BACKGROUND
Growing numbers of patients in hospices across the United States are living and dying as a result of heart failure (HF).1 Over the past two decades, there has been a significant shift in the hospice patient population from those primarily with cancer-related diagnoses, to those of chronic illnesses; of 1.58 million patients approximately 14% are diagnosed with cardiac diseases including HF.1 While the percentage of HF patients admitted to hospice remains relatively steady, the absolute number has increased as growing numbers of hospices across the country contribute to the escalating numbers of HF patients receiving this type of care.1
According to national clinical practice guidelines, hospice can be a valuable alternative for patients with advanced HF requiring end of life care.2,3 Once admitted to hospice, patients with HF may actually live longer (402 vs. 321 days) than they would in non-hospice settings defying the notion that enrolling in hospice hastens patient deaths.4 Nevertheless, while these patients may live longer overall, researchers have documented a number of symptoms that HF patients experience in the hospice setting including pain, dyspnea, edema, and constipation calling into question whether increased length of life is associated with improved quality of life.5–7 Therefore, despite the national clinical practice guideline recommendations for referral, there remain very few evidence-based nursing interventions that guide symptom management for HF patients in hospice beyond that of medication management. In addition, few if any evidence-based interventions are directed towards HF family caregivers in the hospice setting. 8
Instead, current evidence for the efficacy of HF management and interventions in hospice is often borrowed from studies conducted outside of hospice settings or from other chronic illness populations. This is problematic for two potential reasons. Hospice, a capitated form of care, has shown unique patterns of usage 9 resulting in populations that are older and more likely to have Medicare coverage with its defined hospice benefit than those in the general medical population. A second, more critical issue with using evidence from other end stage populations such as cancer or dementia, is the difference in end of life trajectory in these populations.10,11 The degree of uncertainty as to when “end of life” is at hand in HF with its multiple cycles of exacerbation followed by periods of stability, make extrapolating findings out of more predictable trajectories problematic.
Researchers and clinicians recognize there is a critical need for hospice interventional research which is poorly understood despite a growing demand for services.12,13 However, conducting research in hospice populations remains fraught with methodological14 and ethical15 challenges. Methodological challenges involve the necessity of screening large numbers of patients to accrue an adequate sample16, attrition due to death above what is acceptable in healthier populations17, and gatekeeping by both professional and informal caregivers.14 Ethical challenges involve whether to view hospice populations as vulnerable or as autonomous 15,18, how to maintain a valid informed consent when the patient begins to decline 19, and whether research is even morally justifiable in the dying.20 Despite these challenges research is needed in hospice populations to bring evidenced based standards to clinical practice. Until we know what is efficacious and efficient how can we recommend standards of care?
Our plans were to test the feasibility of delivering the COPE psychoeducational intervention to caregivers of patients with HF. COPE is an acronym for Creativity, Optimism, Planning, and Expert Information. The intervention was modified from an approach to supporting family caregivers originally developed by Bucher, Houts, and Ades.21 The intervention was designed to support HF caregiver problem-solving by: 1) using written information about symptoms and symptom management organized to facilitate problem-solving, 2) providing a series of three home visits from nursing to review the written information aiding the caregiver to problem-solve strategies to manage patient symptoms of HF and 3) providing follow-up phone calls to reinforce the learned strategies. 22
This experienced research team was not naïve to the fact that hospice research is difficult. We believed that we had prepared well for a number of contingencies prior to the beginning of our clinical trial. Our team was well versed in the literature, had previous experiences in large studies with hospice cancer patients (SM), and smaller studies with advanced HF patients in the outpatient and acute care settings (CZ). Particular strategies, proven successful in previous studies, were taken to support recruitment and retention, such as cognitive screening for both patient and caregiver prior to obtaining informed consent, conducting the study in a setting which had a history of strong relationships with the research team, utilizing research assistants who were both hospice employees and experienced data collectors, employing an interventionist with hospice nursing experience, and providing a nursing assistant to care for the patient while the interventionist was with the caregiver.
Despite the team expertise and well-defined recruitment strategies the recruitment, both accrual and attrition, remained daunting in this population. The purpose of this paper is to report the challenges of recruiting hospice patients with heart failure and caregivers for our randomized clinical trial: those we anticipated and those we did not.
METHODS
Approvals
The proposal for this study was submitted to the bioethics committee of a large community based home hospice agency. Following approval, it was submitted to the university institutional review board and received approval following a full review.
