Social awareness, social cognition, social communication, social motivation, and autistic mannerisms. The SRS has acceptable levels of internal consistency (.93–.97) and test-retest reliability (.77–.85). Higher scores indicate greater impairment.
Children’s Communication Checklist-2 (CCC-2; Bishop, 2003)
The CCC-2 assesses social communication, including grammar and pragmatics. It consists of 10 scales. We utilized scales related to social language including coherence (failure to provide listener context); initiation (starting interactions); scripted language (delayed echolalia/pedantic speech); context (use of humor and irony and using language in a concrete fashion); and nonverbal communication (constriction in range of affect). Lower scores indicate greater impairment.
Repetitive Behavior Scale (RBS-R; Bodfish, Symons, & Lewis, 1999)
The RBS-R is a 43-item parent-reported measure assessing restricted and repetitive behavior. Subscales include the following behaviors: Stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted interests, and an overall score. The RBS-R has acceptable levels of inter-rater (.88), test-retest (.71), and internal consistency reliability (.78–.91). Higher scores indicate greater impairment.
Internalizing Symptom Measures
Behavior Assessment System for Children (BASC2; Reynolds & Kamphaus, 2004)
The BASC2 is used to evaluate adaptive and problem behaviors of children ages 2–25, and has exhibited acceptable levels of test-retest reliability (.76–.84) and internal consistency (.80–.87). We utilized parent-reported scores from the depression and anxiety scales. Raw scores were used since T-scores are based on gender-norms which may mask sex differences, and sex differences were of interest in the present investigation. Higher scores indicate greater impairment.
Blood draws and OT and AVP assays
We obtained blood samples of 8.0 ml per subject after fasting for at least two hours. Blood was placed in tubes containing EDTA, kept on ice until centrifuging at 4 degrees C, 3300 rpm for 12 minutes. The plasma was aliquoted off and stored at −80 degrees C. Assays were performed using an enzyme immunoassay kit (Assay Designs, Ann Arbor Michigan), which has been validated for humans (OT, Levine et al., 2007; and AVP, Carter, unpublished data). Inter-assay coefficients of variation were 3.47% for OT and 12.26% for AVP; intra-assay coefficients of variation were 3.58% for OT and 3.16% for AVP.
Data Analytic Plan
OT and AVP levels were log transformed to meet normality assumptions. Analysis of Variance (ANOVA) was used to evaluate the main effects of sex, diagnosis, and their interaction on OT and AVP levels, controlling for age, time of blood draw, and medication status. Because not all symptom measures were normally distributed, Spearman’s Rho was used to assess the correlation between autism and internalizing symptom measures and OT and AVP levels, first within each sex and then follow-up exploratory analyses were conducted within each group separately. For RBS variables, correlations were conducted only in the ASD groups due to restricted range in the TYP groups. To account for potential confounding effects of age and time of blood draw, we regressed OT and AVP values on these variables and used the residuals in correlation analyses. False discovery rates (FDR) were calculated to account for multiple testing. However, in interpreting the results we consider both unadjusted and FDR-adjusted values given the preliminary nature of this study and the importance of identifying novel associations to be explored in future research.
Previous work similar to the present study did not find associations between IQ and plasma levels of OT, or differences in OT levels based on medication status (Modahl et al., 1998). Additionally, extremely little is known about relationships between AVP and medication status or IQ. In the present sample, there were no significant associations between IQ and OT or AVP levels (ps range from .157 to .878 for OT and from .313 to .849 for AVP). Girls with ASD on medication showed similar levels of OT, t(17) = .32, p = .757 and AVP, t(16) = .69, p = .501 to girls with ASD not on medication. Boys with ASD on medication showed similar levels of OT levels, t(19) = 1.72, p = .102 but lower levels of AVP, t(19) = 3.03, p = .007 to boys with ASD not on medication. Thus, we conducted secondary exploratory analyses including IQ and medication status in the creation of residuals. Notably, the participants who were on medications differed in the types of medication being taken, each of which may have different effects on neuropeptide levels even within medication class (e.g., Kiss, Bundzikova, Pirnik, & Mikkelsen, 2010). One of our primary goals in this preliminary study was to identify potentially novel associations to explore in future research. Thus, we elected to present findings with and without covariation in order to provide the most comprehensive view of potential associations to provide guidance for future studies.
