Abstract
Objective
To evaluate the extent to which mild disruptions in ovarian function indexed by changes in menstrual cycle length may relate to cardio-metabolic and psychological health in pre-menopausal women.
Methods
Among 804 healthy, regularly-cycling women (ages 25–45, M=35.5 [5.5]), patterns of any change (shortening, lengthening, or increased variability) versus no change in menstrual cycle length were examined in relation to a composite of cardio-metabolic risk and individual risk factors (high-density lipoprotein [HDL], triglycerides, waist circumference, glucose, hypertensive status) as well as in relation to depression indicators (Center for Epidemiologic Studies Depression [CESD] score ≥16 [yes/no], lifetime depression diagnosis [yes/no], lifetime anti-depressant medication use [yes/no]). Models were also explored to test whether changes in menstrual cycle length mediated relations between depression history and cardio-metabolic risk.
Results
In covariate-adjusted models, compared to no change, any change in menstrual cycle length was associated with higher cardio-metabolic risk composite scores and lower HDL (p’s<.05). In addition, compared to no change, any change in menstrual cycle length was associated with a CESD score ≥16, having received a depression diagnosis, and having used an anti-depressant medication (p’s<.05). In exploratory analyses, any change in menstrual cycle length partially mediated the relation between depression history and cardio-metabolic risk (b=0.152, p=.040) which attenuated (b=0.129, p=.083) when any change in menstrual cycle length was covaried.
Conclusions
Findings suggest disruptions in ovarian function marked by subtle changes in menstrual cycle length may relate to aspects of cardio-metabolic, and psychological health among healthy, pre-menopausal women.
Keywords: ovarian function, menstrual cycle length, depression, cardiovascular risk, cardio-metabolic risk, metabolic syndrome
Although risk for cardiovascular disease (CVD) increases during the menopausal transition,1–12 the disease processes underlying the emergence of CVD during this period may begin pre-menopausally as is suggested by studies documenting fatty streaks as well as clinically significant atherosclerotic lesions in young, pre-menopausal women and adolescent girls.13–16 To date, however, factors contributing to atherosclerotic disease development pre-menopausally that may explain variability in CVD post-menopausally are poorly understood. In this context, Kaplan and Manuck have proposed the “precocious acceleration” hypothesis, suggesting that disruptions in ovarian function during the pre-menopausal period (even when mild) can promote atherosclerosis, leading to an accelerated course of disease and increased post-menopausal risk for CVD.17–18 This hypothesis is supported by studies in which women with apparent impairments in ovarian function, marked by anovulation, lower estrogen, and menstrual cycle irregularity, exhibited increased risk for CVD or more problematic CVD risk factor profiles.19–25
Poorer psychological health has been associated with disruptions in ovarian function indexed by menstrual cycle characteristics. Findings drawn from three, largely separate literatures show that i) women with psychiatric disorders frequently experience menstrual cycle abnormalities including increased irregularity and patterns of both shorter and longer cycle length26–29; ii) psychological stress, especially when extreme, can play an etiological role in the cessation of menses30–33; and iii) risk for depressive symptoms increases during peri-menopause, a time when menstrual cycles become less regular due to reproductive aging.34 Poorer psychological health has also been associated with risk for CVD. In particular, depression, including major and minor depressive disorder as well as depressive symptomatology, has been shown to predict incident myocardial infarction, cardiac-specific, and all-cause mortality.35–40 With respect to the examination of cardiovascular risk factors, depression, especially among women, confers risk for the development of metabolic syndrome41–43 and negative psychological factors more broadly are correlated (although not always41–42) with the individual components of metabolic syndrome (i.e., high-density lipoprotein [HDL], triglycerides, waist circumference, glucose, and blood pressure).43–45
To integrate and extend the existing literature, the primary goal of the current study was to evaluate whether mild disruptions in ovarian function among healthy, regularly-cycling women are related to markers of cardio-metabolic health as well as depression. Given the inter-relations between disruptions in ovarian function, cardio-metabolic risk factors, and depression, secondarily, we also explored a model in which disruptions in ovarian function were proposed to play a mediating role (at least partially) in linking depression and cardio-metabolic risk. The study objectives were pursued in a multi-ethnic sample of 804 healthy, regularly-cycling pre-menopausal women in whom we examined disruptions in ovarian function as marked by having experienced any change (i.e., shortening, lengthening, or becoming more variable) compared to no change in menstrual cycle length in relation to standard cardio-metabolic risk factors and in relation to current depressive symptomatology and depression history.
Methods
Participants
The current sample included participants in the Ovarian Aging (OVA) Study, an investigation of the correlates of reproductive aging. Participants were recruited through Kaiser Permanente (KP) of Northern California, a large, integrated health care delivery system that provides medical care to approximately one third of the population of Northern California. The KP membership compared to the population of Northern California is generally representative in its socio-demographic and health-related characteristics, especially when the comparison is limited to those with health insurance.46 Women were included in the OVA Study if they were between 25–45 years of age, had regular menses (i.e., able to predict the start of menses within 5 days), had their uterus and both ovaries intact, self-identified as white, African-American, Latina, Chinese, or Filipina, and were able to speak/read English, Spanish, or Cantonese. Exclusions included the self-report of major medical illnesses, use of medications affecting the menstrual cycle in the 3 months prior to study enrollment, and current pregnancy or breastfeeding.
The OVA Study protocol included an in-person medical history interview, trans-vaginal ultrasound, anthropometric assessment, blood draw, and self-report questionnaires. Of 1019 total participants, 804 women were included in the current analysis. Excluded women (n = 215) were due to the following: 1) menstrual cycle length outside of the 25–35 day range (n = 33); 2) use of a hormonal method of birth control in the past year (n = 25); 3) pattern of change not reflective of shortening, lengthening, or increased variability in menstrual cycle length (n = 127); or 4) missing information on a key variable related to reproductive history, cardio-metabolic health, or reproductive aging (n = 30). In addition, a subset of 553 women with complete questionnaire data were included in analyses examining depression. Questionnaire data were missing for women who were never administered the questionnaires because the questionnaires were not initially included in the study protocol (n = 123) and women who did not return the questionnaires (n = 128). The study protocol was approved by the University of California San Francisco Committee on Human Research as well as the Kaiser Permanente of Northern California Institutional Review Board. Informed, written consent was obtained from all study participants.