Sample and Setting
The sample consisted of the first 648 patients screened for accrual to a study at the hospice over a 16 month time period. The hospice utilized was a large not-for-profit hospice serving south central Florida with an average daily census of >2000 patients; approximately 13% of these patients having a primary diagnosis of HF. According to hospice utilization statistics, the average length of stay for HF patients in this hospice was 120 days with a median length of stay of 30 days. The general overall mean age of the patients was this hospice is 77 years of age, for HF was 81 years.
The study was a small clinical trial piloting the usefulness of a psychosocial intervention for caregivers of hospice HF patients. Based on our estimates from previous studies and anticipated attrition, we targeted a sample of 84 dyads to ensure a final sample of 60 dyads with complete data. Inclusion criteria were adults (+18 years) with a primary diagnosis of HF that was expected to be their cause of death, and an identified family caregiver who provided at least four hours of care per day. Both patient and caregiver had to consent to participate, have at least a sixth grade education, able to read and understand English, and achieve a minimum score of 8 on the Short Portable Mini-mental Status Exam23. Patients were excluded if they had a Palliative Performance Scale 24 score of 30 or less. These criteria helped to insure that patients could reliably report their own symptoms through all three data collection periods and increased the likelihood that patients would survive beyond the end of the study. Because the study focused primarily on management of five common problems, dyspnea, edema, pain, depression and constipation, patients were excluded if they did not have two of these five problems as documented by baseline data collection.
Screening Instruments
Palliative Performance Scale
The Palliative Performance Scale (PPS) 24 was used to evaluate functional status. The PPS was developed specifically for hospice patients and does not include hospitalization as a criterion. It is a single item that generates a score between 0 (dead) and 100 (normal activity level). It has been used successfully in earlier studies25 to predict which patients will survive long enough to benefit from a brief intervention. Concurrent validity was demonstrated using a sample of 213 hospice inpatients24.
Short Portable Mental Status Questionnaire
The Short Portable Mental Status Questionnaire (SPMSQ) 23 was used to screen for mental decline. It is a simple 10-item test of remote memory, knowledge of current events, and mathematical ability. It is administered by the interviewer and scored on the number of errors an individual makes as a measure of levels of mental impairment. The SPMSQ was used to screen patients and caregivers for eligibility for the study.
Data Collection and Analysis
The investigative team kept careful records of the number of patient/caregiver dyads screened, accrued to the study, weeks in the study, reasons for nonaccrual and reasons for attrition. These were entered into a grid daily as the data were collected. Results are presented as frequencies and percentages. Demographic data were not collected unless the patients and caregivers were accrued to the study.
