Abstract
Background
The prevalence of alcohol use during adolescence is concerning given that early age of alcohol initiation is correlated with development of alcohol-related problems later in life. The purpose of this series of studies was to assess whether voluntary ethanol exposure during adolescence would influence ethanol drinking behavior in adulthood using an animal model.
Methods
Pair-housed Sprague-Dawley adolescent (P28-42) rats of both sexes were given single bottle access to one of three solutions in their home cages--10% ethanol in "supersac" (0.125% saccharin and 3% sucrose) (EtOH/SS), supersac without ethanol (SS), or water-- for 30 min. every other day for a total of 8 drinking days, or were left non-manipulated. Animals were non-manipulated thereafter until adulthood (P70) at which time they were given one-bottle, 30 min. limited access tests with 20% ethanol every other day (Exp 1), 10% ethanol in SS (Exp 2) or SS without ethanol (Exp 3).
Results
Adolescent EtOH/SS exposure increased adulthood consumption of EtOH/SS (Exp 2), but not 20% unsweetened ethanol (Exp 1) or SS (Exp 3), with this increase most pronounced at the beginning of the 8 intake day procedure. Access to SS (without EtOH) during adolescence produced an analogous effect, with increased adult SS consumption during the first two intake days, but no increases in either of the ethanol test solutions.
Conclusion
Solution-specific increases in adulthood intake after adolescent exposure are most likely associated with solution acceptance due to familiarity. This is an important consideration for future intake studies assessing the influence of ethanol exposure during adolescence on intake of ethanol in adulthood.
Keywords: Adolescent, Alcohol, Intake, Adult Behavior, Sprague-Dawley Rat
Introduction
The issue of long-term consequences of alcohol exposure during adolescence is important given the prevalence of binge-level alcohol consumption during this developmental period (Johnston et al., 2009). Indeed, in both humans (SAMSHA, 2008) and rodents (Brunell and Spear, 2005; Doremus et al., 2005; Vetter et al., 2007; Vetter-O’Hagen et al., 2009), adolescents drink two- to three-fold more alcohol than their mature counterparts. This pattern of alcohol consumption during adolescence is concerning given that adolescents have been found to be more sensitive to certain adverse consequences of ethanol exposure. For instance, adolescents diagnosed with alcohol use disorders (AUDs) already show cognitive impairments despite a relatively short duration of alcohol exposure (Brown et al., 2000). In adolescent rodents relative to adults, acute exposure to alcohol induces more disruption in long-term potentiation (LTP) (Pyapali et al., 1998; Swartzwelder et al., 1995), whereas repeated alcohol exposure has been reported to result in greater impairments in memory (Markwiese et al., 1998) and learning (Sircar and Sircar, 2005), as well as greater brain damage in certain brain regions (Crews et al., 2000; Pascual et al., 2007) than exposure in adulthood.
Whether the deleterious effects of adolescent alcohol exposure persist into adulthood is a question that still remains, with surprisingly little data generated to date regarding this critical topic. Evidence is beginning to emerge, however, suggesting that repeated exposure to alcohol during adolescence may indeed influence neurobehavioral outcomes in adulthood. For instance, Pascual and colleagues (2007) reported cognitive deficits 20 days after repeated exposure to alcohol intraperitoneally (i.p.) during adolescence, but not following a comparable alcohol exposure regimen administered in adulthood. Similarly, another recent study found impairments in reversal learning in the Morris Water Maze task 30 days after repeated exposure to alcohol intragastrically (i.g.) during adolescence (Coleman et al., 2011). Thus, some evidence suggests that adolescent alcohol exposure may produce long-term cognitive deficits, particularly in terms of behavioral inflexibility.