Results
Blood plasma levels of OT and AVP
Distributions of OT and AVP levels by group are displayed in Figures 1a and 1b, respectively. There were no significant correlations between OT and AVP levels across the entire sample (r = .09, p = .434) or in any of the four groups (rs from = −.01 to .38, ps from .110 to .975). Results of the ANOVA for OT levels showed a significant main effect of sex, F(1, 68) = 4.53, p = .037. Girls exhibited higher OT levels than boys. There was not a significant effect of diagnosis on OT levels, F(1, 68) = 2.28, p = .270, nor was there a significant interaction between diagnosis and sex, F(1, 68) = .07, p = .797. For AVP, there was a main effect of sex, F(1, 67) = 4.24, p = .043. AVP levels were higher for boys. Diagnosis was not significant, F(1, 67) = 1.89, p = .174, nor was the interaction between sex and diagnosis, F(1, 67) = .91, p = .344.
Figure 1.
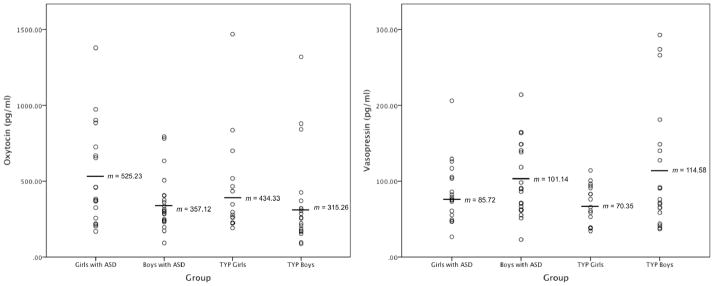
Figures 1a and 1b. Plasma OT and AVP levels by group. Mean scores are marked by horizontal lines. Girls had significantly higher OT levels than boys, but the ASD and TYP groups did not differ. Boys had significantly higher AVP levels than girls, but the ASD and TYP groups did not differ.
ASD and social language symptoms
Means and standard deviations for the CCC2, SRS, and RBS-R can be found in Table 2. As shown in Figure 2a, in all girls, there were significant associations between OT levels and CCC coherence (rs = −.36, p = .033), CCC scripted language (rs = −.43, p = .009), and CCC nonverbal communication (rs = −.35, p = .041). The association between OT and CCC scripted language survived adjustment for multiple comparisons (p = .045); the associations between OT and CCC coherence and CCC nonverbal communication were trend-level after adjustment (both ps = .068). There were no significant associations between AVP and symptom variables. In all boys, there were significant associations between OT and CCC coherence (rs = −.36, p = .022) and CCC nonverbal communication (rs = −.32, p = .046) (Figure 2b). The associations between OT and CCC initiation (rs = −.28, p = .076) and CCC context (rs = −.29, p = .069) were trend-level. After adjustment for multiple comparisons, these significant and trend-level associations were trend-level (ps = .095). There were no associations between AVP and symptom variables in boys. Thus, in boys and girls, high levels of OT were associated with better social communication abilities, AVP levels were not associated with symptoms, and neuropeptide levels were not associated with SRS scores.
Table 2.
Means and SDs of ASD and internalizing symptoms.
| Girls with ASD (n = 19) | Boys with ASD (n = 21) | TYP girls (n = 16) | TYP boys (n = 19) | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| M (range) | SD | M (range) | SD | M (range) | SD | M (range) | SD | |
| SRS | ||||||||
| Social awareness | 9.79 (2–18) | 4.93 | 8.67 (0–18) | 6.04 | 8.00 (2–15) | 4.49 | 9.84 (0–19) | 6.96 |
| Social cognition | 13.68 (1–27) | 8.54 | 11.00 (0–29) | 8.46 | 11.81 (2–27) | 7.98 | 13.53 (0–29) | 11.51 |
| Social communication | 25.16 (1–49) | 16.32 | 19.57 (0–47) | 16.51 | 21.44 (1–48) | 16.51 | 23.79 (0–50) | 20.42 |
| Social motivation | 14.37 (0–30) | 7.57 | 11.10 (0–26) | 8.51 | 11.94 (0–30) | 8.65 | 12.37 (0–30) | 10.45 |
| Autistic mannerisms | 15.21 (0–30) | 9.81 | 11.57 (0–27) | 10.30 | 11.31 (0–30) | 9.70 | 12.16 (0–28) | 11.66 |
| Total | 78.21 (6–143) | 44.97 | 61.90 (0–143) | 47.76 | 64.06 (6–149) | 45.95 | 71.68 (3–148) | 59.59 |
| CCC | ||||||||
| Coherence | 5.84 (3–11) | 2.52 | 6.00 (1–16) | 3.59 | 10.69 (1–18) | 3.94 | 10.32 (0–18) | 4.71 |
| Initiation | 5.21 (1–11) | 2.82 | 5.67 (1–16) | 3.48 | 12.81 (7–19) | 3.15 | 10.68 (1–17) | 4.14 |
| Scripted language | 5.26 (1–12) | 3.23 | 4.29 (1–13) | 3.27 | 11.25 (5–15) | 2.62 | 10.37 (1–15) | 3.77 |