RESULTS
Accrual
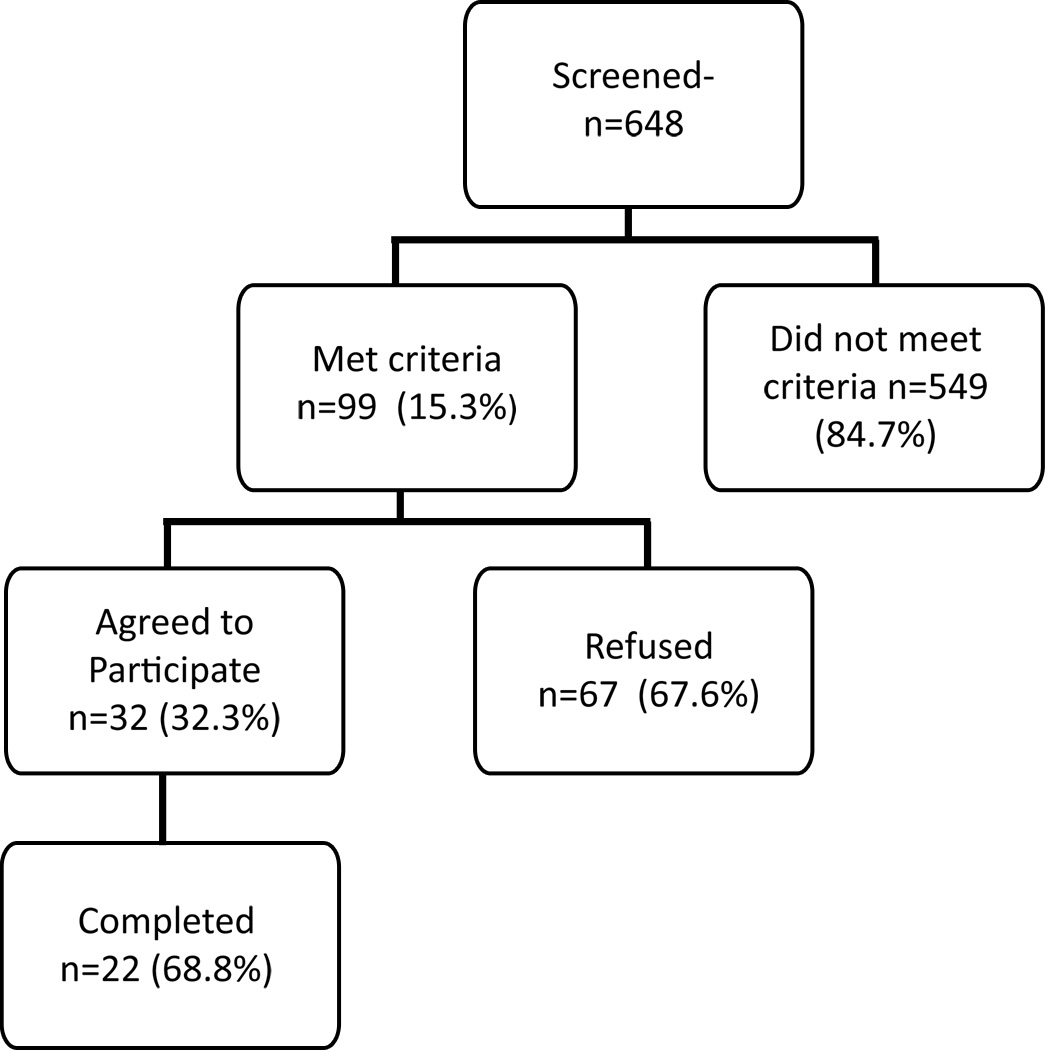
The sample consisted of 648 patients admitted to the hospice with a HF primary diagnosis. Of the 648 screened, only 99 (15.3%) met criteria for the study (Figure 1). Of the 549 who did not meet the criteria, the two most common reasons were that they were not in home care but resided in either an assisted living facility (ALF) or a nursing home or that they had died prior to baseline data collection (See Table 1). Approximately, 13% had less than two of the target symptoms documented in the medical record. Examples of other reasons included no caregiver, altered mental status, or reduced performance status. An additional 10% were excluded for other reasons which included factors such as the patient was actively dying.
Figure 1.
Accrual breakdown for eligible patients
Table 1.
Frequency and Percent of Reasons Screened Patients did not Meet Criteria for Study (n=549)
| Criterion Not Met | Frequency (%) |
|---|---|
| ALF* or Nursing Home Patient | 121 (22) |
| Patient Died Prior to Data Collection | 121 (22) |
| Patient reported less than 2 symptoms | 71 (13) |
| No Caregiver Available | 55 (10) |
| Low Performance Status** | 44 (8) |
| Failed Mental Status Screening | 44 (8) |
| Lacked Education or Spoke No English | 38 (7) |
| Actively Dying | 11 (2) |
| Missing Data | 44 (8) |
ALF = Assisted Living Facility
As measured by the Palliative Performance Scale
Of the 99 patients who met criteria and were contacted about entering the study, 32 (32.3%) agreed to participate. For the 67 who were not accrued, a variety of reasons for refusal were documented. The most common reason given for refusal was simply that they did not want to participate with no further elaboration. The majority of the remaining felt too overwhelmed (either the caregiver or the patient) or that the patient was too sick to participate (Table 2).
Table 2.
Frequency and Percent of Reasons for Refusal of Eligible Dyads to Enter Study (n=66)
| Reason for Refusal | Frequency (%) |
|---|---|
| Does not want to participate | 12 (32) |
| Caregiver overwhelmed | 11 (17) |
| Caregiver believes patient too sick | 10 (16) |
| Patient overwhelmed | 9 (13) |
| Caregiver Refused for patient | 2 (4) |
| No time | 1 (<1) |
| Patient too near death | 1 (1) |
| Unknown reasons | 10 (16) |
Attrition
Patients and caregivers accrued to the study were to give baseline data upon accrual, receive a two week intervention, and then give follow-up data during weeks 4 and 5 of hospice care. Ten patient-caregiver dyads in this initial sample of 32 dropped out of the study before the end of this period. Two were discharged from hospice because of improvement in their symptoms, and others could not be reached or did not want to answer any more questions. The staff had to withdraw one dyad from the study when it became clear that the caregiver suffered cognitive decline. Other reasons for attrition varied and are presented in Table 3.