Another important question is whether adolescent alcohol exposure will increase the propensity to consume alcohol in adulthood. Indeed, early age of initiation of alcohol use in humans has been reported to correlate with increased risk for AUDs in adulthood (Grant and Dawson, 1997), although causality cannot be determined from such correlations. Studies using rats to assess effects of adolescent ethanol exposure on voluntary ethanol intake in adulthood have been inconsistent, with some reports of increases in later ethanol consumption (Pascual et al., 2009; Siciliano and Smith, 2001), contrasting with others that found no increases (Tolliver and Samson, 1991; Vetter et al., 2007). Studies using mice have shown that increased ethanol consumption in adulthood as a result of adolescent ethanol exposure may be mediated by both sex (Strong et al., 2010) and genotype, with adolescent exposed C57BL/J6 mice showing an increase in adult intake, yet this effect did not emerge in DBA2/J mice (Moore et al., 2010). Thus, mere exposure to ethanol during adolescence may not be sufficient to increase subsequent ethanol intake and may be influenced by a number of different variables including differences in experimental paradigms (e.g., ethanol exposure that is experimenter-administered vs voluntarily consumed), as well as genetic and sex differences in susceptibility for continued elevated ethanol intake into adulthood.
The present series of experiments was designed to examine the long-term effects of consumption of a sweetened 10% ethanol solution or the sweetened solution without ethanol during adolescence on intake of two different ethanol solutions [20% EtOH unsweetened (Exp 1) or 10% EtOH sweetened (Exp 2)], as well as the sweetened solution without ethanol (Exp 3) in adulthood. A voluntary, limited access exposure model was chosen because other studies have suggested that experimenter-administered and voluntarily self-administered ethanol may be different in terms of influences on the brain reward systems (Nurmi et al., 1996; Steffenson et al., 2009), as well as motivation to later voluntarily consume ethanol (Gilpin et al., 2012; Walker and Ehlers, 2009). Also, provision of intermittent access to a sweetened ethanol solution resembles human adolescent alcohol consumption in that it is voluntary and does not require water deprivation.
Methods
Subjects
A total of 256 adolescent male and female Sprague-Dawley rats bred and reared in our colony at Binghamton University were used in these experiments. On the day after birth, postnatal day (P) 1, litters were culled to 8-10 pups, with a sex ratio of 6 males and 4 females retained whenever possible. Pups were housed with their mother in a standard clear plastic tub with pine shavings until pair-housed with a littermate at the time of weaning (P21). All animals were maintained in a temperature-controlled vivarium on a 12:12-h light: dark cycle (lights on 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and water. Animals used in this experiment were maintained and treated in accordance with guidelines for animal care established by the National Institutes of Health (8th Ed), using protocols approved by the Binghamton University Institutional Animal Care and Use Committee.
Design & Procedures
Each experiment used a 2 (sex) x 4 (adolescent exposure condition) factorial design (n=12/ group in Exp 1; n=10/group in Exp 2 & 3). Animals were re-housed with a non-littermate partner at P25, thereby allowing assignment of each pair to the same adolescent exposure condition, while also controlling for litter effects by allowing only 1 animal per litter to be placed into any given experimental condition (Holson and Pearce, 1992; Zorilla, 1997).
Adolescent Exposure (P28-42)
Throughout the exposure period, each animal was given access to their assigned solution for 30 min. every other day for a total of 8 drinking days. For each drinking session, pair-housed adolescents were separated by a mesh divider in their home-cage for approximately 10 minutes prior to and after presentation of the experimental solutions. During the 30 minute access period, both animals in each pair were given a single bottle containing one of three solutions: 10% ethanol in "supersac" (0.125% saccharin and 3% sucrose) (EtOH/SS condition), supersac without ethanol (SS condition), or water (H20 condition). Bottles were weighed before and after the 30 minute access period. Ethanol/supersac was chosen because non-water deprived animals readily consume this solution (Broadwater et al., 2010; Ji et al., 2008; Walker et al., 2008). On the last drinking day, tail blood samples were collected for determination of blood ethanol concentrations (BECs). A separate group of animals were not handled throughout the adolescent exposure period except for routine animal care (i.e., cage changes, etc.) [Non-manipulated (NM) condition]. At the end of the exposure period, animals were left undisturbed other than routine colony maintenance (e.g., cage changing) from P43-69 until assessment of ethanol intake in adulthood.
Adult Intake (P70-84)
The intake procedures in adulthood were identical to those used for adolescent exposures, except in adulthood all animals from the four adolescent exposure conditions were exposed to either 20% EtOH in water (Experiment 1), 10% EtOH/ SS (Experiment 2), or SS without ethanol (Experiment 3). Separate animals were used for each experiment.