| Context | 6.16 (1–16) | 3.59 | 5.43 (1–15) | 3.20 | 11.31 (6–16) | 2.77 | 10.42 (0–16) | 4.02 |
| Nonverbal | 3.16 (1–10) | 2.52 | 5.00 (1–15) | 4.02 | 10.94 (5–14) | 2.74 | 9.95 (0–14) | 3.81 |
| RBS | ||||||||
| Stereotyped | 3.67 (0–13) | 3.55 | 5.38 (0–15) | 3.87 | 0.13a (0–2) | 0.52 | 0.37 (0–6) | 1.38 |
| Self-injurious | 2.50 (0–8) | 2.46 | 3.62 (0–12) | 4.08 | 0.07a (0–1) | 0.26 | 0.42 (0–4) | 1.02 |
| Compulsive | 4.72 (0–18) | 5.27 | 7.10 (0–18) | 4.85 | 0.53a (0–4) | 1.25 | 0.26 (0–3) | .73 |
| Ritualistic | 5.33 (0–15) | 4.75 | 7.38 (1–17) | 4.99 | 0.00a (0) | 0.00 | 0.32 (0–3) | .82 |
| Sameness | 9.39 (1–27) | 7.76 | 12.76 (2–31) | 8.09 | 0.20a (0–2) | 0.56 | 0.53 (0–7) | 1.65 |
| Restricted interests | 2.72 (0–10) | 2.63 | 5.29 (1–11) | 2.92 | 0.07a (0–1) | 0.26 | 0.63 (0–5) | 1.57 |
| Overall score | 28.39 (1–77) | 22.31 | 41.52 (18–91) | 23.35 | 1.00a (0–4) | 1.41 | 2.53 (0–19) | 4.72 |
| BASC2 – Parent Report | ||||||||
| Depression (raw) | 17.17b (4–26) | 7.37 | 13.80c (3–31) | 8.33 | 3.20a (0–8) | 2.46 | 4.32 (1–16) | 4.00 |
| Anxiety (raw) | 18.22b (8–26) | 6.25 | 18.60c (6–39) | 8.52 | 7.27a (0–15) | 4.57 | 8.95 (0–25) | 5.94 |
n = 15;
n = 18;
n = 20
Note. ASD = Autism Spectrum Disorder; TYP = Typically Developing; OT = Oxytocin; AVP = Arginine Vasopressin; BASC = Behavior Assessment System for Children; CCC = Children’s Communication Checklist; SRS = Social Responsiveness Scale; RBS = Repetitive Behavior Scale; BASC2 = Behavior Assessment Scale for Children, 2nd Edition.
Figure 2.
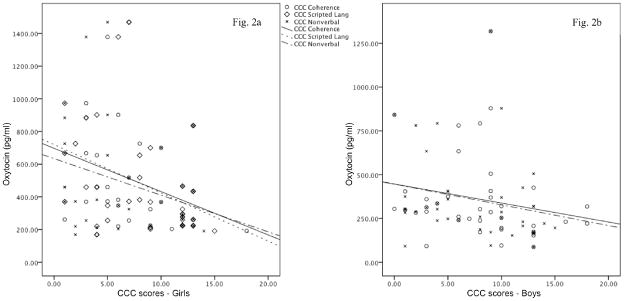
Figures 2a and 2b. Significant associations between Children’s Communication Checklist social language subscale scores and OT levels. Figure 2a displays significant associations in girls; Figure 2b displays significant associations in boys. Higher levels of OT were associated with less impairment on measures of social language.
Next, the same associations were examined in each of the four groups separately, with the inclusion of RBS symptom variables for the ASD groups. In girls with ASD, there were significant associations between OT levels and CCC coherence (rs = −.48, p = .040) and CCC scripted language (rs = −.50, p = .028). Associations were trend-level between OT and RBS ritualistic behavior (rs = .47, p = .051), RBS sameness behavior (rs = .46, p = .058), and RBS restricted interests (rs = .44, p = .070). AVP levels were significantly associated with the RBS self-injurious behavior (rs = .50, p = .042) and RBS overall scores (rs = .51, p = .036); associations between AVP and RBS compulsive behavior (rs = .47, p = .057) and RBS ritualistic behavior (rs = .45, p = .057) were trend-level. In TYP girls, OT levels were associated at a trend-level with CCC scripted language scores (rs = −.50, p = .051). AVP levels were not associated with symptom measures in TYP girls. In boys with ASD, OT levels were not associated with any symptom measures. However, AVP levels were associated with SRS social cognition (rs = −.46, p = .037) and SRS social motivation (rs = −.44, p = .044). AVP levels were associated at a trend-level with RBS self-injurious behavior (rs = −.42, p = .056), and RBS restricted interests (rs = −.39, p = .083). A Fisher r-to-z transformation revealed that the significant and trend-level correlations between AVP and RBS self-injurious behavior significantly differed between girls with ASD and boys with ASD (z = 2.55, p < .01); this was the only RBS variable for which both groups exhibited associations with AVP. Finally, in TYP boys there were significant associations between OT levels and CCC coherence (rs = −.48, p = .036) but no significant associations between AVP and symptom variables. None of these associations survived adjustment for multiple comparisons.