Measures
Menstrual cycle characteristics
In an in-person medical history interview, women were asked to report the number of days in their typical menstrual cycle over the past 12 months in categories: 25–27 days, 28–32 days, and 33–35 days. Women were also asked to report whether their typical menstrual cycle in the past 12 months reflected no change in length or had become shorter, longer, or more variable. In the current analyses, change in menstrual cycle length was coded as having experienced any change in menstrual cycle length (i.e., shortening, lengthening, or increased variability) versus having experienced no change in menstrual cycle length.
Cardio-metabolic risk factors
Cardio-metabolic risk factors, including high-density lipoprotein (HDL), triglycerides, waist circumference, glucose, and hypertensive status were assessed. Assays for HDL, triglycerides, and fasting glucose were performed by Quest Diagnostics (San Jose, CA). Lipids were assayed using enzymatic methods and fasting glucose was assayed by the glucose oxidase method. Waist circumference, taken as the average of 2 measurements, was derived from a standardized anthropometric assessment performed by a study nurse. Lastly, previously diagnosed hypertension (yes/no) and use of anti-hypertensive medications (yes/no) were derived from an in-person medical history interview; endorsement of one or both items was used as a surrogate for measured systolic/diastolic blood pressure which was not assessed in the study protocol. A cardio-metabolic risk composite score was derived for use in the current analyses by taking the mean of the standardized values of each of the five individual cardio-metabolic risk factors (with HDL reversed). Computation of a continuously-measured cardio-metabolic risk composite is consistent with several previous investigations.47–51
Depressive symptomatology and treatment history
Depressive symptoms were measured using the Center for Epidemiological Studies Depression Scale (CESD).52–53 The CESD is a 20-item, self-report questionnaire assessing depressive symptoms over the past week. Each item is scored on a 0–3 point scale. Response choices indicate the frequency with which each symptom (or item) is experienced, ranging from “rarely or none of the time (< 1 day)” scored 0 to “most or all of the time (5–7 days)” scored 3. Following the reversal of scores on 4 positively-worded questions, items are summed to produce a total score (ranging from 0 to 60) with higher values reflecting more depressive symptoms. A total score of ≥16 is a commonly used cut-off due to its correspondence with clinically significant levels of depression.53–54 The CESD is a well-established instrument with adequate reliability55–56 and validity.53,56–60 History of depression diagnosis and treatment were assessed using a standardized set of questions asking women to report whether they had ever received a diagnosis of and/or treatment for depression from a medical professional and the type of treatment that was received. Derived from the CESD and depression diagnosis/treatment history questions, depression variables examined in the current analyses included the CESD total score coded ≥16 (1 = yes, 0 = no), having received a depression diagnosis (1 = yes, 0 = no), and having used an anti-depressant medication (1 = yes, 0 = no). In addition, a lifetime history of depression composite score was computed by coding ever having received a depression diagnosis or used an anti-depressant medication (1 = yes, 0 = no).
Reproductive aging
Reproductive aging was indexed by concentrations of anti-Mullerian hormone (AMH), a biochemical marker of ovarian reserve.61–62 AMH, secreted by the granulosa cells of the pre-antral and small antral follicles of the human ovary,63 plays a key regulatory role in folliculogenesis by inhibiting initial recruitment of primordial follicles into the growing pool of follicles.64–65 Validity for the use of AMH as an indicator of reproductive aging stems from studies showing AMH correlates with the number of primordial follicles66; relates inversely to chronological age in adult women61,67; and predicts menopausal onset68–69 as well as ovarian response in treatments using assisted reproductive technologies70–71. AMH is also stable within and across menstrual cycles72–76 and is not affected by use of oral contraceptives.76–77 AMH was assayed using two commercially available ELISAs from Beckman Coulter (Marseille, France) both of which use a two-site sandwich immunoassay. The Immunotech assay was used for the majority of the sample (84%) until this assay was retired and the second generation assay (Gen II) was used for the remainder of the samples. Among 44 women on whom both assays were performed, regression analyses showed excellent correspondence between the assays (R2 = 0.94) which has also been demonstrated in prior studies.78–79 AMH values based on the Immunotech assay were adjusted using the equation of the line with Immunotech predicting Gen II. The Gen II assay sensitivity was 0.16 ng/mL, the intra-assay coefficient of variation (CV) was 1.4%, and the inter-assay CV was 12.5%.
Statistical Analyses
Logistic regression analyses were performed to evaluate cardio-metabolic risk as well as depression in relation to the odds of being in one of two groups of women: either having experienced a change in menstrual cycle length (i.e., shortening, lengthening, or increased variability over the past 12 months) or having experienced no change in menstrual cycle length. First, a cardio-metabolic risk composite score (representing 5 individual cardio-metabolic risk factors [HDL, triglycerides, waist circumference, glucose, and hypertensive status] with HDL reversed) was examined in relation to group membership. Secondly, in separate models, 4 indicators of current depressive symptomatology and treatment history (CESD score ≥16 [yes/no], depression diagnosis [yes/no], use of anti-depressant medications [yes/no], lifetime history of depression composite score [yes/no]) were examined in relation to group membership. Next, AMH was added to the models to determine whether associations between cardio-metabolic risk/depression and group membership were independent of variability in reproductive aging. All models included covariate-adjustment for age, race/ethnicity (represented by four dummy coded variables with white as the referent), socioeconomic status (SES) indexed by individual-level education (1=<HS/some HS; 2=HS grad/GED; 3=some college/AA/vocational school; 4=college graduate; 5=graduate school [PhD, MS]; 6=professional degree [MD, JD, DDS, MBA]), cigarette smoking (1=current/past smoking, 0=never smoked), physical activity level (indexed by MET-hours of moderate/vigorous activity in a typical week over the past 3 months,80 parity (1=1+ live births, 0=no live births) past use of a hormonal method of birth control (0=no history of use; 1=positive history of use), and menstrual cycle length (1=25–27 days, 2=28–32 days, and 3=33–35 days).