BEC Analysis
Tail blood samples were collected into heparinized tubes, rapidly frozen and maintained at −80 °C until analysis. Samples were assessed for BEC via headspace gas chromotography using a Hewlett Packard (HP) 5890 series II Gas Chromatograph (Wilmington, DE). At the time of assay, blood samples were thawed and 25-μl aliquots were placed in airtight vials, which were then placed in a HP 7694E Auto Sampler that heated each vial for 8 min. prior to extracting and injecting a 1.0 ml sample of the gas headspace into the gas chromatograph. Ethanol concentrations in each sample were determined using HP Chemstation software, which compares the peak area under the curve in each sample with those of standard curves derived from reference standard solutions.
Data Analysis
Prior to analysis, measured fluid amounts were adjusted for leakage based on average data from empty cage controls for each type of solution. Separate repeated measures ANOVAs were used to analyze intake across days in 2 day blocks during adolescence and in adulthood, with Fisher’s LSD planned comparisons used to determine significant differences among adolescent exposure groups within each block. Homogeneity of variance was assessed prior to each ANOVA, with log transformations used to correct violations (adulthood intake in Experiment 1 was the only measure for which such transformations were needed). Correlations were conducted between ethanol intake (g/kg) and BECs determined on the last drinking day for both adolescent and adult consumption periods. Correlational analyses were also used to compare adolescent intake with consumption levels in adulthood.
Results
Adolescent Intake
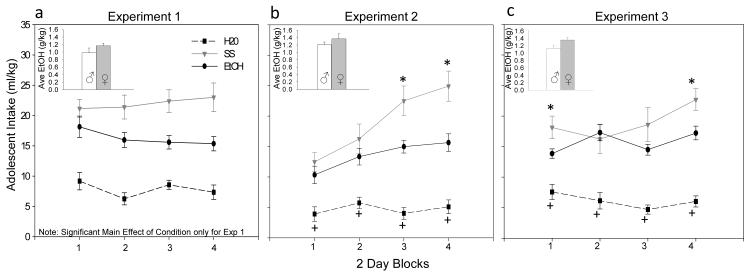
For each experiment, separate 2 (sex) x 3 (adolescent exposure condition: H20, SS and EtOH/SS) repeated measures ANOVAs were used to compare adolescent consumption of each type of solution (ml/kg) across the 4 blocks of 2 intake days. Results were fairly consistent across all three experiments, with a significant main effects of exposure condition [F(2,66)=39.14; F(2,54)= 39.70; F(2,54)= 42.09, p< .01] emerging, where animals given SS drank significantly more than animals given ethanol and water-exposed animals drank the least amount. In experiments 2 & 3, additional significant effects of block [F(3,162)= 14.19; F(3,162)= 2.89, p< .01], and block by condition interaction [F(6,162)= 5.55; F(6,162)= 2.66, p< .01] emerged. SS-exposed animals drank significantly more than EtOH/SS animals on blocks 3 and 4 in experiment 2 (see Fig. 1b) and significantly more on block 1 and 4, with a tendency (p=.06) for more intake on block 3 as well in experiment 3 (see Fig 1c). A significant main effect of sex [F (1,54) = 5.59, p< .05] emerged in experiment 3 only, with females drinking significantly more ml/kg (14.7 ± 1.5) than males (12.6 ± 1.2) regardless of fluid type.
Fig. 1.
Adolescent consumption (ml/kg) of water (H2O), supersac (SS) and 10% ethanol in supersac (EtOH/SS) across four 2-day blocks of intake days during the exposure period (from P28-42) in Experiments 1-3.(a) In Experiment 1, adolescents consumed significantly more EtOH/SS & SS relative to H2O, and more SS than EtOH/SS. (b,c) Adolescents given access to H2O drank significantly less relative to both SS- and EtOH/SS-exposed counterparts (see +) and animals given SS drank significantly more than animals given access to EtOH/SS towards the end of the exposure period in Experiments 2 & 3 and in the beginning of Experiment 3 (see *). Inserts show male and female average EtOH/SS intake (g/kg) collapsed across day during the adolescent exposure period for each experiment. Adolescent females tended to drink more ethanol than males.