When including IQ and medication status as additional covariates, in boys with ASD, the associations between AVP and SRS social cognition (rs = −.62, p = .003) and SRS social motivation (rs = −.62, p = .003) remained significant, and associations between AVP and the following SRS variables emerged as significant: social awareness (rs = −.54, p = .012), social communication (rs = −.49, p = .023), autistic mannerisms (rs = −.54, p = .011), and total score (rs = −.58, p = .006). These did not survive adjustment for multiple comparisons. All other associations between neuropeptide levels and ASD and social language symptoms were non-significant; trend-level associations were present between OT and CCC variables.
Internalizing symptoms
In all girls, there was a significant correlation between OT levels and BASC anxiety (rs = .375, p = .032), but not depression. This association was trend-level after adjustment for multiple comparisons (p = .064). AVP was not associated with either anxiety or depression levels. In all boys, AVP levels were associated with BASC anxiety at a trend level (rs = −.29, p = .076; p = .152 after adjustment for multiple comparisons) but not depression, and OT was associated with neither. When examining each group separately, no significant associations emerged in girls or boys with ASD. For TYP girls, OT levels were significantly associated with parent-reported BASC anxiety (rs = .64, p = .010; p = .020 after adjustment for multiple comparisons) while AVP levels were associated with parent-reported BASC depression scores at a trend level (rs = .48, p = .072; p = .144 after adjustment for multiple comparisons). There were no significant associations in TYP boys.
When including IQ and medication status as additional covariates, the association between BASC anxiety and OT remained significant in TYP girls (rs = −.54, p = .039). The associations in all girls between AVP and BASC anxiety (rs = .37, p = .036) and BASC depression (rs = .36, p = .046) also remained significant. These associations did not survive adjustment for multiple comparisons. All other associations between neuropeptide levels and internalizing symptoms were non-significant; trend-level associations were present between OT and BASC anxiety in all girls, and AVP and BASC depression in TYP girls.
Discussion
This preliminary study examined baseline plasma levels of OT and AVP in girls and boys with and without ASD, and associations between these neuropeptides and symptom variables. Hypotheses of this exploratory study received mixed support. As predicted, overall plasma OT values differed by sex and were higher for girls. Similarly, plasma AVP levels were higher in boys than girls. Contrary to the extant literature, OT plasma levels in persons with ASD were not lower than those found in the TYP group, and there were also no differences in plasma AVP between the ASD and TYP groups. In the current study, high plasma OT values were associated with better social language skills in both boys and girls prior to (and after, in some cases) adjustment for multiple comparisons. While it is difficult to compare studies of peripheral levels with those focusing on central administration of OT, the present findings appear to be consistent with studies finding that intranasal OT infusion improves social language comprehension (Hollander et al., 2007). Findings related to the relationship between plasma neuropeptide levels and internalizing symptoms and repetitive behaviors illustrate the complexity inherent to these relationships, and the potential sexually dimorphic effects of OT and AVP. Specifically, prior to adjustment for multiple comparisons, OT values were positively associated with anxiety in all girls. AVP levels were positively associated with repetitive behavior symptoms in girls with ASD, but were negatively associated with them at a trend level (prior to adjustment for multiple comparisons) in boys with ASD. There was a significant difference between the association between AVP and RBS self-injurious behavior scores in boys and girls with ASD, although it should be noted that the association between AVP and repetitive behaviors in boys with ASD was not significant.
While OT generally is viewed as an anxiolytic and/or a promoter of behaviors with a positive affective tone, in animal and human studies, high OT plasma levels have also been associated with negative emotions involving anxiety and depression. In rats, forced swimming and shaker stress increase plasma OT levels (Gimpl & Fahrenholz, 2001). Chronic stress associated with social isolation also increases the synthesis of OT in prairie voles (Grippo et al. 2007). Women with depression have been shown to display more dysregulated patterns of peripheral OT release and higher plasma OT levels when asked to imagine depression-inducing scenarios (Cyranowski et al., 2008). Based on findings that women reporting difficulties in their social relationships showed elevated plasma OT relative to controls, Taylor et al. (2006) have suggested that OT levels constitute a “biomarker” of social separation or distress in women, and that OT release may provide the impetus for affiliative “tend and befriend” (versus male “fight or flight”) behaviors exhibited by women under stress. Our recent work suggesting that adolescent girls with ASD exhibited particularly high levels of anxiety compared to their male counterparts with ASD as well as TYP girls and boys (Solomon, Miller, Taylor, Hinshaw, & Carter, 2012) underscores the clinical relevance of better understanding the relationship between OT and internalizing symptoms in females with ASD.