To explore a conceptual model of possible inter-relations between disruptions in ovarian function, cardio-metabolic risk factors, and depression, a series of regression analyses were then performed to evaluate whether change in menstrual cycle length might play a mediational role in linking depression to cardio-metabolic risk. In these analyses, depression was represented by the lifetime history of depression composite score, and cardio-metabolic risk was indexed by the cardio-metabolic risk composite score. In accordance with Baron & Kenny,81 conditions for testing for mediation were evaluated first by examining relations between 1) the lifetime history of depression composite score and change in menstrual cycle length (Path A); 2) change in menstrual cycle length and the cardio-metabolic risk composite score, controlling for the lifetime history of depression composite score (Path B); and 3) the lifetime history of depression composite score and the cardio-metabolic risk composite score (Path C). In models in which conditions for testing for mediation were met (i.e., Paths A, B, and C were all statistically significant), mediation was then tested by reevaluating Path C with additional covariate-adjustment for change in menstrual cycle length. Attenuation in Path C was interpreted to indicate that change in menstrual cycle length mediates the relation between the lifetime history of depression composite score and the cardio-metabolic risk composite score. Linear regression was performed when continuously measured outcomes were assessed and binary logistic regression was performed when dichotomously coded outcomes were assessed. All models included covariate-adjustment for the same variables defined in detail above (age, race/ethnicity, SES, smoking, physical activity level, parity, past use of a hormonal method of birth control, menstrual cycle length, and AMH).
Results
Sample description
Eighty-one percent (n = 650) of the sample reported experiencing no change in menstrual cycle length while patterns of shortening, lengthening, and increased variability were reported in 7% (n = 57), 5% (n = 38), and 7% (n = 59) of the sample, respectively. In Table 1, descriptive information for the total sample is provided as well as statistical comparisons between women coded: any change in menstrual cycle length (i.e., shortening, lengthening, or increased variability) and no change in menstrual cycle length. Women on average were 35.3 (SD=5.5) years old and the sample was ethnically diverse (29.7% white, 24% African-American, 22.9% Latina, 19.3% Chinese, and 4.0% Filipina). Regarding the covariates, women who experienced any change compared to no change in menstrual cycle length were more likely to report past use of a hormonal method of birth control (p < .01). Regarding the cardio-metabolic risk variables, women who experienced any change compared to no change in menstrual cycle length had higher cardio-metabolic risk composite scores, lower HDL, and were more likely to have received a hypertensive diagnosis/used anti-hypertensive medication (p’s < .05). Lastly, regarding the depression variables, women who experienced any change compared to no change in menstrual cycle length had higher CESD scores (13.7 versus 10.7), were more likely to have a CESD score ≥16 (p’s <.01), and were more likely to have received a depression diagnosis or used an anti-depressant medication (p’s <.001).
Table 1.
Comparison of women experiencing any change compared to no change in menstrual cycle length.
| Total (n = 804) | Any change (n = 154) | No change (n = 650) | Test Statistic | p | |
|---|---|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | Mean (SD) or % | |||
| Covariates: | |||||
| Age | 35.3 (5.5) | 36.0 (5.7) | 35.2 (5.4) | t = −1.52 | .129 |
| White | 29.7% | 34.4% | 28.6% | χ2 = 2.01 | .157 |
| AA | 24.0% | 28.6% | 22.9% | χ2 = 2.18 | .140 |
| Latina | 22.9% | 18.2% | 24.0% | χ2 = 2.39 | .122 |
| Chinese | 19.3% | 14.3% | 20.5% | χ2 = 3.05 | .081 |
| Filipina | 4.0% | 4.5% | 3.8% | χ2 = 0.16 | .690 |
| Educationg | 3.5 (1.3) | 3.6 (1.2) | 3.5 (1.3) | t = −0.97 | .802 |
| Smoking (% current/past) | 23.9% | 25.3% | 23.5% | χ2 = 0.22 | .640 |
| Physical activity level (MET-hours) | 8.8 (10.3) | 8.4 (9.1) | 8.9 (10.6) | t = 0.48 | .629 |
| Parity (1=1+live births, 0=no live births) | 44.7% | 47.4% | 44.0% | χ2 = 0.58 | .445 |
| Birth control (% past use) | 69.2% | 77.9% | 67.1% | χ2 = 6.87 | .009 |
| Cardio-metabolic factors: | |||||
| Cardio-metabolic composite | 0.0 (0.6) | 0.10 (0.6) | −0.02 (0.6) | t = −2.26 | .024 |
| HDL | 59.6 (15.8) | 57.1 (14.7) | 60.2 (15.9) | t = 2.19 | .029 |
| Triglycerides | 90.7 (60.5) | 93.1 (62.8) | 90.2 (59.9) | t = −0.54 | .588 |
| Waist Circumference | 84.9 (15.5) | 86.7 (16.9) | 84.5 (15.1) | t = −1.61 | .107 |
| Glucose | 87.3 (9.7) | 87.8 (9.1) | 87.2 (9.9) | t = −0.62 | .535 |
| Hypertension (% w dx) | 8.1% | 12.3% | 7.1% | χ2 = 4.64 | .031 |
| Reproductive Aging: | |||||
| AMH (ng/mL) | 3.1 (2.8) | 2.7 (2.6) | 3.2 (2.8) | t = 1.91 | .056 |
| (n = 553) | (n = 107) | (n = 446) | |||
| Depression: | |||||
| CESD score | 11.3 (8.3) | 13.7 (9.8) | 10.7 (7.8) | t = −3.39 | .001 |
| CESD 16+, (1=yes, 0=no) | 27.1% | 37.4% | 24.7% | χ2 = 7.06 | .008 |
| Lifetime depression dx, (1=yes, 0=no) | 8.7% | 18.5% | 6.5% | χ2 = 15.78 | <.001 |
| Lifetime antidepressant use, (1=yes, 0=no) | 8.5% | 18.5% | 6.5% | χ2 = 15.78 | <.001 |
| Lifetime depression composite | 12.5% | 24.1% | 10.0% | χ2 = 15.46 | <.001 |
Education was coded 1=<HS/some HS; 2=HS grad/GED; 3=some college/AA/vocational school; 4=college graduate; 5=graduate school (PhD, MS); 6=professional degree (MD, JD, DDS, MBA).
Are disruptions in ovarian function related to cardio-metabolic risk?
As reported in Table 2, results of logistic regression analyses showed that with each 1-unit increase in the cardio-metabolic risk composite score (reflecting the mean of the standardized values of 5 individual cardio-metabolic risk factors [HDL, triglycerides, waist circumference, glucose, hypertensive status] with HDL reversed) the odds of experiencing any change in menstrual cycle length was increased by 43% (OR = 1.429, 95% CI = 1.063–1.920) compared to experiencing no change in menstrual cycle length. In addition, with each 1 SD increase in HDL the odds of experiencing any change in menstrual cycle length was decreased by 24% (OR = 0.765, 95% CI = 0.628–0.933) compared to experiencing no change in menstrual cycle length. Greater waist circumference and hypertensive status were also associated with an increased odds of experiencing any change compared to no change in menstrual cycle length albeit at the level of a statistical trend (p’s <.10). Triglycerides and glucose were unrelated to categories of any change compared to no change in menstrual cycle length (p’s >.05). All results included adjustment for covariates, including age, race/ethnicity, SES, cigarette smoking, physical activity level, parity, past use of a hormonal method of birth control, and menstrual cycle length. When analyses were repeated with additional covariate-adjustment for AMH, the pattern of results did not change (results not shown), suggesting associations between cardio-metabolic risk and change in menstrual cycle length were not attributable to variability in reproductive aging.