Although slightly different patterns of ethanol consumption emerged, overall exposure levels were generally consistent across the three experiments in terms of g/kg intake, as well as BECs. Adolescent EtOH intakes (g/kg) in each experiment were analyzed via repeated measures ANOVAs across 4 blocks of 2 intake days, with sex as the between subjects factor. A significant main effect of block emerged in Exp 2 and 3 [F(3,54)= 4.95; F(3,54)= 28.82, p< .01], with animals increasing their ethanol intake over blocks. No other significant effects or interactions emerged. Intakes averaged about 1.2 g/kg across all three experiments, with females tending to drink more than males (see inserts in Fig. 1a-c), although these differences did not reach significance in any of the experiments.
Significant correlations between EtOH intake (g/kg) on the last drinking day and BECs emerged in all three experiments [r= .59; r= .85; r=.77, respectively, all p values < .01]. Individual differences in ethanol consumption were evident in all three experiments, with intakes ranging from about 0.5 to 3 g/kg on the last drinking day (in Exp 1, range of: 0.5 - 2.96 g/kg; Exp 2: 0.5 - 1.3 g/kg; Exp 3: 0.26 - 2.1 g/kg). Average BECs were in the moderate range (i.e., 20-90 mg/dl—see Eckardt et al., 1998) on the last drinking day in all three experiments, with notable individual differences, as with the intake data [mean mg/dl ± SEM (range) in Exp 1: 24.1 ± 4.7 (0.5 – 79); Exp 2: 27 ± 0.5 (0 -72); Exp 3: 26 ± 11.5 (1 – 100)].
Experiment 1: Adult Intake of 20% EtOH
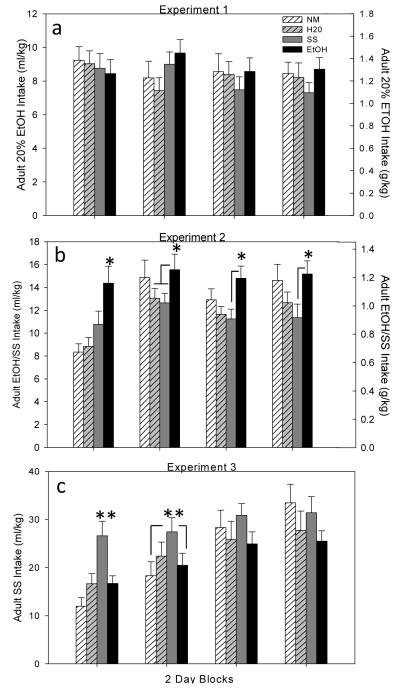
A 2 (sex) x 4 (adolescent exposure: NM, H20, SS or EtOH/SS) repeated measures ANOVA across the 4 blocks of 2 intake days revealed no adolescent exposure effects on adult 20% ethanol (g/kg) consumption (see Fig. 2a). A main effect of sex [F(1,87)= 100.69, p<.01] emerged as expected, with females drinking significantly more 20% ethanol (1.72 g/kg ± .06) in adulthood than their male counterparts (.98 ± .04). Ethanol intake (g/kg) and BECs on the last drinking day were correlated [r= .42, p< .01], with intakes on this last day ranging from 0.47 – 3.6 g/kg, producing BECs from 0 – 52 mg/dl.
Fig. 2.
Blocked adult intake (average of 2 intake days= 1 block) of 20% EtOH (Experiment 1), 10% EtOH in SS (Experiment 2) and Supersac (Experiment 3) of animals in each of the four adolescent exposure conditions. (a) No effect of adolescent exposure condition emerged in Experiment 1. (b) In Experiment 2, animals exposed to EtOH/SS as adolescents drank significantly more EtOH/SS in adulthood (see *), although this effect dissipated across blocks relative to all exposure conditions except SS. (c) In Experiment 3, SS exposure during adolescence significantly increased SS intake in adulthood (see **) in block 1 relative to all other conditions and relative to NM & EtOH/SS exposure conditions in block 2.