Based on findings that there were improvements in recognition of affective prosody in speech with intranasal OT infusion, we examined the relationship between peripheral OT and AVP levels and ASD social language measures. Across boys and girls, we found similar associations between ASD-related language symptoms and OT levels, indicating that less impairment on social language measures was associated with higher levels of OT. That is, higher OT levels may be protective against impairments in social communication. Though we recognize that there is not necessarily a one-to-one relationship between peripheral and central levels of OT, this finding is consistent with treatment studies that have found that intranasal administration of OT improves social language recognition abilities (Hollander et al., 2007). However, given the relationships between OT levels and anxiety in the girls in the present study, along with findings of associations between anxiety and atypical and autism-related language in males with ASD (Solomon, Ozonoff, Carter, & Caplan, 2008), future studies should investigate potential relationships among OT, anxiety, and language dysregulation in males and females with ASD to determine whether individual differences in anxiety levels moderate such associations. Because there are sex differences in many aspects of language, including the autism-related ability to provide adequate listener context (Bitan, Lifschitz, Breznitz, & Booth, 2010; Tenenbaum, Ford, & Alkhedairy, 2010), it is noteworthy that there was a consistent relationship between plasma OT levels and social language abilities across both males and females, though, notably, some of these significant associations did not survive adjustment for multiple comparisons. Still, this raises the possibility that mechanisms of action for OT are such that there is no dimorphism in the language domain.
While the initially significant or trend-level correlations between AVP and repetitive behavior symptoms did not survive adjustment for multiple comparisons, the association between AVP and self-injurious behavior (the only RBS variable for which significant or trend-level associations with AVP were present in both boys and girls with ASD) significantly differed between girls with ASD and boys with ASD. These findings are suggestive of potential sexual dimorphism in that associations between AVP and repetitive behavior symptoms were in the opposite direction for boys versus girls with ASD, with higher AVP levels associated with higher levels of repetitive behaviors in girls with ASD, but lower levels of repetitive behaviors in boys with ASD (although this latter association did not reach statistical significance). Although it has proven challenging to explain the etiology of restricted and repetitive behaviors in ASD, one common view is that they are related to stress (Rodgers, Riby, Janes, Connolly, & McConachie, 2012). The relationship between OT levels and repetitive behaviors in girls is thus consistent with the argument that OT may constitute a biomarker of stress in girls with ASD. Additionally, AVP is associated with the fight or flight response and the present findings may suggest that higher levels of AVP in girls might also be a biomarker for stress related to repetitive behaviors.
One recent position advanced to explain the higher male prevalence of ASD is that the female neuroendocrine system confers “protection” against autistic traits (Carter, 2007). According to this view, the processes mediated by OT and the lack of reliance on AVP make being a girl a protective factor against manifesting autistic-like behavior. While Carter (2007) did not specifically address peripheral levels of OT and AVP, at least at the surface, results of the current study appear to contradict this argument. While we did find that higher OT levels were associated with better social language skills, our findings support taking a more nuanced view of OT’s role. That is, rather than uniformly conferring protection against symptoms, we found that plasma OT levels were related to increased anxiety in girls. Additionally, AVP levels also were significantly related to higher levels of repetitive behaviors in girls, but the direction of similar associations in boys with ASD was reversed. It should be noted that these associations in boys with ASD did not reach significance (ps < .09, prior to adjustment for multiple comparisons), but are suggestive of the utility of exploring the possibility of sexual dimorphism in these domains in future studies.
This preliminary study, which serves as an initial platform for future empirical investigations of sex differences and neuropeptides in ASD, is limited in several ways. First, we measured plasma neuropeptide levels. OT is released into peripheral circulation in a pulsatile fashion. Thus, our approach of using a venipuncture for a single draw, which was based on the fact that we were studying children and adolescents, likely provides an incomplete picture (Cyranowski, 2010). Second, the relationships among brain, CNS, and plasma levels of neuropeptides are not straightforward. While central and peripheral OT levels are believed to be correlated, the relationship may be less clear for AVP (Landgraf & Neumann, 2004). Third, in this early but hypothesis-driven study, we chose to emphasize results prior to adjustment for multiple comparisons. While we also presented results with adjustment for multiple comparisons, we feel that the reduced power resulting from doing so would unreasonably increase the number of false negatives, which is at odds with our goal of identifying new potential relationships and patterns in this developing area of research. We feel that this is especially important in the preliminary stages of investigation of neuropeptides in ASD given the therapeutic potential (and considerable off label use of) OT, as well as the lack of work focusing on females with ASD. Finally, while we conducted secondary exploratory analyses that included IQ and medication status as covariates, it is difficult to fully interpret their effects on the relationships examined in the present study given that little is known about their associations with neuropeptide levels, particularly AVP. Additionally, the participants on medication in the present study differed in the types of medications being taken, each of which could have a different effect on neuropeptide levels even within medication class (e.g., Kiss, Bundzikova, Pirnik, & Mikkelsen, 2010). This is an important issue and one that should be addressed in the design of future, larger studies of neuropeptides in ASD.