Table 2.
Results reported from the final models of separate logistic regression analyses examining cardio-metabolic risk in relation to experiencing any change compared to no change in menstrual cycle length.a
| Any change in menstrual cycle length (n = 154) vs. No change (n = 650) | |||
|---|---|---|---|
| OR | 95% CI | p | |
| Cardio-metabolic risk composite | 1.429 | 1.063 – 1.920 | .018 |
| HDL | 0.765 | 0.628 – 0.933 | .008 |
| Triglycerides | 1.062 | 0.894 – 1.262 | .495 |
| Waist circumference | 1.209 | 0.984 – 1.484 | .071 |
| Glucose | 1.032 | 0.864 – 1.232 | .729 |
| Hypertension dx (1=yes, 0=no) | 1.727 | 0.950 – 3.139 | .073 |
Analyses included covariate-adjustment for age, race/ethnicity, SES, smoking, physical activity level, parity, past use of a hormonal method of birth control, and menstrual cycle length; individual cardio-metabolic risk factors (HDL, triglycerides, waist circumference, and glucose) were standardized prior to entry into the regression models.
Are disruptions in ovarian function related to depression?
As reported in Table 3, results of logistic regression analyses showed that having a CESD score ≥16, having received a depression diagnosis, and having used an anti-depressant medication were associated with a 2.1, 3.0, and 3.1 increased odds, respectively, of experiencing any change compared to no change in menstrual cycle length. Examination of the lifetime history of depression composite score, reflecting endorsement of having received a depression diagnosis or having used an anti-depressant medication [1 = yes, 0 = no] was associated with a 2.6 increased odds of experiencing any change compared to no change in menstrual cycle length. All results included adjustment for covariates, including age, race/ethnicity, SES, cigarette smoking, physical activity level, parity, past use of a hormonal method of birth control, and menstrual cycle length. When analyses were repeated with additional covariate-adjustment for AMH the pattern of results did not change (results not shown) suggesting associations between indicators of depressive symptomatology/treatment history and change in menstrual cycle length were not attributable to variability in reproductive aging.
Table 3.
Results reported from the final models of separate logistic regression analyses examining indicators of depressive symptomatology and psychiatric treatment history in relation to experiencing any change compared to no change in menstrual cycle length.a
| Any change in menstrual cycle length (n = 154) vs. No change (n = 650) | |||
|---|---|---|---|
| OR | 95% CI | p | |
| CESD score ≥16, (1=yes, 0=no) | 2.052 | 1.280 – 3.290 | .003 |
| Lifetime depression dx, (1=yes, 0=no) | 2.986 | 1.569 – 5.682 | .001 |
| Lifetime antidepressant use, (1=yes, 0=no) | 3.061 | 1.576 – 5.946 | .001 |
| Lifetime depression composite, (1=yes, 0=no)b | 2.615 | 1.469 – 4.657 | .001 |
Analyses included covariate-adjustment for age, race/ethnicity, SES, smoking, physical activity level, parity, past use of a hormonal method of birth control, and menstrual cycle length
The lifetime history of depression composite score is coded having received a depression diagnosis or having used anti-depressant medications (yes/no).
Does ovarian function play a mediating role in linking depression to cardio-metabolic risk?
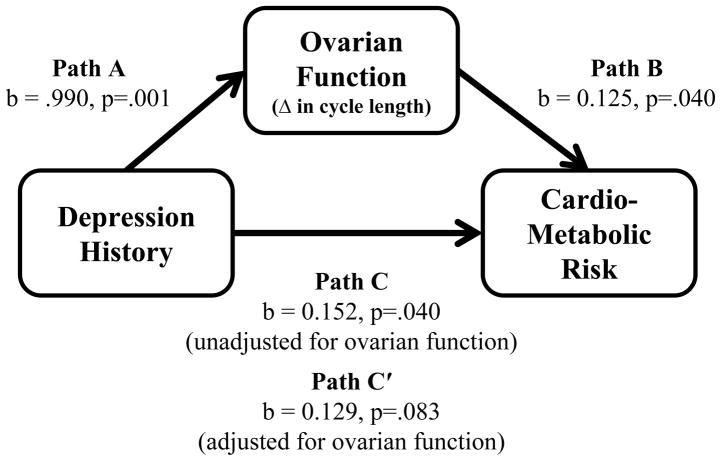
As reported in Table 4, in covariate-adjusted analyses (controlling for age, race/ethnicity, SES, cigarette smoking, physical activity level, parity, past use of a hormonal method of birth control, menstrual cycle length, and AMH), conditions for testing for mediation were met. That is, regarding Path A, the lifetime history of depression composite score was related significantly to change in menstrual cycle length (b = 0.990, p = .001, OR = 2.692, 95% CI = 1.507–4.808). Regarding Path B, change in menstrual cycle length was related significantly to the cardio-metabolic risk composite score (b = 0.125, p = .040), adjusted for the lifetime history of depression composite score. Regarding Path C, the lifetime history of depression composite score was related significantly to the cardio-metabolic risk composite score (b = 0.152, p = .040). When Path C was re-evaluated including additional covariate-adjustment for change in menstrual cycle length, the association between the depression composite score and the cardio-metabolic risk composite score attenuated (b = 0.129, p = .083), suggesting change in menstrual cycle length partially mediates the relation between the lifetime history of depression composite score and the cardio-metabolic risk composite score (see Figure 1).
Table 4.
Results reported from the final models of separate regression equations examining paths in the proposed meditational model to determine whether ovarian function indexed by change in menstrual cycle length mediates the relation between depression history and cardio-metabolic risk.a
| Path A | Path B | Path C | Path C′ | ||||
|---|---|---|---|---|---|---|---|
| Depressionb → Ovarian Fxc | Ovarian Fxc → Cardio-metabolic Riskd | Depressionb → Cardio-metabolic Riskd (unadjusted for ovarian function) | Depressionb → Cardio-metabolic Riskd (adjusted for ovarian function) | ||||
| b | p | b | p | b | p | b | p |
| .990 | .001 | 0.125 | .040 | 0.152 | .040 | 0.129 | .083 |
Analyses included covariate-adjustment for age, race/ethnicity, SES, smoking, physical activity level, parity, past use of hormone-containing medication for birth control, menstrual cycle length, and AMH.