Table 3.
Frequency and Percent of Reasons for Withdrawing from Study (N=10)
| Reasons for Attrition | Frequency (%) |
|---|---|
| Patient discharged from hospice; improved | 2 (20) |
| No response to phone calls; unable to reach for follow-up data collection | 2 (20) |
| Patient did not want to answer any more questions | 2 (20) |
| Caregiver felt overwhelmed with home and hospice responsibilities | 1 (10) |
| Wanted to wait until after holidays to continue (outside of time parameters) | 1 (10) |
| Patient no longer willing to participate; no reason given | 1 (10) |
| Caregiver found to be demented; staff withdrew dyad from study | 1 (10) |
Discussion
The purpose of this paper was to report the challenges of recruiting hospice patients and caregivers for our randomized clinical trial, those challenges we anticipated and those we did not. Of the first 648 HF patients admitted to the hospice after the start of the study, only 32 (5%) were accrued and 22 (3.4%) ultimately completed the study. This came at an unanticipated cost to the grant as resources were primarily directed at screening rather than delivery of the intervention. Although this accrual number is very low, it is consistent our earlier studies of hospice cancer patients, in which 5% were accrued26. Our numbers were slightly lower in this study with 3.4% being accrued. In both cases, the patients were being accrued at local hospices; the primary difference was their diagnosis. However the dyads who were recruited in a cancer center rather than in a hospice setting had only slightly better accrual rates at 5.5% being accrued. 16 It is also in keeping with HF studies where a clinical trial at a large, urban, primary care center reported a 4% accrual rate at one site.27 We believed that our approach to recruiting patients from a large, progressive hospice would offset the challenges associated with smaller hospices. Still, our results highlight the need for large catchment areas for HF hospice studies. With 80% of hospices reporting an average yearly census of less than 500 patients total this presents a unique challenge to HF hospice studies.1
Accrual
Ineligible Dyads
Of the 648 HF patients admitted to the hospice during the timeline for this study, nearly 85% did not meet the criteria of the study. The most common reasons were that the patient died before the research team screened their record, that the patient already was admitted to a nursing home or assisted living facility (ALF) and thus had no consistent family caregiver, or the patient did not report at least two symptoms. Given that baseline data collection was to have occurred within 24–72 hours of hospice admission, it was unfortunate that 22% died before study admission because this means that these patients and caregivers had very limited opportunity to benefit from the palliative care services offered by the hospice, other than for crisis family support and bereavement services. Late referral to hospice has been reported in a number of previous studies for HF patients (and in general).5,10
Compared to a previous study of HF patients in an urban area, our study was not impacted by lack of physician support or competition from other studies.27 Still, late referral to hospice has been attributed to a number of factors including lack of provider buy in to hospice as an appropriate delivery of care system until often very late in the disease trajectory.28–31 Our study underscores the need for continued efforts in developing evidence-based approaches to enhance timely referral to hospice. Remarkably, the same percentage of patients in ALFs or nursing homes was consistent with the earlier cancer study26 suggesting that admission to hospice while in ALFs or nursing homes is reaching similar proportions for heart failure as with cancer. Another 10% of heart failure patients lived alone in private homes having no caregivers available, another statistic that is consistent with the earlier cancer study.26 This is not a surprising finding given that the study occurred in Florida where many retirees live. Commonly, a married couple retires and moves to Florida, away from extended family, and when one dies, the other is left alone with no one to serve as caregiver when he or she becomes seriously ill. Thus, lack of a caregiver is a fairly common problem in this hospice requiring tailored interventions with minimal resources.
It was interesting that our initial screening strategies led to about 13% of patients declared ineligible due to documentation of less than the minimum of two symptoms required for enrollment. In this hospice, an admission team sees the patient initially and does the admission paperwork. As the study progressed, the screeners were advised to review the patient’s chart to verify the presence or absence of symptoms. This increased accrual because patients did not always reveal all of their symptoms to the admission staff members. If patients are far enough along on the HF disease trajectory to warrant hospice, it seemed likely that they would have multiple symptoms as has been reported elsewhere5,32, and on further investigation, we found that many did. However, a group of these patients apparently did have only one symptom at the time of admission to the hospice. This may reflect the current guideline directed medical management which should result in decreased symptoms, symptom volatility33 or it could also reflect lack of documentation of symptoms in the medical record.