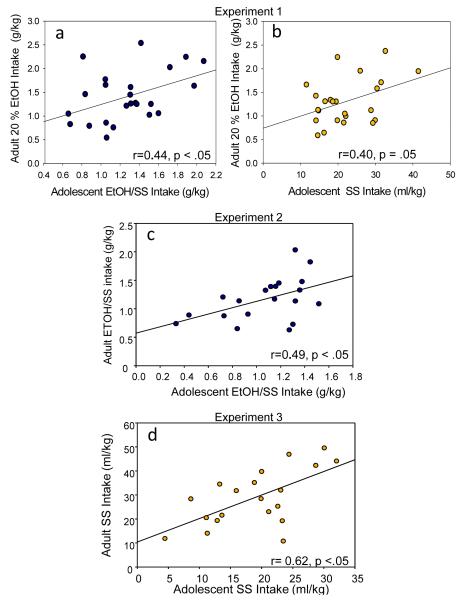
When assessing correlations between average adolescent intake and average 20% EtOH drinking in adulthood, a significant positive correlation emerged between adolescent EtOH/SS intake and adult 20% EtOH intake [r=0.44, p< .05], as well as a marginally significant tendency for adolescent SS consumption to correlate positively with adult 20% EtOH intake [r=0.40, p= .05] (see Fig. 3a,b). H20 consumption in adolescence did not correlate with 20% EtOH consumption in adulthood [r= 0.3, p=.11].
Fig. 3.
Significant correlations between adolescent and adult intake in Experiments 1-3. (a,b) In Experiment 1, intake of EtOH/SS and SS during adolescence was correlated with adulthood intake of 20% EtOH. (c,d) Experiment 2 and 3 show solution specific correlations, with significant correlations between adolescent and adult intake of EtOH/SS (Exp 2) and adolescent and adult intake of SS (Exp 3).
Experiment 2: Adult Intake of 10% EtOH in SS
A 2 (sex) x 4 (adolescent exposure: NM, H20, SS or EtOH/SS) repeated measures ANOVA of 10% EtOH/ SS (g/kg) intake across the 4 blocks of days in adulthood revealed significant main effects of condition [F(3,72)= 4.23, p<.01], sex [F(1,72)= 28.93, p<.01] and block [F(3,216)= 13.03, p<.01], as well as a significant block x condition interaction [F(9,216)= 2.64, p<.01]. Animals exposed to EtOH/SS as adolescents generally drank more EtOH/SS than the other groups, although the effect dissipated over blocks, with animals exposed to EtOH/SS as adolescents drinking significantly more EtOH/SS in adulthood relative to all other exposure conditions on block day 1, relative to H2O and SS on block day 2, and relative to only SS-exposed animals on block days 3 & 4 (see Fig. 2b). Adult females drank significantly more EtOH/SS (1.16 ± .05) than their male counterparts (0.83 ± .04).
EtOH/SS intakes (g/kg) and BECs on the last drinking day were significantly correlated [r= .65, p<.01], with intakes on this day ranging from 0.25 to 2.6 g/kg and BECs ranging from 0 - 74 mg/dl.
When assessing correlations between average adolescent intake and average EtOH/SS drinking in adulthood, a significant positive correlation emerged between adolescent EtOH/SS intake and adult EtOH/SS intake [r=0.49, p< .05] (see Fig. 3c). SS or H20 consumption in adolescence did not correlate with amount of EtOH/SS consumed in adulthood [r= 0.22, p=.35; r=-0.01, p=.98, respectively].
Experiment 3: Adult Intake of SS
A 2 (sex) x 4 (adolescent exposure: NM, H20, SS or EtOH/SS) repeated measures ANOVA across 4 blocks of SS (ml/kg) intake in adulthood revealed significant main effects of condition [F(3,72)= 4.04, p<.01], sex [F(1,72)= 43.95, p<.01] and block [F(3,216)= 35.67, p<.01], as well as significant block x condition [F(9,216)= 3.37, p<.01] and block x sex [F(3,216)= 3.44, p<.05] interactions. Animals exposed to SS during adolescence drank significantly more SS in adulthood relative to all other adolescent exposure groups during the first block and relative to animals in EtOH/SS and NM exposure condition in block 2, with no significant group effects evident in the last two blocks (see Fig 2c). Reminiscent of the other two experiments, adult females generally drank significantly more SS (30 ml/kg ± 2.0) than their male counterparts (17.8 ± 0.9), with females increasing their intake of SS slightly more rapidly across days than males (data not shown).