Depression was represented by the lifetime history of depression composite score coded having received a depression diagnosis or having used anti-depressant medications (yes/no).
Ovarian function was indexed by change in menstrual cycle length coded any change vs. no change.
Cardio-metabolic risk was represented by the cardio-metabolic risk composite score reflecting the mean of standardized values of 5 individual risk factors (HDL, triglycerides, waist circumference, glucose, hypertensive status) with HDL reversed.
Figure 1.
Mediational model depicting the attenuation in the association between depression history and cardio-metabolic risk following adjustment for ovarian function.*
* Depression history was represented by the lifetime history of depression composite score coded having received a depression diagnosis or having used anti-depressant medications (1 = yes, 0 = no); ovarian function was indexed by change in menstrual cycle length coded any change (shorter, longer, or more variable) vs. no change; cardio-metabolic risk was represented by the cardio-metabolic risk composite score reflecting the mean of standardized values of 5 individual risk factors (HDL, triglycerides, waist circumference, glucose, hypertensive status).
Discussion
In cross-sectional analyses of 804 healthy, regularly-cycling pre-menopausal women, results indicated that any change in menstrual cycle length in the previous 12 months versus no change was associated with increased cardio-metabolic risk and depression. Associations were independent of statistical control for age, race/ethnicity, SES, cigarette smoking, physical activity level, parity, past use of a hormonal method of birth control, and menstrual cycle length. When AMH, a marker of ovarian reserve, was additionally covaried results did not change, suggesting variability in reproductive aging at least in this relatively young (mean age of 35) pre-menopausal sample of women does not account for associations between changes in menstrual cycle length and cardio-metabolic risk/depression. Lastly, results of exploratory analyses (albeit based on cross-sectional data) supported the proposed conceptual model suggesting that ovarian function may play a mechanistic role in linking depression to cardio-metabolic risk as demonstrated by results in which any change compared to no change in menstrual cycle length partially mediated the relation between lifetime history of depression and the cardio-metabolic risk composite score.
Each one-unit increase in the cardio-metabolic risk composite score (reflecting the composite of five individual cardio-metabolic risk factors, including HDL [reversed], triglycerides, waist circumference, glucose, and hypertensive status) was associated with a 43% increased odds of experiencing any change compared to no change in menstrual cycle length. This finding is consistent with prior studies in which menstrual cycle irregularity has been shown to be an independent risk factor for CVD.21–23,25 However, irregularity has been defined inconsistently and commonly represents more extreme patterns (e.g., very short [<21 days] or very long [≥40 days] cycles) that may reflect an underlying clinical condition such as polycystic ovarian syndrome. Other prior studies have also reported a longer menstrual cycle length was associated with CVD risk factors, including higher BMI and lower HDL19,82–83 although menstrual cycle length and not change in menstrual cycle length was examined in these studies. Thus, the current findings are unique in suggesting more subtle changes in menstrual cycle length among healthy, regularly-cycling women are also related to cardio-metabolic risk and that change in menstrual cycle length even independently of menstrual cycle length itself may contribute to this risk.
Indicators of depressive symptomatology and treatment history were associated with experiencing any change compared to no change in menstrual cycle length. Associations were present across a range of depression indicators representing normative variability in depressive symptoms as well as more clinically significant outcomes. In particular, the lifetime history of depression composite score, reflecting having ever received a depression diagnosis or having ever used an anti-depressant medication, was associated with a 2.6-fold increase in the odds of experiencing any change compared to no change in menstrual cycle length. Our findings are generally consistent with previous studies showing women with psychiatric disorders disproportionately experience abnormalities in menstrual cycle characteristics reflected by irregular cycles as well as patterns of both shorter and longer length.26–29 However, as mentioned previously with respect to the examination of cardio-metabolic risk, menstrual cycle length and not change in menstrual cycle length was examined in these studies.
All models were re-evaluated additionally including AMH as a covariate to account for the possibility that self-reported changes in menstrual cycle length may reflect accelerated reproductive aging which has itself been recently linked to depressive symptomatology and psychological stress.84–85 In descriptive analyses, mean levels of AMH were lower among women reporting any change compared to no change in menstrual cycle length which is consistent with studies suggesting menstrual cycles initially shorten with age.86–87 However, the inclusion of AMH in multivariate models did not account for associations between any change compared to no change in menstrual cycle length and cardio-metabolic risk/depression, indicating that reproductive aging did not make a significant contribution to the current model. It remains possible, however, that as the current sample ages and the number of women experiencing changes in menstrual cycle length increases, variability in reproductive aging may become a more prominent factor in explaining these changes.
The current study had several significant weaknesses, including that patterns of change in menstrual cycle length were self-reported and retrospective with women reporting changes in a typical cycle over the past 12 months. Also, depression indicators, having received a depression diagnosis and use of an anti-depressant medication, were derived from a self-report questionnaire and were not confirmed by other sources such as a diagnostic psychiatric interview or medical records. In addition, because of the small number of women in each change category (i.e., shortening, lengthening, or increased variability) we could not analyze these groups separately. Most notably, because the analyses were cross-sectional, the direction of association between the variables of interest cannot be determined. In particular, while we proposed on an exploratory basis a conceptual model suggesting that depression may negatively impact ovarian function as well as cardio-metabolic risk (possibly via disruptions in ovarian function), it is plausible that associations are actually bi-directional. That is, depressive symptomatology may also result from disruptions in ovarian function and cardio-metabolic risk factors may play a role in promoting depressive symptoms.88–89 A primary strength of the current study was its novel emphasis on examining disruptions in ovarian function among women who are healthy and regularly-cycling. In addition, the sample itself was well-characterized in terms of reproductive history, cardio-metabolic risk factors, and potential confounding variables.
Conclusions
On the whole, results provide indirect support for Kaplan and Manuck’s “precocious acceleration” hypothesis which proposes that even mild disruptions in ovarian function pre-menopausally may promote atherosclerosis.17–18 In summary, findings from the current study suggest that subtle disruptions in ovarian function marked by changes in menstrual cycle length are observable even in healthy, regularly cycling women and these changes (compared to no change) are associated with increased cardio-metabolic risk and depression. In addition, exploratory analyses suggest changes in menstrual cycle length may partially mediate relations between depression and cardio-metabolic risk although this finding should be considered preliminary only due to the cross-sectional nature of the data. Future longitudinal studies are needed to more fully examine the complex inter-relations between reproductive, cardio-metabolic, and psychological health in women and to delineate the mechanisms linking these areas.