Refusal to Participate
Most of the reasons given for refusal to participate in the study centered around the caregivers. In 16% of the cases, no specific reason was given. But when a reason was given, it tended to be that the caregiver felt overwhelmed, had no time or believed the patient to be too ill to be in a study. This finding is supported by a recent review of hospice research which highlighted the ways in which caregivers function as one level of gate-keepers.14 Unfortunately, limiting access to patients who may want to participate in research for altruistic satisfaction may deprive that very person of a meaningful activity in their end of life. 34
Attrition
Once accrued to the study, 10 (31%) of the original 32 withdrew from the study prior to its completion. This attrition is high for any study but is better than an earlier study conducted to test the COPE intervention. In a cancer group, only 38% of the dyads accrued completed the study, resulting in a 62% rate of attrition 26. The most common reason for attrition in the cancer group was the patient dying (21%) or becoming too debilitated to continue (29%). It was this high attrition that caused the investigators in this project to shorten the intervention time to decrease attrition, particularly from patient decline and death. This change in protocol apparently was successful because the attrition among these HF patients was half of what it was for the cancer patients; in fact, two of the HF patients improved enough to be discharged from hospice care. Thus, it would seem that shortening the protocol achieved its purposes and that possibly HF patients are likely to survive longer than cancer patients and drop out of a study at a lower rate. However, this is still a very high rate of attrition, making this population very costly to study. In addition, the population of hospice patients with HF, while growing in numbers, is already much smaller than hospice patients with cancer, causing attrition to have a greater potential impact on the study results.
Intervention
Based on our final sample size, we were not able to fully measure the outcomes we had selected for the intervention. We had hoped to measure the effect size of the intervention impact on caregiver burden, depression and anxiety, caregiver knowledge, patient symptoms and quality of life but were unable to accomplish this as reported elsewhere.22 Therefore, the hospice caregiver intervention science remains without proven interventions.
The increasingly standard use of the electronic medical record may facilitate accurate accounting of patient and caregiver changes at the point of direct care. For example, standardization of measures used by clinicians giving direct patient care to assess symptoms and quality of life may allow for randomized clinical trials to be conducted using already existing data being collected in direct patient care. It will be essential to decrease the burden of data collection from these hospice patients and caregivers if we are to do this important work.
Limitations
As with any feasibility study, we recognized issues in our methodology across recruitment and enrollment. For example, we originally intended to rely on documentation of two symptoms in the medical record to accrue patients into the study. After a period of time, we challenged our own findings of the high number of patients with only one symptom documented so the screeners began to use the medical record sent from the physician to verify presence or absence of symptoms. As a result, we were able to find additional eligible patients.
Conclusion
Our approach to this study was based on our background with hospice research as well as consideration of lessons learned from other studies in HF research. We anticipated and planned for an older population, cognitive impairment, reduced functional status, caregivers as gatekeepers (both provider and family). We believed that due to the high volume of HF patients in this large hospice setting, we could achieve an adequate sample size for the study. We expected that we would be testing feasibility of the COPE intervention in the HF population in hospice. In fact, what we were testing was the feasibility of recruitment and accrual of patients for a HF study in hospice.
Feasibility will remain a challenge unless funding sources recognize the value-added of symptom management and quality of life studies in hospice for prevention of emergency department visits and reducing caregiver burden; allowing for the higher cost of research with seriously ill patients. On the other hand, efforts to either upstream palliative care or minimize the occurrence of late referral to hospice will allow patients and caregivers to enroll in hospice prior to reaching the state of being overwhelmed or exhausted.
This study contributes to the discussion of best value research methods for hospice evidence. We must continue to question ourselves: are large national databases a more feasible means of data collection and analysis than the direct, patient, caregiver contact methods? Or is the patient the best source of current information about symptom status and quality of life. Further use of qualitative methods may provide additional insight at the level of the patient as well as the caregiver.
In a study of massage for cancer patients, Gorman and colleagues wrote that intervention studies in hospice require a “humble restructuring of expectations” (35 p. 195). We humbly agree. Nevertheless, we must not admit defeat; we must continue to advocate for these patients and their families. We recognize that there is high burden for all involved and, it is our responsibility as nurses and researchers to remain committed to alleviating the burden and improving quality of care.