A significant positive correlation emerged between amount of SS consumed in adolescence with consumption of SS in adulthood [r= 0.62, p<.01] (see Fig. 3d), with a tendency for adolescent consumption of EtOH/SS to correlate with SS intake in adulthood as well [r= 0.4, p=.07]. Adolescent H20 consumption was not correlated with adult SS intake [r= -0.08, p=.74].
Discussion
Adolescent ethanol exposure via voluntary access to EtOH/SS increased adulthood consumption of EtOH/SS (Exp 2), an effect that appears to be specific to the EtOH/SS solution in that no elevations were observed in adult intake of 20% unsweetened ethanol (Exp 1) or SS alone (Exp 3). However, access to SS without EtOH during adolescence produced a similar effect, elevating later consumption of SS, but not either of the ethanol test solutions. In both cases, elevated intakes were most prominent in adulthood during early intake sessions, and dissipated as non-manipulated and water-exposed control animals began to consume more of each solution across days. Given that these increases were most prominent during the first part of the eight day intake procedure, increased consumption of EtOH/SS in adulthood after voluntary consumption of the solution during adolescence appears to be related to solution acceptability, perhaps related to familiarity of the test solution, rather than an ethanol-specific effect.
This model of ethanol access resulted in consistent ethanol intake levels during adolescence across all three experiments, producing average intakes of 1.2 g/kg ethanol during the 30 min access periods and BECs in the moderate range on the last drinking day. These levels are consistent with previous studies measuring voluntary access of EtOH/SS during adolescence (Broadwater et al., 2010; Ji et al., 2008). However, this voluntary consumption model produced BECs well below forced exposure approaches such as repeated intraperitoneal injection of 3 g/kg (Pascual et al, 2007; 2009; Sherrill et al., 2011), intragastric administration up to 5 g/kg (Coleman et al., 2011; Fleming et al., 2012; Maldonado-Devincci et al., 2010), and vapor inhalation exposures producing BECs between 200-260 mg/dl (Conrad and Winder, 2011; Diaz-Granados and Graham, 2007) that were used in some other studies assessing various long-term effects of adolescent ethanol exposure. Of these studies, only two investigated voluntary alcohol consumption in adulthood. Pascual and colleagues (2009) found increased consumption after adolescent exposure to 3 g/kg i.p., although the effect of adolescent ethanol exposure emerged only during the last two out of the 5 different drinking test paradigms utilized. Maldonado-Devincci et al. (2010) also found increased sweetened alcohol consumption in adulthood after intragastric ethanol exposure to 1.5, 3 and 5 g/kg during adolescence, with males exposed to the highest dose showing the most pronounced elevations in adult alcohol consumption. While it is possible that later elevations in consumption of even unsweetened ethanol might have emerged had higher adolescent exposure levels been attained in the current study, it is difficult to encourage consumption levels higher than those obtained here in a limited access drinking model using non-deprived, outbred rats. While we have promising data showing that a social intake model may support binge level BECs in some, but certainly not all Sprague-Dawley rats (Truxell et al., 2011 RSA abstract), another useful approach may be to utilize other strains or species that more readily consume ethanol to determine consequences of adolescent binge level ethanol consumption on adult ethanol intake (e.g., Strong et al., 2010).
Within the framework of the current study, another approach that was used to assess how different ethanol exposure levels can influence adulthood drinking was to correlate individual differences in adolescent ethanol consumption with later drinking in adulthood. These analyses revealed significant solution specific correlations, with adolescent EtOH/SS correlated with adult consumption of EtOH/SS (Exp 2), but not EtOH alone (Exp 1). A similar relationship also emerged between SS exposure during adolescence and SS consumption in adulthood (Exp 3). Together, these findings support the suggestion that adult consumption of a particular solution is related to amount of that solution consumed during adolescence. From such correlational analyses, however, it is not possible to dissociate whether these effects are driven by amount consumed or reflect inherent and relatively stable individual differences in intake propensity. What is clear is that the effect is solution specific, with no effect of adolescent water consumption (i.e., fluid consumption per se) on adult drinking in any of the 3 experiments. A significant positive correlation also emerged between adult 20% EtOH intake (Exp 1) and adolescent EtOH/SS consumption, as well as a marginally significant trend with adolescent SS consumption. Thus, propensity to consume sweet solutions (SS & EtOH/SS) appears to be related to subsequent unsweetened ethanol consumption, an effect that is consistent with previous reports of correlations between intake of solutions sweetened with saccharin and ethanol intake (Kampov-Polevoy et al., 1990; Overstreet et al., 1993).