Acknowledgments
Funding: Preparation of this manuscript and the research described here were supported by NIH/NICHD and NIH/NIA (R01 HD044876); NIH/NIA (K08 AG03575); NIH/UCSF-CTSI (UL1 RR024131); Brain and Behavior Research Foundation; and Robert Wood Johnson Foundation (045820).
Footnotes
The authors have nothing to disclose.
References
- 1.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke, The Framingham Heart Study. Stroke. 2009;40(4):1044–1049. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuster LT, Rhodes DJ, Gostout BS, Grossardt BR, Rocca WA. Premature menopause or early menopause: Long-term health consequences. Maturitas. 2010;65(2):161–166. doi: 10.1016/j.maturitas.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y, Hong XM, Wilker E, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196(2):590–597. doi: 10.1016/j.atherosclerosis.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parashar S, Reid KJ, Spertus JA, Shaw LJ, Vaccarino V. Early menopause predicts angina after myocardial infarction. Menopause-J N Am Menopause Soc. 2010;17(5):938–945. doi: 10.1097/gme.0b013e3181e41f54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colditz G, Willett W, Stampfer M, Rosner B, Speizer F, Hennekens C. Menopause and the risk of coronary heart disease in women. N Engl J Med. 1987;316:1105–1110. doi: 10.1056/NEJM198704303161801. [DOI] [PubMed] [Google Scholar]
- 6.Park HA, Park JK, Park SA, Lee JS. Age, menopause, and cardiovascular risk factors among Korean middle-aged women: The 2005 Korea National Health and Nutrition Examination Survey. J Womens Health. 2010;19(5):869–876. doi: 10.1089/jwh.2009.1436. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen BK, Nilssen S, Heuch I, Kvale G. Does age at natural menopause affect mortality from ischemic heart disease. J Clin Epidemiol. 1997;50(4):475–479. doi: 10.1016/s0895-4356(96)00425-8. [DOI] [PubMed] [Google Scholar]
- 8.vander Schouw YT, vander Graaf Y, Steyerberg EW, Eijkemans MJC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347(9003):714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 10.Palmer JR, Rosenberg L, Shapiro S. Reproductive factors and risk of myocardial infarction. Am J Epidemiol. 1992;136(4):408–416. doi: 10.1093/oxfordjournals.aje.a116513. [DOI] [PubMed] [Google Scholar]
- 11.Fioretti F, Tavani A, Gallus S, Franceschi S, La Vecchia C. Menopause and risk of non-fatal acute myocardial infarction: an Italian case-control study and a review of the literature. Hum Reprod. 2000;15(3):599–603. doi: 10.1093/humrep/15.3.599. [DOI] [PubMed] [Google Scholar]
- 12.Lin JW, Caffrey JL, Chang MH, Lin YS. Sex, menopause, metabolic syndrome, and all-cause and cause-specific mortality cohort analysis from the Third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2010;95(9):4258–4267. doi: 10.1210/jc.2010-0332. [DOI] [PubMed] [Google Scholar]
- 13.Dawson JD, Sonka M, Blecha MB, Lin WJ, Davis PH. Risk factors associated with aortic and carotid intima-media thickness in adolescents and young adults The Muscatine Offspring Study. J Am Coll Cardiol. 2009;53(24):2273–2279. doi: 10.1016/j.jacc.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strong JP, Malcom GT, McMahan CA, et al. Prevalence and extent of atherosclerosis in adolescents and young adults - Implications for prevention from the pathobiological determinants of atherosclerosis in youth study. JAMA-J Am Med Assoc. 1999;281(8):727–735. doi: 10.1001/jama.281.8.727. [DOI] [PubMed] [Google Scholar]
- 15.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults - Evidence from intravascular ultrasound. Circulation. 2001;103(22):2705–2710. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 16.Sutton-Tyrrell K, Lassila HC, Meilahn E, Bunker C, Matthews KA, Kuller LH. Carotid atherosclerosis in premenopausal and postmenopausal women and its association with risk factors measured after menopause. Stroke. 1998;29(6):1116–1121. doi: 10.1161/01.str.29.6.1116. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan J, Manuck S. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause - The Journal of the North American Menopause Society. 2008;15(4):768–776. doi: 10.1097/gme.0b013e31815eb18e. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: A primate continuum. Ilar J. 2004;45(2):89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- 19.Matthews KA, Santoro N, Lasley B, et al. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91(5):1789–1795. doi: 10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]
- 20.Gorgels W, vanderGraaf Y, Blankenstein MA, Collette HJA, Erkelens DW, Banga JD. Urinary sex hormone excretions in premenopausal women and coronary heart disease risk: A nested case-referent study in the DOM-cohort. J Clin Epidemiol. 1997;50(3):275–281. doi: 10.1016/s0895-4356(96)00367-8. [DOI] [PubMed] [Google Scholar]
- 21.de Kleijn M, van der Schouw Y, van der Graaf Y. Reproductive history and cardiovascular disease risk in postmenopausal women. A review of the literature. Maturitas. 1999;33:7–36. doi: 10.1016/s0378-5122(99)00038-9. [DOI] [PubMed] [Google Scholar]
- 22.Solomon C, Hu F, Dunaif A. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- 23.Punnonen R, Jokela H, Aine R, Teisala K, Salomaki A, Uppa H. Impaired ovarian function and risk factors for atherosclerosis in premenopausal women. Maturitas. 1997;27(3):231–238. doi: 10.1016/s0378-5122(97)00040-6. [DOI] [PubMed] [Google Scholar]
- 24.Bairey Merz C, Johnson B, Sharaf B. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–419. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- 25.Gast GCM, Grobbee DE, Smit HA, Bueno-de-Mesquita HB, Samsioe GN, van der Schouw YT. Menstrual cycle characteristics and risk of coronary heart disease and type 2 diabetes. Fertil Steril. 2010;94(6):2379–2381. doi: 10.1016/j.fertnstert.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 26.Barron ML, Flick LH, Cook CA, Homan SM, Campbell C. Associations Between Psychiatric Disorders and Menstrual Cycle Characteristics. Arch Psychiatr Nurs. 2008;22(5):254–265. doi: 10.1016/j.apnu.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisaga K, Petkova E, Cheng JF, Davies M, Feldman JF, Whitaker AH. Menstrual functioning and psychopathology in a county-wide population of high school girls. J Am Acad Child Adolesc Psychiatry. 2002;41(10):1197–1204. doi: 10.1097/00004583-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Joffe H, Kim DR, Foris JM, et al. Menstrual dysfunction prior to onset of psychiatric illness is reported more commonly by women with bipolar disorder than by women with unipolar depression and healthy controls. J Clin Psychiatry. 2006;67(2):297–304. doi: 10.4088/jcp.v67n0218. [DOI] [PubMed] [Google Scholar]