What’s New?
Caregivers of patients with heart failure often act as gatekeepers to participation in studies suggesting the need for earlier interventions to reduce caregiver burden.
Creativity and past successes in hospice research does not predict future success in this complex patient population
Broad inclusion criteria will be essential to maximize number of potential participants across a large catchment area.
Acknowledgments
The authors would like to acknowledge the National Institute of Nursing Research 1R21NR 011224-01A1 for funding of this study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cheryl Zambroski, University of South Florida, College of Nursing.
Harleah Buck, School of Nursing, The Pennsylvania State University, 201 Health and Human Development East, University Park, PA 16802.
Chris Garrison, St. Petersburg College Nursing Department, St. Petersburg, FL USA.
Susan C. McMillan, Thompson Professor of Oncology Nursing, College of Nursing, University of South Florida, Tampa, FL 33612.
References
- 1.NHPCO facts and figures: hospice care in American. 2011 http://www.nhpco.org/files/public/Statistics_Research/2011_Facts_Figures.pdf, 2012.
- 2.Jessup M, Abraham WT, Casey DE, et al. 2009 Focused Update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53(15):1343–1382. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 3.Heart Failure Society of America. Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16(6):475–539. [Google Scholar]
- 4.Connor SR, Pyenson B, Fitch K, Spence C, Iwasaki K. Comparing hospice and nonhospice patient survival among patients who die within a three-year window. J Pain Symptom Manage. 2007;33(3):238–246. doi: 10.1016/j.jpainsymman.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Zambroski CH, Moser DK, Roser LP, Heo S, Chung ML. Patients with heart failure who die in hospice. Am Heart J. 2005;149(3):558–564. doi: 10.1016/j.ahj.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 6.McMillan SC, Dunbar SB, Zhang W. The Prevalence of Symptoms in Hospice Patients in End-Stage Heart Disease. J Hosp Palliat Nurs. 2007;9(3):124–131. [Google Scholar]
- 7.Janssen DJ, Spruit MA, Wouters EF, Schols JM. Daily symptom burden in end-stage chronic organ failure: a systematic review. Palliat Med. 2008;22(8):938–948. doi: 10.1177/0269216308096906. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz KA, Lynn J, Dy SM, et al. Evidence for Improving Palliative Care at the End of Life: A Systematic Review. Ann Intern Med. 2008;148(2):147–159. doi: 10.7326/0003-4819-148-2-200801150-00010. [DOI] [PubMed] [Google Scholar]
- 9.Han B, Remsburg RE, McAuley WJ, Keay TJ, Travis SS. National trends in adult hospice use: 1991–1992 to 1999–2000. Health affairs (Project Hope) 2006;25(3):792–799. doi: 10.1377/hlthaff.25.3.792. [DOI] [PubMed] [Google Scholar]
- 10.Bain KT, Maxwell TL, Strassels SA, Whellan DJ. Hospice use among patients with heart failure. Am Heart J. 2009;158(1):118–125. doi: 10.1016/j.ahj.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Hupcey JE, Penrod J, Fenstermacher K. Review Article: A Model of Palliative Care for Heart Failure. Am J Hosp Palliat Med. 2009;26(5):399–404. doi: 10.1177/1049909109333935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NIH Consensus Development Program. Vol 21. Washington, D.C.: National Institute of Nursing Research; 2004. NIH State-of-the-Science Conference Statement on improving end-of-life care; pp. 1–26. [PubMed] [Google Scholar]
- 13.Hupcey JE, Penrod J, Fogg J. Heart failure and palliative care: implications in practice. J Palliat Med. 2009;12(6):531–536. doi: 10.1089/jpm.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wohleber AM, McKitrick DS, Davis SE. Designing Research With Hospice and Palliative Care Populations. Am J Hosp Palliat Med. 2012;(5):335–345. doi: 10.1177/1049909111427139. [DOI] [PubMed] [Google Scholar]
- 15.Casarett D, Ferrell B, Kirschling J, et al. NHPCO Task Force Statement on the Ethics of Hospice Participation in Research. J Palliat Med. 2001;4(4):441–449. doi: 10.1089/109662101753381566. [DOI] [PubMed] [Google Scholar]