Enhancement of adult intake levels as a result of solution familiarity is reminiscent of a previous study that found adolescent exposure to sucrose and sucrose-milk solutions increased consumption of both of those solutions in adulthood relative to water controls (Pian et al., 2009). Unlike our results, that previous study found a significant effect of adolescent exposure to sweetened solutions (without ethanol) on adult ethanol intake, although elevated ethanol intake in adulthood was only found when 2.5% EtOH was combined with 10% sucrose; when the concentration of EtOH was increased to 10%, animals that were exposed to sweetened solutions during adolescence showed similar (sucrose-exposed animals) or reduced consumption (sucrose milk-exposed) of 10% EtOH combined with 10% sucrose. Thus, the increased consumption of the lower concentration (2.5%) of sweetened EtOH seen in the Pian et al. (2009) study may still be attributable to familiarity to sweetened solutions, with the effect diminishing as ethanol concentration is increased, attenuating the sweet taste. When interpreted this way, these data are consistent with our findings that adolescent SS exposure did not elevate adult ethanol intake (Exp 1 & 2). The results of the current study extend Pian et al.’s familiarity effect on adult intake to include adolescent exposure to a sweetened solution that contains ethanol. These results are important given that human adolescents typically initiate alcohol use with sweetened ethanol solutions (Copeland et al., 2007), and perhaps indicate that acceptability of such solutions will be later maintained in individuals exposed to sweetened ethanol as adolescents. An important caveat to the results of the present study, however, is that it is unknown whether similar solution acceptability effects would be observed in animals not given access to the solutions until adulthood and later assessed for intake after the same exposure-to-consumption test interval. Given that adult rats do not voluntarily consume as much ethanol as adolescents (Brunell and Spear, 2005; Doremus et al, 2005; Vetter and Spear, 2007), comparing the effects of ethanol exposure between adolescents and adults utilizing a self-administration model inherently confounds amount of exposure with exposure age, limiting interpretability of the data. For instance, if the increase in ethanol intake seen after adolescent exposure was not evident following self-administration in adults, it would not be possible to decipher if this effect was driven by age of exposure or the relative lower ethanol intake evident in the animals self-administering ethanol in adulthood.
This series of studies suggests that solution acceptance associated with prior familiarization to the solution is an important factor that should be considered when assessing the influence of ethanol exposure during adolescence on ethanol intake in adulthood. Even in experiments that are not using a voluntary exposure method, animals experience cues associated with odor from expired alcohol (Molina et al., 1984), which provide some degree of familiarization to the alcohol cue during a later voluntary access period. Although challenging to control for, such familiarization effects may be detected through utilization of different ethanol concentrations and/or solutions at test in adulthood from that which animals were given access to as adolescents, as in the current study. Other models besides simple voluntary access, like operant intake procedures that can more readily assess motivational properties of alcohol intake (see Samson and Czachowski, 2003 for review), could also be utilized to determine whether increases in ethanol intake in adulthood after adolescent exposures are due to solution familiarity and/or biological alterations that influence ethanol’s rewarding properties.