- 29.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668–674. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Pasternak A, Brooks PG. The long-term effects of the Holocaust on the reproductive function of female survivors. J Mimim Invasive Gynecol. 2007;14(2):211–217. doi: 10.1016/j.jmig.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Whitacre F, Barrera B. War amenorrhea. JAMA. 1944;124:399–403. [Google Scholar]
- 32.Bethea CL, Centeno ML, Cameron JL. Neurobiology of Stress-Induced Reproductive Dysfunction in Female Macaques. Mol Neurobiol. 2008;38(3):199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meczekalski B, Podfigurna-Stopa A, Warenik-Szymankiewicz A, Genazzan AR. Functional hypothalamic amenorrhea: Current view on neuroendocrine aberrations. Gynecol Endocrinol. 2008;24(1):4–11. doi: 10.1080/09513590701807381. [DOI] [PubMed] [Google Scholar]
- 34.Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN) Psychol Med. 2011;41(9):1879–1888. doi: 10.1017/S003329171100016X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anda R, Williamson D, Jones D, et al. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of United States adults. Epidemiology. 1993;4(4):285–293. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Aromaa A, Raitasalo R, Reunanen A, et al. Depression and cardiovascular diseases. Acta Psychiatr Scand. 1994;89:77–82. doi: 10.1111/j.1600-0447.1994.tb05807.x. [DOI] [PubMed] [Google Scholar]
- 37.Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93(11):1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- 38.Ford DE, Mead LA, Chang PP, Cooper-Patrick L, Wang NY, Klag MJ. Depression is a risk factor for coronary artery disease in men - The precursors study. Arch Intern Med. 1998;158(13):1422–1426. doi: 10.1001/archinte.158.13.1422. [DOI] [PubMed] [Google Scholar]
- 39.Penninx B, Beekman ATF, Honig A, et al. Depression and cardiac mortality - Results from a community-based longitudinal study. Arch Gen Psychiatry. 2001;58(3):221–227. doi: 10.1001/archpsyc.58.3.221. [DOI] [PubMed] [Google Scholar]
- 40.Pratt LA, Ford DE, Crum RM, Armenian HK, Gallo JJ, Eaten WW. Depression, psychotropic medication, and risk of myocardial infarction: Prospective data from the Baltimore ECA follow-up. Circulation. 1996;94(12):3123–3129. doi: 10.1161/01.cir.94.12.3123. [DOI] [PubMed] [Google Scholar]
- 41.Goldbacher EM, Bromberger J, Matthews KA. Lifetime History of Major Depression Predicts the Development of the Metabolic Syndrome in Middle-Aged Women. Psychosom Med. 2009;71(3):266–272. doi: 10.1097/PSY.0b013e318197a4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaysina D, Pierce M, Richards M, Hotopf M, Kuh D, Hardy R. Association between adolescent emotional problems and metabolic syndrome: The modifying effect of C-reactive protein gene (CRP) polymorphisms. Brain Behav Immun. 2011;25(4):750–758. doi: 10.1016/j.bbi.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pulkki-Raback L, Elovainio M, Kivimaki M, et al. Depressive Symptoms and the Metabolic Syndrome in Childhood and Adulthood: A Prospective Cohort Study. Health Psychol. 2009;28(1):108–116. doi: 10.1037/a0012646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luppino FS, Dortland A, Wardenaar KJ, et al. Symptom Dimensions of Depression and Anxiety and the Metabolic Syndrome. Psychosom Med. 2011;73(3):257–264. doi: 10.1097/PSY.0b013e31820a59c0. [DOI] [PubMed] [Google Scholar]
- 45.Whittaker KS, Krantz DS, Rutledge T, et al. Combining Psychosocial Data to Improve Prediction of Cardiovascular Disease Risk Factors and Events: The National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation Study. Psychosom Med. 2012;74(3):263–270. doi: 10.1097/PSY.0b013e31824a58ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gordon N. How Does the Adult Kaiser Permanente Membership in Northern California Compare with the Larger Community? Oakland, CA: Kaiser Permanente Division of Research; 2006. [Google Scholar]
- 47.Agarwal S, Jacobs DR, Jr, Vaidya D, et al. Metabolic syndrome derived from principal component analysis and incident cardiovascular events: The Multi Ethnic Study of Atherosclerosis (MESA) and Health, Aging, and Body Composition (Health ABC) Cardiol Res Pract. 2012 doi: 10.1155/2012/919425. Epub 2012 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanson RL, Imperatore G, Bennett PH, Knowler WC. Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes. 2002;51(10):3120–3127. doi: 10.2337/diabetes.51.10.3120. [DOI] [PubMed] [Google Scholar]
- 49.Hillier TA, Rousseau A, Lange C, et al. Practical way to assess metabolic syndrome using a continuous score obtained from principal components analysis. Diab tologia. 2006;49(7):1528–1535. doi: 10.1007/s00125-006-0266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kekalainen P, Sarlund H, Pyorala K, Laakso M. Hyperinsulinemia cluster predicts the development of type 2 diabetes independently of family history of diabetes. Diabetes Care. 1999;22(1):86–92. doi: 10.2337/diacare.22.1.86. [DOI] [PubMed] [Google Scholar]
- 51.Lempiainen P, Mykkanen L, Pyorala K, Laakso M, Kuusisto J. Insulin resistance syndrome predicts coronary heart disease events in elderly nondiabetic men. Circulation. 1999;100(2):123–128. doi: 10.1161/01.cir.100.2.123. [DOI] [PubMed] [Google Scholar]
- 52.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977 Sum;1(3):385–401. 1977. [Google Scholar]
- 53.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. Am J Epidemiol. 1977;106(3):203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 54.Breslau N. Depressive symptoms, major depression, and generalized anxiety - a comparison of self-reports on CESD and results from diagnostic interviews. Psychiatry Research. 1985;15(3):219–229. doi: 10.1016/0165-1781(85)90079-4. [DOI] [PubMed] [Google Scholar]
- 55.Nunnally JC. Psychometric theory. 1967. Psychometric theory: (1967) [Google Scholar]
- 56.Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clinical Gerontologist. 1986 Jun;5(1–2):119–136. 1986. [Google Scholar]
- 57.Blazer DG, Landerman LR, Hays JC, Simonsick EM, Saunders WB. Symptoms of depression among community-dwelling elderly African-American and White older adults. Psychol Med. 1998;28(6):1311–1320. doi: 10.1017/s0033291798007648. [DOI] [PubMed] [Google Scholar]
- 58.Clark VA, Aneshensel CS, Frerichs RR, Morgan TM. Analysis of effects of sex and age in response to items on the CES-D scale. Psychiatry Research. 1981;5(2):171–181. doi: 10.1016/0165-1781(81)90047-0. [DOI] [PubMed] [Google Scholar]