- 16.Ransom S, Azzarello LM, McMillan SC. Methodological issues in the recruitment of cancer pain patients and their caregivers. Res Nurs Health. 2006;29(3):190–198. doi: 10.1002/nur.20129. [DOI] [PubMed] [Google Scholar]
- 17.Schneider S, Kub JK, Hughes MT, et al. Barriers to Research Participant Retention in a Longitudinal Study of End-of-Life Decision Making. J Hosp Palliat Nurs. 2010;12(3):177–183. [Google Scholar]
- 18.Dobratz MC. Issues and dilemmas in conducting research with vulnerable home hospice participants. J Nurs Scholarsh. 2003;35(4):371–376. doi: 10.1111/j.1547-5069.2003.00371.x. [DOI] [PubMed] [Google Scholar]
- 19.Lawton J. Gaining and maintaining consent: ethical concerns raised in a study of dying patients. Qual Health Res. 2001;11(5):693–705. doi: 10.1177/104973201129119389. [DOI] [PubMed] [Google Scholar]
- 20.Duke S, Bennett H. Review: A narrative review of the published ethical debates in palliative care research and an assessment of their adequacy to inform research governance. Palliat Med. 2010;24(2):111–126. doi: 10.1177/0269216309352714. 2010. [DOI] [PubMed] [Google Scholar]
- 21.Bucher JA, Houts BS, Ades T. Family Caregiving. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 22.McMillan SC, Small BJ, Haley WE, Zambroski CH, Buck HG. The COPE Intervention for Caregivers of Patients with Heart Failure: An Adapted Intervention. J Hosp Palliat Nurs. doi: 10.1097/NJH.0b013e31827777fb. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 24.Anderson F, Downing GM, Hill J, Casorso L, Lerch N. Palliative performance scale (PPS): a new tool. J Palliat Care. 1996;12(1):5–11. [PubMed] [Google Scholar]
- 25.McMillan SC, Small BJ, Weitzner M, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer. 2006;106(1):214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]
- 26.McMillan SC, Weitzner MA. Methodologic issues in collecting data from debilitated patients with cancer near the end of life. Oncol Nurs Forum. 2003;30(1):123–129. doi: 10.1188/03.ONF.123-129. [DOI] [PubMed] [Google Scholar]
- 27.Pressler SJ, Subramanian U, Shaw RM, Meyer LE, Stoudemire K, Gradus-Pizlo I. Research in patients with heart failure: challenges in recruitment. Am J Crit Care. 2008;17(3):198–203. [PubMed] [Google Scholar]
- 28.Zambroski CH. Hospice as an alternative model of care for older patients with end-stage heart failure. J Cardiovas Nurs. 2004;19(1):76–83. doi: 10.1097/00005082-200401000-00012. quiz 84–75. [DOI] [PubMed] [Google Scholar]
- 29.American Society of Clinical Oncology. The Debate in Hospice Care. J Oncol Pract. 2008;4(3):153–157. doi: 10.1200/JOP.0838503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlson MDA, Morrison RS, Bradley EH. Improving Access to Hospice Care: Informing the Debate. J Palliat Med. 2008;11(3):438–443. doi: 10.1089/jpm.2007.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGorty EK, Bornstein BH. Barriers to physicians’ decisions to discuss hospice: insights gained from the United States hospice model. J Eval Clin Pract. 2003;9(3):363–372. doi: 10.1046/j.1365-2753.2003.00406.x. [DOI] [PubMed] [Google Scholar]
- 32.Adler ED, Goldfinger JZ, Kalman J, Park ME, Meier DE. Palliative care in the treatment of advanced heart failure. Circ J. 2009;120(25):2597–2606. doi: 10.1161/CIRCULATIONAHA.109.869123. [DOI] [PubMed] [Google Scholar]
- 33.Fitzsimons D, Strachan PH. Overcoming the challenges of conducting research with people who have advanced heart failure and palliative care needs. Eur J Cardiovasc Nurs. 2012;11(2):248–254. doi: 10.1016/j.ejcnurse.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Terry W, Olson LG, Ravenscroft P, Wilss L, Boulton-Lewis G. Hospice patients' views on research in palliative care. Intern Med J. 2006;36(7):406–413. doi: 10.1111/j.1445-5994.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 35.Gorman G, Forest J, Stapleton SJ, et al. Massage for cancer pain: a study with university and hospice collaboration. J Hosp & Palliat Nurs. 2008;10(4):191–197. doi: 10.1097/01.njh.0000319160.89854.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]