There are many clinically significant avenues for future neuropeptide research in ASD. Although the challenges to the development of pharmaceuticals derived from OT and AVP are enormous, there has been recent progress in the creation of a first generation small-molecule OT receptor agonist (Ring et al., 2010). One of the most important implications of the present study relates to the importance of testing seemingly “face valid” conclusions about the roles of OT and AVP, and to the necessity of including girls in studies of neuropeptides in ASD – including treatment trials – given their potential unique response patterns and under-representation in ASD research.
Acknowledgments
This work was supported by K08 MH074967-01 from NIMH and a BIRCWH award (K12 HD051958) to Marjorie Solomon. Statistical support was made possible by UL1 RR024246 from the National Center for Research Resources, a component of the NIH and NIH Roadmap for Medical Research.
Footnotes
The authors report no conflicts of interest.
References
- Al-Ayadhi LY. Altered oxytocin and vasopressin levels in autistic children in Central Saudi Arabia. Neurosciences. 2005;10:47–50. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders–4th Edition Text Revised. 4. Washington, D.C: American Psychiatric Association; 2000. [Google Scholar]
- Amico JA, Challinor SM, Cameron JL. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): Evidence for functionally independent oxytocinergic pathways in primates. Journal of Clinical Endocrinology and Metabolism. 1990;71:1531–1535. doi: 10.1210/jcem-71-6-1531. [DOI] [PubMed] [Google Scholar]
- Andari E, Duhamel J, Zalla T, Herbrecht E, Leboyer M, Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proceedings of the National Academy of Sciences. 2010;107:4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A, Gessa GL. Central functions of oxytocin. Neuroscience & Biobehavioral Reviews. 1991;15:217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- Asplund R, Aberg H. Diurnal variation in the levels of antidiuretic hormone in the elderly. Internal Medicine. 1991;229(2):131–134. doi: 10.1111/j.1365-2796.1991.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swabb DF. The stress system in depression and neurodegeneration: Focus on the human hypothalamus. Brian Research Reviews. 2008;57:531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Hollander E, Ludwig NN, Kolevzon A, et al. Oxytocin selectively improves empathic accuracy. Psychological Science. 2010;2:1426–1428. doi: 10.1177/0956797610383439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Bishop D. Children’s Communication Checklist. 2. San Antonio, TX: Pearson; 2003. [Google Scholar]
- Bitan T, Lifshitz A, Breznitz Z, Booth JR. Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. Journal of Neuroscience. 2010;30:11576–11585. doi: 10.1523/JNEUROSCI.1245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodfish JW, Symons FJ, Lewis MH. Western Carolina Center Research Reports. 1999. The Repetitive Behavior Scale-Revised. [Google Scholar]
- Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: A transnasal approach to the human brain. Nature Neuroscience. 2002;5:514–516. doi: 10.1038/nn849. [DOI] [PubMed] [Google Scholar]
- Boso M, Emanuele E, Politi P, Pace A, Arra M, Nemi SU, et al. Reduced plasma apelin levels in patients with autistic spectrum disorder. Archives of Medical Research. 2007;38:70–74. doi: 10.1016/j.arcmed.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Carter CS. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behavioural Brain Research. 2007;176:170–186. doi: 10.1016/j.bbr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson J-Å, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: A study with oxytocin and estrogen receptor-α and -β knockout mice. Proceedings of the National Academy of Science. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. The Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2002. [Google Scholar]
- Cyranowski JM, Hofkens TL, Frank E, Seltman H, Cai HM, Amico JA. Evidence of dysregulated peripheral oxytocin release among depressed women. Psychosomatic Medicine. 2008;70:967–975. doi: 10.1097/PSY.0b013e318188ade4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Wiegant VM, Westenberg HG. Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. Journal of Psychiatric Research. 2008;42:192–198. doi: 10.1016/j.jpsychires.2006.11.009. [DOI] [PubMed] [Google Scholar]
- de Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138:947–955. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biological Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;332:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Yang SH, Chan AW, Young LJ. Production of germline transgenic prairie voles (Microtus ochrogaster) using lentiviral vectors. Biological Reproduction. 2009;81:1189–1195. doi: 10.1095/biolreprod.109.077529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebstein RP, Isreal S, Lerer E, Uzefosky F, Shalev I, Gritsenko I, et al. Arginine vasopressin and oxytocin modulate human social behavior. Values, Empathy, & Fairness across Social Barriers. 2009;1167:87–102. doi: 10.1111/j.1749-6632.2009.04541.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: Plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiological Reviews. 