- 59.Hertzog C, Van Alstine J, Usala P, Hultsch D, Dixon R. Measurement properties of the Center for Epidemiological Studies Depression Scale (CES-D) in older populations. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1990;2(1):64–72. [Google Scholar]
- 60.Knight RG, Williams S, McGee R, Olaman S. Psychometric properties of the Centre for Epidemiologic Studies Depression Scale (CES-D) in a sample of women in middle life. Behav Res Ther. 1997;35(4):373–380. doi: 10.1016/s0005-7967(96)00107-6. [DOI] [PubMed] [Google Scholar]
- 61.van Rooij IAJ, Broekmans FJM, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–987. doi: 10.1016/j.fertnstert.2004.11.029. [DOI] [PubMed] [Google Scholar]
- 62.La Marca A, Sighinolfi G, Radi D, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16(2):113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 63.Weenen C, Laven JSE, von Bergh ARM, et al. Anti-Mullerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. doi: 10.1093/molehr/gah015. [DOI] [PubMed] [Google Scholar]
- 64.Visser JA, Themmen APN. Anti-Mullerian hormone and folliculogenesis. Mol Cell Endocrinol. 2005;234(1–2):81–86. doi: 10.1016/j.mce.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 65.Gruijters MJG, Visser JA, Durlinger ALL, Themmen APN. Anti-Mullerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211(1–2):85–90. doi: 10.1016/j.mce.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 66.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 67.Seifer DB, Baker VL, Leader B. Age-specific serum anti-Mullerian hormone values for 17,120 women presenting to fertility centers within the United States. Fertil Steril. 2011;95(2):747–750. doi: 10.1016/j.fertnstert.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 68.Broer S, Eijkemans M, Scheffer G, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96(8):2532–2539. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 69.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-Mullerian Hormone as a Predictor of Time to Menopause in Late Reproductive Age Women. J Clin Endocrinol Metab. 2012;97(5):1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Majumder K, Gelbaya TA, Laing I, Nardo LG. The use of anti-Mullerian hormone and antral follicle count to predict the potential of oocytes and embryos. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):166–170. doi: 10.1016/j.ejogrb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 71.Yates A, Rustamov O, Roberts S, et al. Anti-Mullerian hormone-tailored stimulation protocols improve outcomes whilst reducing adverse effects and costs of IVF. Hum Reprod. 2011;26(9):2353–2362. doi: 10.1093/humrep/der182. [DOI] [PubMed] [Google Scholar]
- 72.Fanchin R, Taieb J, Lozano DHM, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20(4):923–927. doi: 10.1093/humrep/deh688. [DOI] [PubMed] [Google Scholar]
- 73.Hehenkamp WJK, Looman CWN, Themmen APN, de Jong FH, te Velde ER, Broekmans FJM. Anti-Mullerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–4063. doi: 10.1210/jc.2006-0331. [DOI] [PubMed] [Google Scholar]
- 74.La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21(12):3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 75.Rustamov O, Pemberton PW, Roberts SA, et al. The reproducibility of serum anti-Mullerian hormone in subfertile women: within and between patient variability. Fertil Steril. 2011;95(3):1185–1187. doi: 10.1016/j.fertnstert.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 76.Streuli I, Fraisse T, Pillet C, Ibecheole V, Bischof P, de Ziegler D. Serum antimullerian hormone levels remain stable throughout the menstrual cycle and after oral or vaginal administration of synthetic sex steroids. Fertil Steril. 2008;90(2):395–400. doi: 10.1016/j.fertnstert.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 77.Deb S, Campbell BK, Pincott-Allen C, Clewes JS, Cumberpatch G, Raine-Fenning NJ. Quantifying effect of combined oral contraceptive pill on functional ovarian reserve as measured by serum anti-Mullerian hormone and small antral follicle count using three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2012;39(5):574–580. doi: 10.1002/uog.10114. [DOI] [PubMed] [Google Scholar]
- 78.Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 2010;362(1–2):51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 79.Nelson SM, La Marca A. The journey from the old to the new AMH assay: how to avoid getting lost in the values. Reprod Biomed Online. 2011;23(4):411–420. doi: 10.1016/j.rbmo.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 80.Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 81.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. Journal of Personality & Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 82.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89(6):2622–2631. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 83.Rubba F, Mattiello A, Chiodini P, et al. Menstrual cycle length, serum lipids and lipoproteins in a cohort of Italian Mediterranean women: Findings from Progetto ATENA. Nutr Metab Carbiovasc Dis. 2008;18(10):659–663. doi: 10.1016/j.numecd.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Bleil ME, Adler NE, Pasch LA, et al. Psychological stress and reproductive aging among pre-menopausal women. Hum Reprod. doi: 10.1093/humrep/des214. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bleil ME, Adler NE, Pasch LA, et al. Depressive symptomatology, psychological stress, and ovarian reserve: A role for psychological factors in ovarian aging? Menopause. doi: 10.1097/gme.0b013e31825540d8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mitchell ES, Woods NF, Mariella A. Three stages of the menopausal transition from the Seattle Midlife Women’s Health Study: toward a more precise definition. Menopause-J N Am Menopause Soc. 2000;7(5):334–349. doi: 10.1097/00042192-200007050-00008. [DOI] [PubMed] [Google Scholar]
- 87.Van Voorhis BJ, Santoro N, Harlow S, Crawford SL, Randolph J. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstet Gynecol. 2008;112(1):101–108. doi: 10.1097/AOG.0b013e31817d452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raikkonen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: Antecedent or consequence? Metab-Clin Exp. 2002;51(12):1573–1577. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- 89.Pan A, Keum N, Okereke OI, et al. Bidirectional Association Between Depression and Metabolic Syndrome A systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35(5):1171–1180. doi: 10.2337/dc11-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]