Acknowledgments
The research presented in this paper was supported by NIAAA grant U01AA019972-NADIA Project
References
- Broadwater M, Varlinskaya EI, Spear LP. Chronic intermittent ethanol exposure in early adolescent and adult male rats: effects on tolerance, social behavior, and ethanol intake. Alcohol Clin Exp Res. 35:1392–1403. doi: 10.1111/j.1530-0277.2011.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr., He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Winder DG. Altered anxiety-like behavior and long-term potentiation in the bed nucleus of the stria terminalis in adult mice exposed to chronic social isolation, unpredictable stress, and ethanol beginning in adolescence. Alcohol (Fayetteville, N.Y.) 2011;45:585–593. doi: 10.1016/j.alcohol.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland J, Stevenson RJ, Gates P, Dillon P. Young Australians and alcohol: the acceptabllity of ready-to-drink (RTD) alcoholic beverages among 12-30-year-olds. Addiction (Abingdon, England) 2007;102:1740–1746. doi: 10.1111/j.1360-0443.2007.01970.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, III RCS, Knapp DJ. Binge Ethanol Consumption Causes Differential Brain Damage in Young Adolescent Rats Compared With Adult Rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Kalant H, Koob GF, Li TK, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcohol Clin Exp Res. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent Binge Drinking Leads to Changes in Alcohol Drinking, Anxiety, and Amygdalar Corticotropin Releasing Factor Cells in Adulthood in Male Rats. Plos One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal Of Substance Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicology And Teratology. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behavioural Pharmacology. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings, 2008 (NIH Publication No. 09-7401) National Institute on Drug Abuse; Bethesda, MD: 2009. [Google Scholar]
- Kampov-Polevoy AB, Kasheffskaya OP, Sinclair JD. Initial acceptance of ethanol: gustatory factors and patterns of alcohol drinking. Alcohol (Fayetteville, N.Y.) 1990;7:83–85. doi: 10.1016/0741-8329(90)90065-k. [DOI] [PubMed] [Google Scholar]
- Maldonado-Devincci AM, Badanich KA, Kirstein CL. Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol (Fayetteville, N.Y.) 2010;44:57–66. doi: 10.1016/j.alcohol.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Molina JC, Serwatka J, Spear NE. Changes in alcohol intake resulting from prior experiences with alcohol odor in young rats. Pharmacology, Biochemistry, And Behavior. 1984;21(3):387–391. doi: 10.1016/s0091-3057(84)80100-8. [DOI] [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL. Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcohol Clin Exp Res. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmi M, Ashizawa T, Sinclair JD, Kiianmaa K. Effect of prior ethanol experience on dopamine overflow in accumbens of AA and ANA rats. European Journal Of Pharmacology. 1996;315:277–283. doi: 10.1016/s0014-2999(96)00650-4. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Kampov-Polevoy AB, Rezvani AH, Murrelle L, Halikas JA, Janowsky DS. Saccharin intake predicts ethanol intake in genetically heterogeneous rats as well as different rat strains. Alcohol Clin Exp Res. 1993;17:366–369. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. The European Journal Of Neuroscience. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal Of Neurochemistry. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Milk consumption during adolescence decreases alcohol drinking in adulthood. Pharmacology Biochemistry and Behavior. 2009;94:179–185. doi: 10.1016/j.pbb.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyapali GK, Sik A, Penttonen M, Buzsaki G, Turner DA. Dendritic properties of hippocampal CA1 pyramidal neurons in the rat: intracellular staining in vivo and in vitro. The Journal Of Comparative Neurology. 1998;391:335–352. doi: 10.1002/(sici)1096-9861(19980216)391:3<335::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Samson HH, Czachowski CL. Behavioral measures of alcohol self-administration and intake control: rodent models. International Review Of Neurobiology. 2003;54:107–143. doi: 10.1016/s0074-7742(03)54004-1. [DOI] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behavioural Brain Research. 2011;225:104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano D, Smith RF. Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiology & Behavior. 2001;74:637–643. doi: 10.1016/s0031-9384(01)00623-0. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent Rats Exposed to Repeated Ethanol Treatment Show Lingering Behavioral Impairments. Alcohol Clin Exp Res. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacology, Biochemistry, And Behavior. 2009;92:68–75. doi: 10.1016/j.pbb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. "Binge" drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Hormones And Behavior. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2007 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-34, DHHS Publication No. SMA 08-4343) Rockville, MD: 2008. [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Samson HH. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacology, Biochemistry, And Behavior. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Truxell EM, Varlinskaya EI, Spear LP. A new “social drinking” paradigm reveals individual differences in intake. Alcohol Clin Exp Res. 2011;35:237. Summary of 2011 RSA Posters/Abstracts. [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time Course of Elevated Ethanol Intake in Adolescent Relative to Adult Rats Under Continuous, Voluntary-Access Conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol And Alcoholism (Oxford, Oxfordshire) 2009;44:547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behavioral Neuroscience. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Developmental Psychobiology. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]