2001;8:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Green LA, Fein D, Modahl C, Feinstein C, Waterhouse L, Morris M, et al. Oxytocin and autistic disorder: Alterations in peptide forms. Biological Psychiatry. 2001;50:609–613. doi: 10.1016/s0006-3223(01)01139-8. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Gerena D, Huang J, Kumar N, Shah M, Ughreja R, et al. Social isolation induces behavioral and neuroendocrine disturbances relevant to depression in female and male prairie voles. Psychoneuroendocrinology. 2007;32:966–980. doi: 10.1016/j.psyneuen.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2008;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin enhances the encoding of positive social memories in humans. Biological Psychiatry. 2008;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, Rinehart NJ, Tonge BJ, Lambert TJ, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorder. Biological Psychiatry. 2010;67:692–649. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Microsatellite instability generates diversity in brain and sociobehavioral traits. Science. 2005;308:1630–1634. doi: 10.1126/science.1111427. [DOI] [PubMed] [Google Scholar]
- Hammock EA, Young LJ. Oxytocin, vasopressin and pair bonding: Implications for autism. Philosophical transactions of the Royal Society of London Series B, Biological Sciences. 2006;361:2187–2198. doi: 10.1098/rstb.2006.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Bartz J, Chaplin W, Phillips A, Sumner J, Soorya L, et al. Oxytocin increases retention of social cognition in autism. Biological Psychiatry. 2007;61:498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Hollander E, Novotny S, Hanratty M, Yaffe R, DeCaria CM, Aronowitz BF, et al. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology. 2003;28:193–198. doi: 10.1038/sj.npp.1300021. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: Searching for the social brain. Annual Review of Neuroscience. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Insel TR, O’Brian DJ, Leckman JF. Oxytocin, vasopressin, and autism: Is there a connection? Biological Psychiatry. 1999;45:145–157. doi: 10.1016/s0006-3223(98)00142-5. [DOI] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: A review of oxytocin, vasopressin, and affliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neuroscience Letters. 2007;24:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Paredes J, Yee JR, Pournajafi-Nazarloo H, Bales KL, Carter CS. Neuroendocrine and behavioural responses to exposure to an infant in male prairie voles. Journal of Neuroendocrinology. 2012;24:874–886. doi: 10.1111/j.1365-2826.2012.02301.x. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Curley JP. Vasopressin, oxytocin and social behavior. Current Opinion in Neurobiology. 2004;14:777–783. doi: 10.1016/j.conb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Young LJ, Gonen D, Veenstra-VanderWeele J, Courchensne R, Courchesne E, et al. Transmission disequilibrium testing of arginine vasopressin receptor 1A (AVRPR1A) polymorphisms in autism. Molecular Psychiatry. 2002;7:503–507. doi: 10.1038/sj.mp.4001125. [DOI] [PubMed] [Google Scholar]
- Kiss A, Bundzikova J, Pirnik Z, Mikkelsen JD. Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. Journal of Neuroscience Research. 2010;88:677–685. doi: 10.1002/jnr.22226. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Cushing BS, Carter CS, Wu J, Ottinger M. Sex and species differences in plasma oxytocin using an enzyme immunoassay. Candian Journal of Zoology. 2004;82:1194–1200. [Google Scholar]
- Landgraf R, Neumann ID. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Frontiers in Neuroendocrinology. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Bouvard MP, Launay JM, Tabuteau F, Waller D, Dugas M, et al. Brief report: A double-blind study of naltrexone in infantile autism. Journal of Autism and Developmental Disorders. 1992;22:309–319. doi: 10.1007/BF01058158. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Goodman WK, North WG, Chappell PB, Price LH, Pauls DL, et al. Elevated cerebrospinal fluid levels of oxytocin in obsessive-compulsive disorder. Comparison with Tourette’s syndrome and healthy controls. Archives of General Psychiatry. 1994;51:782–792. doi: 10.1001/archpsyc.1994.03950100030003. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. Journal of Human Genetics. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, et al. The Autism Diagnostic Observation Schedule-Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–85. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Macintosh KE, Dissanayake C. Annotation: The similarities and differences between autistic disorder and Asperger’s disorder: A review of the empirical evidence. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2004;45:421–434. doi: 10.1111/j.1469-7610.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiology & Behavior. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, et al. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: A preliminary report. Biological Psychiatry. 2006;60:892–895. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Modahl C, Green LA, Fein D, Morris M, Waterhouse L, Feinstein C, et al. Plasma oxytocin levels in autistic children. Biological Psychiatry. 1998;43:270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- Naber F, van Ijzendoorn MH, Deschamps P, van Engeland H, Bakermans-Kranenburg MJ. Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: A double-blind within-subject experiment. Psychoneuroendocrinology. 2010;35:1583–1586. doi: 10.1016/j.psyneuen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Neumann JD, Kromer SA, Toschi N, Ebner K. Involvement of the brain oxytocin system in stress coping: Interactions with the hypothalamic-pituitary-adrenal axis. Progress in Brain Research. 2000;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Cacioppo JT, Morris JS, Malarkey WB, Berntson GG, Devries AC. Oxytocin increases autonomic cardiac control: Moderation by loneliness. Biological Psychology. 2011;86:174–180. doi: 10.1016/j.biopsycho.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Griffith EM. Neuropsychological function and the external validity of Asperger syndrome. In: Klin A, Volkmar FR, Sparrow SS, editors. Asperger Syndrome. New York: Guilford Press; 2000. [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Young WS, Lee HJ, et al. Oxytocin receptor knockout mice display deficits in the expression of autism-related behaviors. Hormones & Behavior. 2012;61:436–444. doi: 10.1016/j.yhbeh.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJG, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Archives of General Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. 2. Circle Pines, MN: American Guidance Service; 2004. [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, et al. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58:69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Rodgers J, Riby DM, Janes E, Connolly B, McConachie H. Anxiety and repetitive behaviours in autism spectrum disorders and Williams syndrome: A cross-syndrome comparison. Journal of Autism and Developmental Disorders. 2012;42:175–180. doi: 10.1007/s10803-011-1225-x. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. SCQ: Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schächinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin during the initial states of romantic attachment: Relations to couples’ interactive reciprocity. Psychoneuroendocrinology. 2012;37:1277–1285. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can relase oxytocin in humans. Proc Biol Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. Journal of Autism and Developmental Disabilities. 2012;42:48–59. doi: 10.1007/s10803-011-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff S, Carter C, Caplan R. Formal thought disorder and the autism spectrum: Relationship with symptoms, executive control, and anxiety. Journal of Autism and Developmental Disorders. 2008;38:1474–1484. doi: 10.1007/s10803-007-0526-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Kruesi MJ, Rettew DC, Listwak SJ, et al. Cerebrospinal fluid neurochemistry in children and adolescents with obsessive-compulsive disorder. Archives of General Psychiatry. 1992;49:29–36. doi: 10.1001/archpsyc.1992.01820010029004. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Gonzaga GC, Klein LC, Hu P, Greendale GA, Seeman TE. Relation of oxytocin to psychological stress responses and hypothalamic-pituitary-adrenocortical axis activity in older women. American Psychosomatic Society. 2006;68:238–245. doi: 10.1097/01.psy.0000203242.95990.74. [DOI] [PubMed] [Google Scholar]
- Tennenbaum HR, Ford S, Alkhedairy B. Telling stories: Gender differences in peers’ emotion talk and communication style. British Journal of Developmental Psychology. 2010;29:238–245. doi: 10.1348/2044-835X.002003. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GM, Franhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, et al. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- van Londen L, Kerkhof GA, van den Berg F, Goekoop JG, Zwinderman KH, Frankhuijzen-Sierevogel AC, et al. Plasma arginine vasopressin and motor activity in major depression. Biological Psychiatry. 1998;43:196–204. doi: 10.1016/S0006-3223(97)80433-7. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ferris CF, de Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proceedings of the National Academy of Sciences. 1994;91:400–404. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietila J, Goedken RJ, Folstein SE, et al. Examination of AVPR1a as an autism susceptibility gene. Molecular Psychiatry. 2004;9:968–972. doi: 10.1038/sj.mp.4001503. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- Wermter A, Kamp-Becker I, Hesse P, Schulte, Köme G, Strauch K, et al. Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. American Journal of Medical Genetics Part B. 2009;153:629–639. doi: 10.1002/ajmg.b.31032. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulation of social behavior. Proceedings of the National Academy of Sciences. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biological Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Yirmiya N, Rosenberg C, Levi S, Salomon S, Shulman C, Nemanov L, et al. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: Mediation by socialization skills. Molecular Psychiatry. 2006;11:488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- Ylisaukko-oja T, Alarcón M, Cantor RM, Auranen M, Vanhala R, Kempass E, et al. Search for autism loci by combined analysis of Autism Genetic Resource Exchange and Finnish families. Annals of Neurology. 2006;59:145–155. doi: 10.1002/ana.20722. [DOI] [PubMed] [Google Scholar]


